Introduction
Chest surgery in adults is associated with a high incidence of intense pain in the hours and days following surgery,1,2 and is also associated to high chronic pain rates.1,3 The best described management standard in the literature for postoperative pain in this setting is thoracic epidural catheter for continuous analgesic infusions.4,5 However, the paravertebral block (PVB) technique has been an analgesic strategy broadly equated with epidural analgesia in terms of clinical outcomes.1,2
The great advantages offered by PVB compared with the standard management in chest surgery is greater hemodynamic stability and a lower risk of complications derived from anticoagulation6; in addition, like the epidural technique, it allows the insertion of catheters with the advantages of continuous analgesia. Despite the above, a wide variability has been observed in the execution of the PVB technique,7-9 which limits its systematic application.
To incorporate PVB into routine anesthetic practice and guarantee the continuity of adequate analgesia through-out the postoperative period (POP), it is necessary to know which execution technique is associated with satisfactory analgesia in patients undergoing thoracotomy.
The objective of this review is to describe the different techniques of PVB placement used in adult patients undergoing open thoracotomy and which may perform better for post-operative analgesic management.
Methods
Systematic literature review with qualitative synthesis: We considered observational, analytical, and experimental studies, both prospective and retrospective, published without language restriction, including patients over 18 years old undergoing open chest surgery and using PVB as a single analgesic technique or in comparison with other techniques; in addition, the studies reported the block placement method and performed an analysis of the effectiveness thereof in the POP.
The analyzed interventions were the different techniques of analgesic PVB in single puncture or combined with continuous paravertebral space blockage by catheter in the different studies, namely: surface anatomy-guided PVB (PVB-AS) before surgery; ultrasound-guided preoperative PVB (PVB-US) with both flat and off-plane approaches; neurostimulation-guided PVB (PVB-NE) (these approaches include both single blocks and blocks associated with catheter insertion for continuous block-age), and PVB by direct surgeon visualization (PVB-C) with anesthetic injection and/or catheter insertion.
The main assessed outcome was postoperative analgesia measured by visual analog scale (VAS) and opioid consumption equivalent to morphine. The other assessed outcomes were the frequency of quality assessment of the block and the incidence of complications associated with the blockage.
Information sources and research strategy
Articles were researched without initial time restriction, but up until July 2018. The research was performed using Ovid Medline, Ovid Embase, Cochrane Central Register of Controlled Trials (CENTRAL), LILACS, and Scientific Electronic Library Online (Scielo), using a combination of controlled terms such as Medical Subject Heading (MeSH), Emtree and free text terms with various synonyms in English. The terms "Paravertebral block," "anatomic landmark technique," "paravertebral blockage," "Para-vertebral catheter," "block technique," "Catheter insertion," "Ultrasonography," "Transcutaneous Electric Nerve Stimulation," "postoperative pain," "thoracic surgical procedures," "Operative surgical procedures," "thoracic surgery," "Thoracotomy," and "Thorax/Surgery" were used.
The high sensitivity strategy was used (Annex 1CDC). In addition, manual and gray literature reference searches from controlled clinical trials were performed, and other systematic reviews of literature published in clinical trial registry databases, such as Clinicaltrials.gov, World Health Organization WHO International Clinical Trials Registry Platform Search Portal, controlled-trials.com, The National Institutes of Health, the Association of the British Pharmaceutical Industry European Medicines Agency, opengrey.eu, openSIGLEs (GreyNet International), and Google Scholar were performed. We also searched for abstracts published at conferences of major congresses in the area.
Selection of studies
The database of the studies and the collection of information from the bibliographic databases were carried out using the Mendeley program. Repeated studies and studies published in languages other than English or Spanish were eliminated. Two evaluators (EV-G and LMP-M) blindly and independently reviewed the study titles and abstracts resulting from the searches. One study was included in the full-text phase, when either reviewer considered it potentially eligible. Selected studies were then reviewed in full text by the 2 reviewers independently (EV and LP) and eligibility criteria were assessed for final inclusion. Disagreements were resolved via consensus, and in cases of no consensus, a third evaluator resolved on eligibility (FDC-A).
Data extraction and management
Data extraction from the studies was performed independently by the 2 initial reviewers in a pre-established format in Microsoft Excel, as well as using the tables offered by the RevMan 5.3 program. Disagreements were resolved through discussion. In cases of disagreement, a third reviewer was brought in to resolve it (FDC-A). The main data collection strategy used the variables contained in the Poblation - Intervention - Comparator - Outcome (PICO) question, as suggested by the Cochrane manual for this type of reviews.
Quality assessment
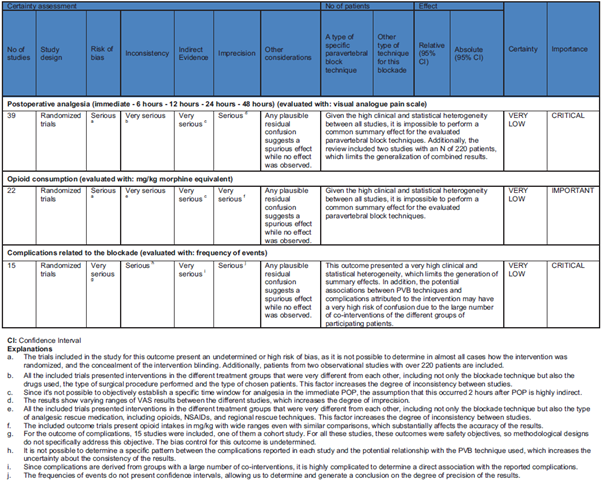
To assess the quality of the chosen studies, 2 investigators (EV-G and LMP-M) independently applied the Cochrane collaborative risk assessment tool, considering random allocation, allocation concealment, blinding of intervention, presence of complete data in the results, selective or non-selective results presentation, and other biases. This process was applied to the included clinical trials. In addition, the evidence was evaluated through each outcome through the system proposed by the Grading of Recommendation Assessment, Development and Evaluation (GRADE) Working Group, and that evidence was classified as follows: high, moderate, low, and very low. Several traditional GRADE factors were analyzed: bias risk, imprecision, inconsistency, absence of direct evidence, and publication bias. The Guideline Development Tool platform was used for this process.
Effect measurement
Pain VAS data were taken in the immediate POP at 6, 12, 24, and 48hours, from the intervention groups of each study where VAS was used. In addition, the consumption of opioid equivalent to morphine was reported at 24 and 48-hour POP. The results are reported by averages and standard deviation and are presented by means of bar and whiskers graphs for each technique used. For the remaining qualitative outcomes, frequencies, and incidences were presented.
Results
Characteristics of the studies
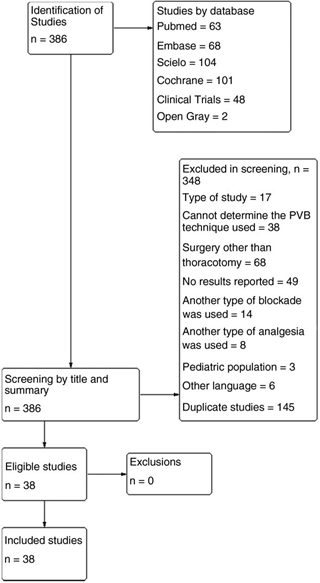
The research of the different bibliographic sources allowed to identify 386 studies, 348 of which were excluded. In total, 38 articles entered the systematic review (Fig. 1), 36 of which were controlled clinical trials and 2 were cohort type observational studies: Hutchins et al10 and Komatsu et al11 conducted a retrospective study of 185 patients undergoing thoracotomy, in which a paravertebral block with catheter was placed directly by the treating surgeon; the Hutchins study10 was conducted prospectively on 35 patients undergoing thoracotomy for lung transplantation, where the treating anesthesiologist inserted a US-guided paravertebral catheter. The other studies included in the review were 36 controlled clinical trials, 4 of which had PVB intervention in the 2 compared groups, making variations in the drug used: Ammar and Mahmoud12 compared the use of bupivacaine vs. the mixture of bupivacaine with magnesium sulfate in PVB-US; Bauer et al13 compared ropivacaine vs. ropivacaine plus Sufentanil in PVB-US; Dutta et al14 and Hassan and Mahran15 compared local anesthetic with and without steroid. In the other studies, PVB intervention was compared with another type of intervention strategy, with epidural analgesia as the main comparison, followed by intravenous analgesia with PCA (patient controlled analgesia) with fentanyl or morphine-type opioids, and intercostal or pleural blocks. The study by Fibla et al16 compares PVB in 2 types of thoracotomy: anterior vs. posterolateral. Moreover, in 2009, the same author17 evaluated PVB performed before surgery and after closing the thoracotomy. Garutti et al18 compared the preoperative PVB performed by the anesthesiologist with the PVB performed by the surgeon. Kaya et al19 compared single injection PVB vs. multiple thoracic injections.
The PVB placing technique used in 13 of the studies was PVB-AS.18,20-31 The main technique used by researchers in these studies was guidance through anatomical references previously taken by surface anatomy and, in a few cases, prior ultrasound marking on puncture site. Loss of resistance with both air and saline was performed, according to institutional guidelines or as established in the study protocol.
The preoperative US-guided technique was performed in 7 studies10,12-15,32,33 using high-frequency linear ultra-sound probes (10-16MHz). The main ultrasound technique was flat puncture10,12-14,33 with ultrasound location of the vertebrae's spinous processes, the transverse costal ligaments, the pleura, and the paravertebral spaces. The procedure was not only performed before the surgical incision, but it also required previous training in the ultrasound technique.
The insertion of a paravertebral catheter by the surgeon during the procedure (PVB-C) was performed in 18 studies,11,16-18,34-47 making it the most representative PVB technique, as it was evaluated in 46% of the studies included in the review. This approach was the one with the greatest variation throughout the included studies, given that the puncture technique changed substantially according to the surgeon's degree of surgical visualization during the procedure; in addition, the moments when the blockage was performed were highly inconsistent, given that in some studies it was performed at the beginning of the surgery, in others before the thoracotomy (by thoracoscopy before placement)25,26,28,34,48 and in others at the end of the surgical procedure.
Only the study carried out by Kaya et al19 used neurostimulation as a guide for the PVB, with a 50mm Stimuplex needle, an initial current of 2.5mA, 0.3ms, and 1 Hz, to stimulate the intercostal muscles and determine when to introduce the perineural catheter.
During most of the included studies, a catheter was left for continuous analgesic perfusion, except in the studies of Ammar et al,12 Li et al,33 Neuburger et al31, and Zhang et al,41 which were single-injection procedures in the paravertebral space. In the studies of Hill et al5 and Kaya et al,19 a single paravertebral injection block was performed in several thoracic spaces without leaving a catheter for continuous infusion. The table of abstracts of the included studies is shown in Annex 2 (CDC).
Risk of bias between studies
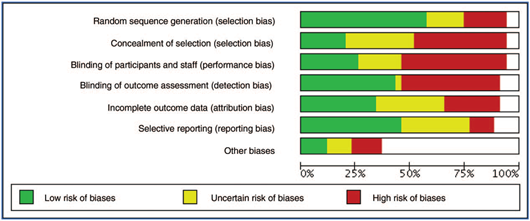
In 28 of the studies included in the review, patients were randomly assigned to the different groups; in 9 of the studies it was not possible to establish this factor, and in the 2 cohort studies (due to their observational nature) this criterion was not met. Over 50% of the studies included in the review did not meet the criteria of allocation concealment and patient and/or treatment staff blinding. The other bias assessment criteria were also not met in most studies or it was not possible to determine a control method, given the quality of the report. Figure 2 shows the summary of biases between studies and Annex 3 (CDC) reports the biases for each study.
Results synthesis
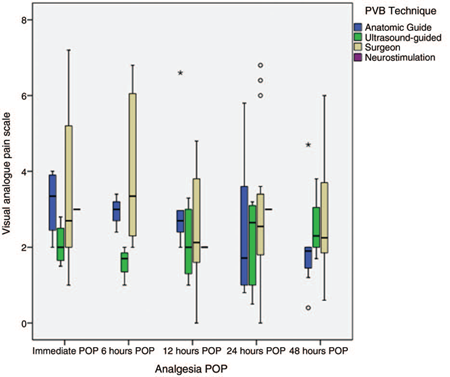
Postoperative analgesia.Figure 3 shows the mean VAS summary measure for the 4 techniques used. A table of all VAS reports for each study can be found in Annex 4 (CDC).
Source: Authors.
Figure 3 Pain control according to the paravertebral blockade technique. The results are expressed in averages with their maximum and minimum range. POP = postoperative, PVB = paravertebral block.
For the PVB-AS technique (n = 393), the mean pain in VAS was less than or equal to 4 in the immediate POP in the studies that reported it, as well as in the 6, 12, 24, and 48 post-operative hours in almost all the studies, except in that of Ouerghi et al21 and Kosiński et al,26 where POP pain had a high variability. Both studies compared epidural block versus PVB-AS, finding a difference in favor of epidural.
For the PVB-US technique (n = 279), it was found that studies that reported immediate POP pain10,12,13,32 had a mean pain according to the equivalent VAS of less than 3, and for 6, 12, 24, and 48 hours did not report pain averages greater than 4 in VAS. Ammar and Mahmoud12 and Li et al,33 who analyzed PVB with single puncture, showed VAS at all times at less than 3. The PVB-US technique showed the most consistency in analgesic results among the included studies.
Studies where PVB was performed under the surgeon's direct visualization (n = 771) reported the greatest variation in POP pain outcomes. The series of studies reported by the Fibla et al group16,17,43,44 found that this analgesic technique generated POP pain in VAS of no less than 5 to 6, as did the Tamura et al group40 and Perttunen et al,37 with VAS pain between 6 and 8, a trend that was maintained throughout the postoperative stay, that is at 6, 12, 24, and 48 of the POP. On the contrary, studies that achieved pain control, with VAS levels of less than 3 from the immediate POP, demonstrated maintaining the control trend until completing the 48 hours of the POP.
Opioid consumption. Opioid consumption in the different studies was evaluated inconsistently, since its measurement was highly variable even within the same study. Figure 4 shows the summary of morphine consumption for the POP techniques, and Annex 5 (CDC) shows the morphine consumption report for each study.
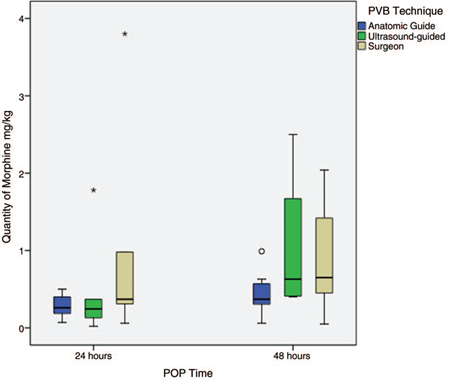
Source: Authors.
Figure 4 Consumption of morphine-equivalent opioid for each technique. The results are expressed in averages with their maximum and minimum range. POP=postoperative, PVB=paravertebral block.
Only in 8 of the PVB-AS studies was it possible to determine the consumption of opioids of morphine equivalents.18,21-23,26,27,30,31 Such studies saw a paravertebral catheter used as a POP analgesic technique.
On the other hand, 7 of the studies conducted by PVB-US reported the amount of morphine equivalent used for analgesic rescue.10,12-15,32,33 The study by Li et al33 showed a very high morphine consumption at 24 and 48hours (1.78 and 2.5 mg/kg, respectively). In this study, the PVB consisted of a single injection of ropivacaine 20cm3 plus dexamethasone, with no analgesic scheme other than a PCA of Sufentanil, compared with Ammar's study,12 which was also a single injection of bupivacaine 12 cm3 plus magnesium sulfate with a multimodal analgesia scheme, with a 0.42 mg/kg morphine equivalent consumption at 48 hours.
Opioid consumption was reported in only 7 of the studies that evaluated PVB-C as an analgesic technique,18,37,41,42,45,47,49 with high variability in the amount of morphine equivalent for pain management. The study by Baki et al42 reported a morphine equivalent consumption of 3.8 mg/kg at 24hours; however, approximately 50% of the patients who underwent PVB in this study had a poor location of the paravertebral catheter, which increased the need for analgesic rescue. No analgesic rescues are reported in the neurostimulation-guided PVB study.
None of the studies reported the time needed to request analgesic rescues in the evaluated groups.
Block quality evaluation frequency. The quality of the block was assessed and reported in 10 (25.6%) of the studies included in this review, mainly those that performed PVB-AS. The main evaluation method reported was the use of cold temperature to determine the degree of dermatomic block, followed by the puncture or pin prick method. Most of the included studies were clinical trials, and it is difficult to establish whether those who did not report the use of a specific blocking test technique would have effectively performed it within each other's working protocol.
Associated complications. In 5 of the studies conducted by PVB-AS, some type of complication was reported in the PVB group. The main reported complication was nausea and POP vomiting, followed by hypotension and urinary retention. Only 1 case of inadvertent epidural puncture and 3 cases of pleural puncture were reported.28
Only 3 patients included in the PVB-US studies reported any type of procedure-associated complication, mainly presenting hypotension, followed by POP nausea and vomiting, as well as symptomatic bradycardia. In 7 of the PVB-C studies, associated complications were reported, with nausea and vomiting as the main complications, followed by urinary retention and hypotension. Kadomatsu et al34 reported 1 case of arrhythmia and 2 cases of paravertebral hematoma requiring surgical management. Perttunen et al37 reported 4 cases of pruritus, 13 cases of drowsiness and 1 case of dyspnea (Annex 6 CDC).
Figure 5 shows the summary of biases found through the different outcomes according to the GRADE system.
Discussion
In the last decade, PVB has been shown to be an option comparable with the best described standard, the continuous thoracic epidural for post-thoracotomy pain control.5,44,50-52 In this systematic review we found variable analgesia among the 4 most frequently applied PVB techniques in clinical practice. A qualitative assessment showed a trend of superiority (VAS of <4) for the PVB-US and PVB-AS techniques, compared with the PVB technique under direct surgeon visualization, where an VAS of 6 or less was observed.
The analgesic superiority of PVB-US and PVB-AS over PVB under direct surgeon visualization can be explained by several reasons. In preventive analgesia, it is known that a preoperative axonal block reduces pain to a greater extent than its application after surgical incision. This blockage of the sodium channels avoids central sensitization and is associated with lower VAS pain scores, as well as with lower opioid consumption.53
There was a tendency for the PVB-US technique to provide better POP analgesia with a VAS frequency of 3.3 or less at 6, 12, 24 and 48 hours after the thoracotomy. This approach was associated with lower VAS in the immediate POP (2 hours) in the studies that measured this outcome, in comparison the other techniques. A recent review reports how using US can improve the rate of successful blockage in regional anesthesia and therefore explain this lower VAS trend.54
For PVB-AS, the qualitative analysis shows a tendency of pain in VAS comparable with PVB-US at 6, 12, 24, and 48 hours. This satisfactory analgesia gives PVB-AS a current utility in clinical practice. Although the growing use of US and its advantages have been described, the adequate analgesia also observed with PVB-AS is an alternative in centers with little experience in ultrasound. One potential reason behind the efficacy of PVB-AS, according to authors such as Costache et al,55 is that there is the possibility of blocking the spinal root in the paravertebral space even if the space is not actually penetrated, remaining in "paraspinal" location and in proximity to the transverse costal ligament.
This indirect way to reach the paravertebral space and the possible diffusion of the local anesthetic to the epidural space has also been described for blocks such as the erector plane of the spine (ESP) and the retrolaminar block.56-58 In the case of PVB-AS, satisfactory analgesia can be achieved despite not visualizing the needle, with the tip remaining in or near the paravertebral space. Costache's proposal55 is interesting in that it posits abandoning the belief that it is indispensable to always access the paravertebral space with the tip of the needle to achieve satisfactory analgesia, as it can be conversely achieved through proximity.
The analgesia observed in PVB trials under direct surgeon visualization, both in the immediate POP and in the first 48 hours, was the least satisfactory (VAS of 6 or less). The results in the latter group were divergent, with studies showing levels of early postoperative VAS between 5 and 7, such as the findings of Fibla et al,44 Tamura et al40 and Perttunen et al,37 with results favoring the use of epidural analgesia, which was the control used, a trend that was maintained in POP pain assessments.
As for opioid consumption, given that this outcome showed inconsistency and high variability, a qualitative assessment was only possible at 24 and 48 hours, where opioid consumption was comparable among the 3 techniques. However, the most extreme and variable values were found in the PVB group under direct surgeon visualization.42,45 On the other hand, higher doses of opioid rescue requirement were reported in the Li et al33 and Ammar and Mahmoud12 studies, which used single-injection PVB without placing the catheter for continuous analgesia. This supports the importance of continuous paravertebral catheter blockage or of resorting to alternatives that prolong the blockage, such as liposomal bupivacaine.59,60 It is worth highlighting that a tendency was observed upon 48 hours following PVB-US application toward increased morphine requirements, even above those catheters placed with an anatomical technique. It may be argued that, to ensure local anesthetic irrigation, a location close to the paravertebral space of the catheter with surface anatomy may provide satisfactory continuous analgesia; this could prove true even if the catheter is located close to the paravertebral space, in a compartment that allows a longer length of a multi orifice catheter to advance, for example, the undiagnosed location of the catheter in the ESP; this may provide it with some stability and explain a lower analgesic requirement once the anesthetic of the initial injection has been cleared.56,58
When evaluating how the dermatomic distribution was measured (i.e., the blockage quality), it is observed that it was highly infrequent (25.6%). Dermatomic sensory loss with pin-prick or ice was proven in PVB-US (2/7), PVB-AS (4/13), PVB-C (3/ 18), and PVB-NE (1/1). However, in the last decade there has been a tendency to increase blockage testing in a significant number of PVB trials in chest surgery (77%), with pin-prick or post-blockage temperature.12,15,19,20,26,28,40 This practice allows us to know if the blockage is really going to be useful beyond technique, to properly design a balanced anesthesia and analgesia plan based on it.
Recently, the dermatomic testing of PVB performed by Uppal et al61 showed that a single injection with ultrasound guidance allows blocking a similar number of dermatomes, compared with multiple injections of PVB (5 on average) after a single injection of 25 mL of local anesthetic. Based on the above, the testing of dermatomic sensory loss with pin-prick or ice is systematically recommended at least 20 to 30 minutes after PVB, as a quality standard.
In general, the complications reported with the different PVB techniques were low and minor. This low complication frequency is comparable with the one reported in previous studies.5,62 In Luyet's trial,28 3 cases of pleural injection were reported vs. no case in the PVB-US technique. The same study reported a case of epidural injection. Similarly, in the PVB-AS technique, Kosiński et al26 reported dyspnea in 11% of cases following blockage, which can be attributed to a possible pneumothorax, among other causes.
Norum and Breivik,5 in a systematic review of PVB vs. epidural in 2010, reports a frequency of intravascular injection of up to 3.8%, pleural puncture of 1.1% and pneumothorax of 0.5% for PVB. No reports of intravascular injection were found in the studies included in this review.
Limitations
The main limitation of the analysis was the lack of a detailed description of the different PVB placement techniques in the analyzed studies and, moreover, the lack of evidence from studies that directly compared 2 PVB techniques with each other. The major reference for comparing each technique reported in the literature is the thoracic epidural.40,62 Norum et al5 have reported comparable efficacy of the 3 PVB techniques in relation to the epidural; however, this group of authors also points out that some PVB comparisons in chest surgery with epidurals positioned below T7 may result in an "unfair" favorable result for PVB, especially considering the high heterogeneity of reporting the PVB technique.
By the time these results were reported, the technique that showed better analgesia and more homogeneous results in this review, that is, the PVB-US, had not yet reached a statistically significant number for a location of the paravertebral space and the positioning of a catheter that remained stable in that space or in its proximity. The only randomized clinical trial found that compared 2 different PVB techniques was the study by Garutti et al,18 where the PVB-AS technique was analyzed (by an anesthesiologist) vs. catheter insertion under direct surgeon visualization, and it was concluded that there were no significant differences in the effectiveness of the blockage for both techniques, but there was a halving of opioid consumption when the surgeon inserted the catheter. PVB advantages over epidural have been described, such as a better hemodynamic profile and lower risk of epidural hematoma.52 With the use of ultrasound, new comparisons are warranted to establish the best PVB technique and more accurately define its potential advantages over the standard thoracic epidural, ideally in the preoperative.
Another limitation for the proposed objective of this systematic review was the poor description of the different PVB techniques, which impacts both the evaluation of analgesic efficacy and technique reproducibility, as well as on the wide variability that can be found between medical centers using paravertebral block as an option for pain relief in chest surgery.
We believe that the quality of the included trials is generally similar, with intermediate-to-high risk of bias, as reported in the GRADE table.
In this sense, primary research type clinical trials are necessary to compare the different techniques of PVB localization, especially the potential sono-anatomical windows offered by ultrasonography for this purpose. Including observational studies and a comparison of their estimates with available clinical trials in this systematic review may lead to potential biases and additional heterogeneity. Therefore, the results should be interpreted cautiously and in light of these limitations.
In addition, in our opinion, with the advent of derived analgesic techniques such as blocking the ESP, the comparison of such technique with PVB may be the objective of a primary investigation of high clinical impact.
Conclusion
The systematic review suggests that ultrasound or anatomy-guided techniques are superior in analgesia compared with PVB under direct surgeon visualization. However, its effectiveness must be systematically verified before the anesthetic act. The increasing use and trend toward superior blocking rates and the safety of the PVB-US merit further research into the best sono-anatomy for its approach.
Ethical responsibilities
Protection of people and animals. the authors state that the procedures followed conformed to the ethical standards of the responsible human experimentation committee and in accordance with the World Medical Association and the Declaration of Helsinki.
Data confidentiality. the authors declare that they have followed their workplace protocols on the publication of primary patient data.
Right to privacy and informed consent. because the work is based on secondary data, no informed consent is necessary.











 text in
text in