In their editorial, Gómez-Duarte1 suggests that the association of dipyrone with multiple adverse effects should lead to reconsider its use. We are of the opinion that such position neglects part of the available evidence, although we recognize that based on such evidence different views arise with regard to its use.2,3 Dipyrone is an analgesic agent widely used by the Colombian anesthesiologists. In our country, we have accumulated a vast experience in the area of acute postoperative pain and it is fundamental to assess the available evidence to determine the role of dipyrone in the practice of anesthesia in Colombia.
The safety profile of dipyrone is apparently no different from that of the non-steroidal anti-inflammatory drugs (NSAIDs), and is probably less harmful for the gut and the kidneys. Kotter et al4 conducted a systematic review on the safety of dipyrone in less than 2-week cycles, including randomized clinical controlled trials and comparing dipyrone against other analgesic agents. A total of 79 trials with almost 4000 participants were included, with the primary end-point being the incidence of adverse effects. There were no differences between Dipyrone and NSAIDs (relative risk 0.91, 95% confidence interval [CI] 0.79-1.05). It should be highlighted that the most salient negative aspect was that only 17% of the trials included in this paper had a low risk of bias, but in contrast, the strengths of this review included the high sensitivity of the search and the large number of participating patients. Gaertner et al5 conducted a systematic review assessing the impact of dipyrone in the management of cancer pain in adults (3 clinical trials and 1 observational study with a total of 252 patients); they documented a similar incidence of adverse effects for dipyrone and NSAIDs, with both medications being equally effective.5 Furthermore, Konij-nenbelt-Peters et al6 indicated in a study using VigiBase (the largest and most comprehensive pharmaco-vigilance database worldwide), that dipyrone could be safer for the GI tract and the kidneys, as compared to NSAIDs (Table 1). Some estimates have even concluded that the risk of fatal adverse effects with dipyrone is lower than with diclofenac, mainly due to the lower incidence of gastrointestinal bleeding when using dipyrone (25 vs 592 deaths per 100 million users).7 It should be mentioned that the safety profile might be comparable in the short term only, since there is not enough evidence supporting the use of dipyrone in the medium or long term.
Table 1 Odds ratio for reporting gastrointestinal and renal adverse effects6.
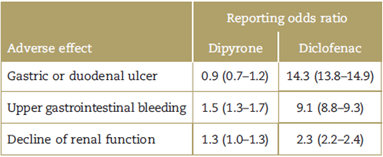
Source: Konijnenbelt-Peters et al.6
With regard to agranulocytosis, there are specific risk factors such as the dose, the time of exposure, and the population risk (associated with the presence of certain human leukocyte antigen alleles that increase the risk mainly in Caucasians).8 This happens in less 1 in 1 million prescriptions issued9 and is much less frequent in the pediatric population,3 although the evidence is still incipient. Many of the studies suggesting higher frequencies have been criticized because the incidence estimates were based on sporadic reports of adverse event records; in fact, due to the incomplete and sometimes selective reporting, it is not possible to estimate reliable incidences based on these sources of information.
In Latin America, according to the findings of the LATIN10 case-control study conducted between 2002 and 2005 in 7 hospitals in Brazil, Argentina, and Mexico, the documented incidence of agranulocytosis was of 0.38 cases per 1 million inhabitants/year. Huber et al11 conducted another case-control trial in Germany, through active surveillance in 51 hospitals in Berlin, from 2000 to 2010. The total standardized incidence of agranulocytosis by age and gender was 0.96 per 1 million inhabitants/year (9% CI, 0.95-0.97), with a mean treatment duration of 6 days; the cases identified were classified as "likely" or "possible", in keeping with the criteria of the World Health Organization, but there were no "certain" cases identified. In this paper, the most frequent indications for dipyrone were head ache and acute postoperative pain. Stamm-schulte et al12 analyzed the spontaneous reports of dipyrone-associated agranulocytosis in Germany. Out of 161 reports, 1/4 were on off-label prescriptions and 2/3 developed after 6 weeks of continuous and intermittent use (69.5% of the cases presented after 1 week); further-more, half of the cases received other concomitant medications involving the risk of developing agranulocytosis. Hence, there is a need to consider the limitations of the available evidence, particularly because of the type of epidemiological design used in these trials.13 Notwith-standingthese considerations, it is not appropriate to veto the medication on the basis of avoiding agranulocytosis, since the absolute risk seems to be low, though it seems advisable to regulate its use in specific clinical situations.
Moreover, the risk of hypersensitivity reactions-an-other fear leading to limit the use of dipyrone-is similar to that of diclofenac14 and even lower than penicillin.15 The incidence of agranulocytosis and hypersensitivity reactions should not be reason to veto the medication in the context of acute postoperative pain, when the anesthesiologist administers a single dose or a short cycle in a controlled environment.
The case report mentioned by Gómez-Duarte1 in his editorial refers to a patient that received the medication for more than 2 weeks. It is our opinion that the medication was used for an extended period of time. The Instituto Nacional de Vigilancia de Medicamentos y Alimentos (Invima-National Institute of Food and Drugs Surveillance) recommends avoiding the use of dipyrone for more than 1 week, in addition to stating that it should be administered by medical prescription only.16 Dipyrone has specific indications for safe use (fever that fails to respond to other therapies, intestinal colic-type pain, cancer pain, or other severe pain where other medications are not indicated); if the right doses are administered (doses commonly used in anesthesia: 10-20 mg/kg-doses recommended by the manufacturer: 6-16 mg/kg- maximum daily dose: 3-4g)17-19 and its administration is limited to a single dose or cycles of not more than 3 to 5 days, dipyrone may be used since agranulocytosis is considered to be a rare event in our population. We did not find any information supporting the use of higher than the abovementioned doses.
Notwithstanding the available evidence, and based on national pharmacovigilance data, the Invima indicates that dipyrone is a second-line analgesic-antipyretic agent in case of moderate-to-severe pain or fever that have failed to respond to other pharmacological (non-narcotic analgesics) and non-pharmacological options. We agree that dipyrone should not be an over-the-counter medication, that its use should be under medical supervision, and that, just as with any other drug, the patient should be informed about any potential adverse effects. Considering the high frequency of use (including first-line analgesic agent), and the gaps and limitations of the available evidence, we should then encourage the development of further studies to establish with certainty the safety profile in the Colombian population. For the time being, the available data and the experience accumulated with the use of the drug in Colombia suggest that a single dose, or a short cycle are safe in our population, particularly for acute postoperative pain.











 text in
text in 


