Introduction
Postoperative nausea and vomiting (PONV) are common issues arising after general anesthesia in the post-anesthesia care unit (PACU). 1,2 Research has enabled the identification of several independent risk factors (RF) for PONV associated with the patient, with anesthesia, and with the surgical procedure.3-5
Severe PONV, together with intraoperative awareness and postoperative pain, were strongly associated with patient dissatisfaction following anesthesia6; likewise, postoperative vomiting was the least desirable postoperative effect, together with incision pain and retching caused by the endotracheal tube.7
PONV may increase healthcare costs by extending the PACU stay and increasing the readmission of postsurgical patients after discharge from ambulatory surgery.8,9 Furthermore, severe nausea and vomiting may lead to adverse effects such as dehydration, electrolyte imbalance, opening of the surgical wound, bleeding under the skin flaps, aspiration of gastric contents, mediastinal emphysema, subcutaneous emphysema, pneumomediastinum and pneumothorax.10-13
The incidence of PONV reported between 1936 and 1990 ranged from 9% to 43% in different case series and reviews in Europe, North America, and Australia (from neonates to adults, both inpatients, and outpatients), with the use of inhaled general anesthesia (with classic and modern inhaled anesthetic agents), balanced general anesthesia, combined (regional and general) anesthesia, spinal, and local.14,15 However, another multicenter study reported a range between 39% and 73% in adults (mostly operated under general anesthesia and others using regional techniques).16
There are few reports of PONV in patients operated on under general anesthesia in Latin America; the incidence rates reported for hospitals in Colombia and Cuba are 10.9%17 and 15.4%,18 respectively. In Brazil, a study reported 18.5% of patients experiencing nausea and 8.5% vomiting during the postoperative period.19 In Peru, descriptive PONV studies in patients receiving general anesthesia are practically non-existent20; likewise, in our institution, most patients programed for cholecystectomy received general balanced anesthesia. This is why we decided to undertake this research project with the primary objective of establishing the cumulative incidence of PONV during the first 24hours after surgery. The secondary objectives were to determine the RF involved, the characteristics of PONV, the number of RF experienced by the patients according to the simplified Apfel21 scale and the quality of the postoperative antiemetic prophylaxis.
Methods
Study population
All adult patients over 18 years old, who were programed to undergo elective cholecystectomy (conventional and laparoscopic) under general balanced anesthesia at the Hospital EsSalud Talara, were selected. To estimate the sample, a pilot study was conducted (from October to December 2014) which found a cumulative incidence of PONV of 0.3. Hence, the necessary minimum sample size was estimated at 225 patients, in order to obtain a cumulative PONV incidence with a 12% interval amplitude and a 95% confidence level. In addition, the losses were estimated at 8%.
The patients that met all the inclusion criteria and gave their informed consent to participate from January 2015 to December 2016 were then selected. All patients with a body mass index (BMI) over 40kg/m2 (the simplified Apfel scale did not include patients with a BMI > 40), with previous antiemetic therapy, patients that requested to be discharged before the next 24hours after surgery, any patients who for any reason were unable to answer the interview and those programmed for elective surgery other than cholecystectomy under general anesthesia were excluded.
Drugs used in anesthesia
All the agents used were part of the usual anesthesia regimen of the surgical center. No premedication was administered and an intravenous induction with propofol or midazolam, fentanyl, and rocuronium was used. Sevoflurane or isoflurane were administered for maintenance. The neuromuscular block was reversed with neostigmine (<2.5mg) as needed. Analgesia was started intraoperatively with metamizole and/or ketoprofen. The postoperative antiemetic and analgesia prophylaxis was selected and prescribed by the treating surgeon and initiated in the PACU.
Data collection
At the time of admission to the surgical center, the following information was recorded through anamnesis and review of the medical record: age, gender, weight, size, BMI, programed surgery, RF according to the simplified Apfel scale for adults, American Society of Anesthesiologists physical status classification,22 and Goldman cardiac risk index. Patients were asked for their informed consent. The time of admission to the PACU marked the beginning of the 24-hour observation period. The duration of anesthesia and the antiemetic prophylaxis prescribed were recorded in the medical record.
PONV assessment
The assessment was conducted at the end of the observation period. The presence of the various PONV events (nausea without vomiting [N] nausea and vomiting [NV] and episodes of vomiting with no previous nausea [V W/O N] was assessed by questioning patients using a simple and clear language, in order to try to reduce any memory biases).
Nausea was defined as the desire to vomit with no expulsive muscle movements,15 while vomiting is the forceful oral expulsion of the gastrointestinal contents.15 The episode where no gastric contents were expulsed was called retching,15 and in this study was considered as vomiting. Similarly, retching and vomiting were grouped under the term emetic episodes (EE).23
In order to assess nausea (patients with N and NV) a 10 point visual analog scale (VAS) was used, with 0 representing no nausea and 10 the worst nausea sensation ever experienced.24 Hence, the patients expressed an overall score for the nausea experienced during the observation period. In the case of vomiting, the number of episodes was quantified21 patients with NV and V W/O N).
The quality of antiemetic prophylaxis was assessed based on the variables indicating the presence of antiemetic prophylaxis and adjusted prophylactic therapy, based on the patients' RF according to the Apfel scale. This adjustment was assessed with the regimen described by Apfel in which antiemetics are prescribed in accordance with the number of RF present in hospitalized patients, including pharmacological associations and the feasibility of administering total intravenous therapy.3
Statistical methods
The statistical analyses were conducted using StatCrunch and StatsDirect software.3 If more than 5% of the data were missing, the multiple imputation method was indicated. The corresponding univariate and bivariate analyses were conducted, including a normality test of the numerical variables (age, weight, age, size, BMI, duration of anesthesia) using the Kolmogorov-Smirnov test with Lilliefors correction, with a view to selecting the corresponding parametric and non-parametric tests. The statistical tests in the bivariate analysis included the calculation of Spearman correlation coefficients, Chi-square tests, Mann-Whitney tests and Student's t test. Finally, a binary logistics regression was performed to construct an association model according to the identification of statistically and clinically significant variables. Then, to obtain the adjusted model, the non-relevant variables were eliminated, any confounding factors were identified, the goodness of the adjustment was verified and a residue analysis was carried out.
Ethical considerations
The study followed the ethical principles of the Declaration of Helsinki, with the patients consenting to participate in the research project, and the STROBE guidelines to report observational research. The clinical database was confidential, the participants' data could not be disclosed except upon express authorization. The study protocol was approved by the hospital directorate.
Results
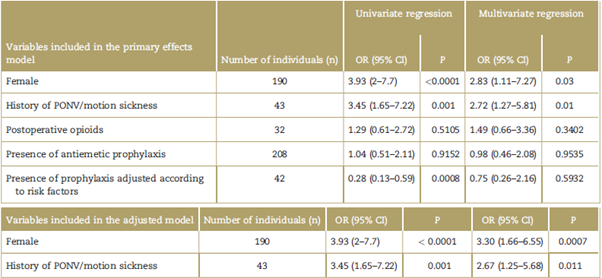
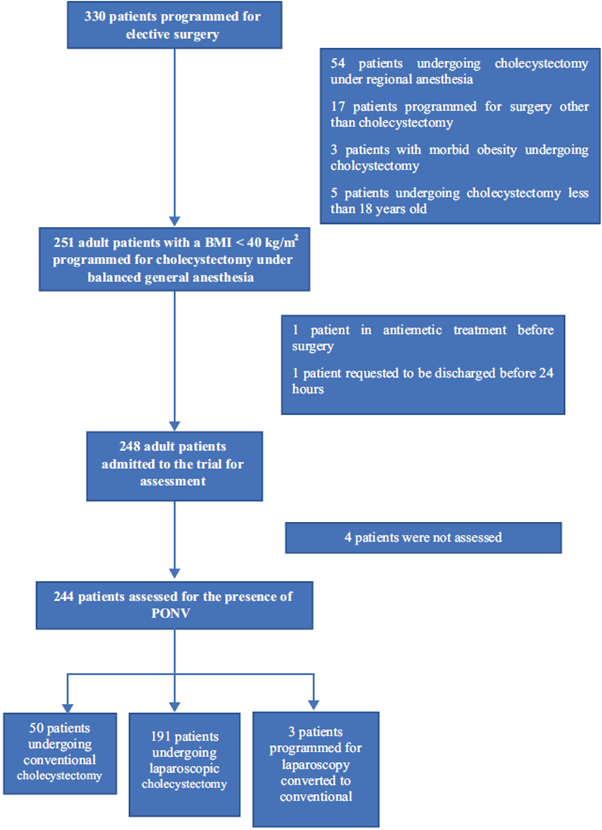
A total of 248 patients were included but 4 (1.6%) were not assessed, so the total number of patients assessed for the presence of PONV was 244 (the inclusion and exclusion processes are shown in Fig. 1). 77.9% were females, the mean age was de 44 years and the mean BMI was 27.2kg/m2 (see the baseline characteristics in Table 1).
Source: Authors.
Figure 1 Inclusion and exclusion flow diagram of the patients participating in the research project. BMI= Body Mass Index, PONV=Postoperative nausea and vomiting.
Table 1 Patient characteristics.
These figures represent the number of patients and percentages (%) unless otherwise stated. ASA=American Society of Anesthesiologists, IQR= interquartile range, PONV= postoperative nausea and vomiting. Other pathologies: hypothyroidism, migraine, dyslipidemia, Chronic coronary heart disease, and rheumatoid arthritis, SD = standard deviation. Source: Authors.
Descriptive and univariate analysis
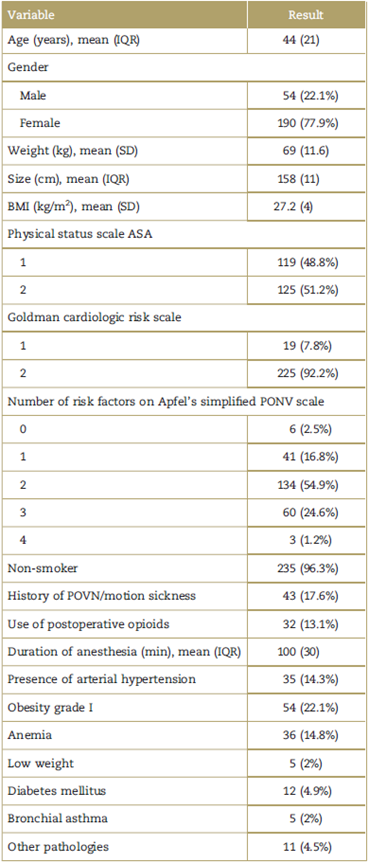
During the observation period, 124 patients experienced PONV, representing a cumulative incidence of 0.51 (95% confidence interval [CI] 0.45-0.57) in 24hours. The different types of PONV are shown in Fig. 2.
Source: Authors.
Figure 2 Types of PONV event. Note: The percentages are expressed with regard to the total study population (244 patients). PONV= postoperative nausea and vomiting.
With regard to the number of EE experienced, 63 patients (25.82%) experienced between 1 and 4 EE, 15 (6.15%) between 5 and 8 EE and 3 (1.23%) more than 8 EE. Likewise, in terms of the intensity of nausea (patients with N and NV), 41 (16.8%) reported a score between 1 and 4, the same number a score between 5 and 7, and 32 (13.11%) from 8 to 10 points in the VAS.
With regard to the antiemetic pharmacological prophylaxis (Table 2), most patients received prophylaxis during the postoperative period (85.25%), but only 42 (17.21%) received prophylaxis adapted to the patient's RF.3 Metoclopramide was used in 203 (83.2%) and dimenhydrinate in 5 (2.05%) (1 patient received both antiemetic agents). With regard to the patients experiencing PONV (124), 85.5% received antiemetic prophylaxis, but only 12.1% received rescue therapy (which was not prescribed in the postoperative indications in any of the cases). Likewise, 135 patients (55.33%) presented 2 RF in Apfel's simplified scale for PONV. In addition, the percentage of incidental cases of PONV are shown, for patients presenting with 0,1,2,3, and 4 RF (16.67%, 24.39%, 51.11%, 71.19%, and 66.67%, respectively).
Table 2 Patients presenting with PONV based on the number of risk factors in the simplified Apfel scale and exposure to antiemetic pharmacological prophylaxis.
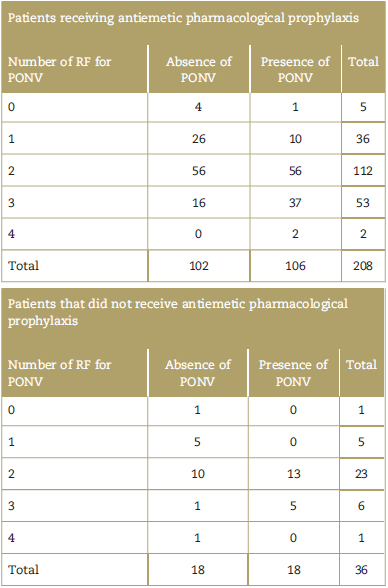
PONV=postoperative nausea and vomiting, RF=risk factors.
Source: Authors.
A normality test of the quantitative variables was also conducted, which indicated that weight and BMI variables followed a normal distribution.
Bivariate analysis
The Spearman coefficient values between the variables of duration of anesthesia and nausea intensity and the number of EE showed very weak correlations. Similarly, the cumulative incidence homogeneity test of PONV in patients undergoing conventional cholecystectomy, versus laparoscopic cholecystectomy, was not significant (P=0.065, odds ratio [OR] 1.78 95% CI 0.96-3.31).
The variables showing an association with the presence of PONV (any type of event) were female gender, history of PONV or motion sickness, and the exposure to prophylactic treatment adjusted according to the RF of the Apfel scale present in the patient (Table 3). The variables showing an association with the presence of nausea (patients experiencing N and NV) were: female gender (P < 0.001, OR 4.63 95% CI 2.25-9.52), history of PONV or motion sickness (P < 0.001, OR 4.22 95% CI 2.01-8.85), and exposure to adjusted prophylactic therapy (P = 0.0003, OR 0.25 95% CI 0.11-0.55). With regard to EE (patients affected with NV and V W/O N), there was statistical significance with the female gender (P < 0.001, OR 8.52 95% CI 2.95-24.56), absence of smoking (P=0.03), history of PONV or motion sickness (P < 0.001, OR 4.06 95% CI 2.05-8.06), and exposure to adjusted prophylactic therapy (P = 0.0003, OR 0.17 95% CI 0.06-0.50).
Table 3 Independent variables and their association with the presence of PONV as a dependent variable.
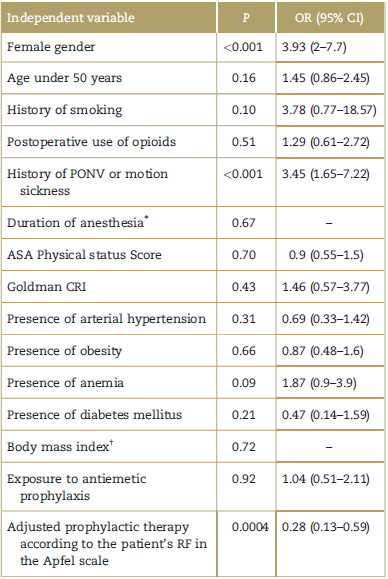
All of the statistically significant tests were conducted with the Chi square test, unless otherwise indicated. ASA= American Society of Anesthesiologists, CI=confidence interval, CRI = cardiac risk index, OR=odds ratio, PONV=postoperative nausea and vomiting.
* Mann-Whitney U test.
† Student's t test.
Source: Authors.
The variable PONV was not associated with the presence of antiemetic prophylaxis, but it was associated with the adjusted prophylactic therapy variable (Table 3).
Multivariate analysis
A binary logistics regression analysis (Table 4) was conducted, developing a model of primary effects with the statistically significant variables in the bivariate analysis (female gender, history of PONV/motion sickness, and presence of adjusted prophylaxis based on RF) and those of high clinical relevance (use of postoperative opioids, and receiving antiemetic prophylaxis) for the presence of PONV. Based on this higher complexity model, the non-relevant variables that were not related to the outcome and were not confounding factors were deleted, in order to obtain a model more adjusted to the principle of parsimony or economy. Then the adjustment of the Hosmer-Lemeshow goodness was estimated, which resulted in a non-significant P value (0.6155) and showed lack of disparity between the boxes for observed and expected results. Finally, a residue analysis was con-ducted (Pearson, Pearson standardized, Deviance) which failed to show any atypical data.
Discussion
This research project found a high cumulative incidence of PONV during the first 24hours after surgery, and this may be due to several reasons. First of all, due to the use of volatile anesthetic agents for the general anesthesia administered.5 Second, due to the type of surgery performed-cholecystectomy-(laparoscopic in 78.28% of the patients and conventional in 21.72%). On previous studies of patients undergoing these surgical procedures, a significant incidence was also reported. For instance, a systematic review reported a combined adjusted OR of 1.9 (1.36-2.68) based on 4 research projects in patients undergoing cholecystectomy; the same review also found a combined OR of 1.37 (1.07-1.77) in 8 patients undergoing laparoscopic surgery.4 Other 2 studies in patients under-going laparoscopic cholecystectomy reported an incidence of 69% with an adjusted OR of 3.23 (1.55-6.74) in the first one,25 and an OR of 2.85 (1.4-5.81) in the second one.26 Based on the above findings, notwithstanding the fact that most types of surgeries did not show to be independent predictors for PONV, laparoscopic cholecystectomies and gynecological procedures were considered exceptions.3
There are a number of previous studies reporting much lower incidences of PONV in the same period of observation (both in inpatients and outpatients receiving general anesthesia), which was probably due to the fact that they experienced different types of surgeries (not exclusively laparoscopic surgery or cholecystectomy).18,27,28 Likewise, previous research projects that included other types of anesthesia different from general anesthesia, also reported lower cumulative incidences,17,19,29 except for 1 case in which patients were not allowed to receive pharmacological prophylaxis.30 Moreover, a Peruvian study reported cumulative PONV incidences of 0.24 and 0.14 in laparoscopic and conventional cholecystectomy, respectively; however, this trial was retrospective and hence could have been subject to under registration of PONV.20
Other cause for the high incidence found could be the deficient antiemetic prophylaxis, since when analyzing the total number of patients receiving such prophylaxis, there were 113, 53, and 2 with 2,3, and 4 RF for PONV in the Apfel scale, respectively. However, almost all patients received prophylaxis with 1 single agent, which fails to comply with the risk-adapted multimodal approach (in accordance with the number of RF present in the patient) using drug combinations, and the availability of total intravenous anesthesia.3 Likewise, the preoperative assessment for the risk of PONV and the development of treatment guidelines adapted to the local setting, have been recently recommended.31
Most patients received 10 mg of metoclopramide prophylactically, a dose that proved to be effective in cesarean sections under neuraxial anesthesia32; but another review did not consider it effective to reduce the incidence of PONV.33 Moreover, the patients treated with dimenhydrinate antiemetic prophylaxis, did not receive the recommended dose (1mg/kg IV).33
Being a female and having a history of PONV or motion sickness, represented RF for the patients in our institution. One of the limitations of the trial is the absence of smoking which could not be assessed as a RF, since only 9 patients (3.7%) were smokers, making it difficult to consider in the analysis of logistic regression, since a low number of events per variable may lead to problems in the logistic model.34 The small number of smokers could be the result of the low consumption of cigarettes35 and the declining trend of smoking over the last few decades in Peru.36 This means that any future trials should consider a larger study population or a population with a higher incidence of smoking.
The use of postoperative opioids was not a RF in our institution, where low doses of tramadol were administered to 32 patients (13.11%). 65.63% of these patients received the drug BID (twice a day from Latin bis in dei), and 18.75% only once, 15.63% TID (3 times a day,-ter in dei- from Latin); and in no case was the maximum recommended dose for adults and adolescents reached or exceeded (400 mg/day).37 Along the same lines, the crossed validation study of the Apfel scale in 2 hospitals reported that this RF could be questionable, since it was only significant at 1 center that used higher doses (20 mg of oxycodone vs. 100mg of tramadol), and in most patients (80% vs. 10%) as compared with the other institution.21
In terms of the internal validity, the study patients in the sample were enrolled in a continuous and uninterrupted manner. However, despite the measures used in the patient history, there could have been a memory bias because a number of patients has already experienced PONV in previous surgeries, and these patients reported more accurately the characteristics of the adverse event, in contrast to those patients who had not experienced any PONV. Finally, in terms of the external validity, the results of the trial may be generalized to adult patients undergoing cholecystectomy under balanced general anesthesia in other healthcare institutions. However, the results cannot be extrapolated to patients receiving anesthesia, other than general anesthesia, or to pediatric or adolescent patients.
The following recommendations should be highlighted: (1) conduct the assessment using a PONV risk scale during the preoperative anesthesia consultation; (2) develop local hospital guidelines to prescribe prophylactic pharmacological and rescue regimens based on the risk of experiencing PONV; and (3) enter these regimens into the immediate postoperative indications.
Conclusion
This trial identified a high cumulative incidence of PONV in adult patients undergoing balanced general anesthesia for cholecystectomy: most of these patients had 2 RF according to the Apfel scale. The most frequent PONV-like adverse events were vomiting accompanied by nausea. The RF for PONV were being a female, and having a history of PONV or motion sickness. The antiemetic pharmacological prophylaxis showed some deficits in terms the doses and risk-associated adjustment; likewise, only a small number of affected patients received rescue antiemetic therapy, because it was not prescribed in the postoperative indications.
Ethical responsibilities
Protection of persons and animals. The authors declare that the procedures met the ethical standards of the committee responsible for experiments on humans and were consistent with the World Medical Association and the Declaration of Helsinki.
Confidentiality of the information. The authors declare that they have followed the protocols of their institution on the disclosure of patient data.
Right to privacy and informed consent. The authors have received the informed consent of all patients and/or subjects herein discussed. This document is under custody of the corresponding author.











 text in
text in