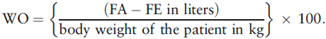Introduction
The enhanced recovery after surgery (ERAS) program comprises strategies and interventions aimed at attenuating the response to surgical stress and to enable the patient to return to his or herbaseline status and to family and social activities in the shortest possible time. The key elements are scientific evidence, multidisciplinary work, adequate communication among the actors involved in the care and management of patients, and the auditing processes on the implementation of the various strategies.1 Protocols for the different adult diagnostic and therapeutic procedures have been published. In general, these comprise between 17 and 23 strategies that involve the complete perioperative process, which have positively impacted complications, morbidity, and healthcare costs.2
Due to the physiological differences and to the lower morbimortality rates in children, it is difficult to adapt the adult ERAS protocols to the pediatric population.3 However, observational studies in colorectal surgery with ERAS,4 have documented similar outcomes to those observed in adults. Thus, the applicability and adaptation of these strategies is effective in reducing postoperative complications, shortening the hospital stay, earlier initiation of enteral nutrition, and lower healthcare costs in children.
The purpose of this article was to conduct a narrative literature review regarding the current evidence on the various strategies, within the framework of enhancing recovery in pediatric surgery, specifically in the context of major abdominal surgery, focusing primarily on colon surgery.
Methods
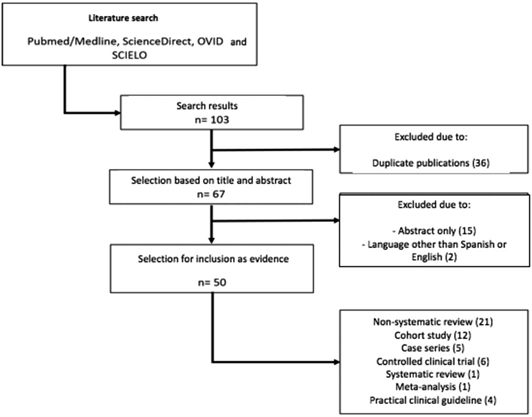
A literature search was conducted in various databases, including Pubmed/Medline, ScienceDirect, OVID, Scielo, using the words "surgery", "pediatric", "recovery", "anesthesia", "anaesthesia", "analgesia", "enhanced recovery", "fast track", "ERAS program," and "major abdominal procedures". The search and selection of the articles was conducted in an independent fashion, including meta-analysis, systematic reviews, clinical trials, observational studies and review articles. The date of publication was not a limitation. Both English and Spanish articles were included. Gray literature was not taken into account (Fig. 1).
Historical evolution
Henrik Kehlet introduced the concept of ERAS. He attributed the prolonged recovery to organic dysfunctions resulting from surgical stress and endocrine-metabolic changes.2 By acknowledging that no individual perioperative intervention can modify these physiological disorders, Kehlet and Mogensen published a study on the viability and efficiency of a multimodal rehabilitation regimen to promote the post-operative recovery of patients undergoing open sigmoidectomy. Using a combination of regional analgesia, oral feeding, and early postoperative ambulation, shortened the hospital stay from 10 to 2 days.5,6
Since the creation of the ERAS society in 2010, several publications have been made with specific recommendations for various surgical procedures.7 For several years, the focus on adapting ERAS strategies has been the pediatric population. Although the evidence is scanty, recently not only was the development of a protocol for enhanced recovery for children undergoing gastrointestinal surgery published,8 but also de results of its application, evidencing the reduction in the level of Intravenous (IV) fluids administered (FA), the reduction in the doses of opioids, faster initiation of oral feeding, shorter hospital stays, and lower healthcare costs.4
Physiological context
One of fundamental goals of the ERAS protocol is to reduce the response to surgical stress. Such response is represented by hormonal and metabolic changes resulting in hematological, immunological, and endocrine alterations; it is characterized by an elevation in counter-regulatory hormones (cortisol, growth hormone, glucagon, and catecholamines) induced by the activation of the hypothalamic-pituitary-adrenal axis, and an initial prevalence of pro inflammatory cytokines (Interleukin-1 and Interleukin-6), followed by anti-inflammatory cytokines. This response is stronger as the surgical trauma increases, and accounts for the development of insulin resistance in some patients.7
Strategies
Following is a discussion of some of the most important strategies included in the ERAS protocols in the context of major abdominal surgery (Table 1).
Parents and patient education
Children are more vulnerable to emotional stresses following surgery, and therefore the pre-operative preparation is important, not just by administering anxiolytics, but also facilitating postoperative recovery (compliance with the indications for early ambulation, respiratory therapy, inter alia). It is indispensable to get the parents involved in the education process, since their own stress may be transferred to the child, and this raises the level of anxiety. The surgical procedure to be conducted, the anesthetic technique, the likelihood of postoperative pain, the management after surgery, and any potential risks and complications must all be discussed. However, make sure that the education methodology is specific, accurate, and age appropriate.9,10
Colon preparation
Elective intestinal anastomosis is a surgical procedure frequently used in pediatric surgery. It is intended to restore intestinal continuity (closure of ileostomy or colostomy), to cure an inflammatory disease, or to correct an anatomical or functional congenital malformation in the colorectal region.11
Mechanical bowel preparation (MBP) in colorectal surgery is done using antibiotics and/or oral or rectal enemas for mechanical intestinal cleansing. Nevertheless, this is nowadays challenged with the argument of major collateral damage, in addition to the lack of scientific support.12 Such practice has been changing and it is estimated that only 1/4 of patients receive mechanical preparation before colorectal surgery.
Studies comparing the infectious complications in patients with mechanical preparation of the colon without the simultaneous administration of oral antibiotics in colorectal surgery,13 colostomy closure14 and augmentation cystoplasty,15 found no differences in the incidence of surgical site infection, intra-abdominal abscesses, and anastomotic leaks, but there was a longer hospital stay and greater discomfort. Other studies in colon surgery without MBP found that the risk of surgical site infection or anastomotic leakages does not increase, but there is a reduction in the length of stay and less patient discomfort.16,17
In 2015, Rangel published a literature review, and informed about the best current evidence for the prevention of infectious complications in colorectal surgery in children; hence, the conclusion was that the MBP is of no benefit. Furthermore, the use of non-absorbable oral antibiotics before surgery (neomycine and metronidazol) without MBP, significantly reduces the infectious complications and the length of stay.18,19
Pre-operative fasting
Prolonged fasting is dangerous and may result in a metabolic and immune response that triggers protein catabolism, insulin resistance, and decreased intravascular volume.20 In addition, it generates stress, irritability, and discomfort in children.
Encouraging the intake of clear liquids with carbohydrates (3 mL/kg or 1 ounce per each 10 kg of weight) up to 1 hour before the induction of anesthesia, avoids these undesirable effects and improves patient satisfaction.21,22 Such benefits extend into the postoperative period, by decreasing the length of stay and favoring bowel movement.20,23 For this reason, it is important that the doctor prescribes the time, the type, and the quantity of liquid to be administered to the patient before surgery.
Anxiety relief
Controlling pre-operative anxiety reduces the use of analgesics, the frequency of delirium and postoperative maladaptive behaviors.24,25 The most frequently used medications in pediatric care for this purpose are: midazolam, la clonidine, and dexmedetomidine.26,27 (Table 1). Non-pharmacological measures are also effective, such as the use of videos and music therapy.26
Evaluating the risk of thromboembolism
The risk of venous thromboembolism must be established in children, in order to decide which preventive measures are most appropriate (pharmacological and non-pharmacological). The international guidelines suggest the use of gradient compression stockings or pneumatic compression device in individuals over 13 years old, regardless of the risk of a thromboembolic event. Postoperative early ambulation is recommended. 28
Surgical site infection prevention
Surgical site infection prevention (SSI) is the most common healthcare-associated infection. It prolongs length of stay, increases by 2 to 11 fold the risk of mortality, and raises costs. Healthcare institutions should implement pre-operative antibiotic prophylaxis guidelines based on the surgical procedure, the most frequently SSI-associated pathogens, and the bacterial resistance profile.29 Furthermore, the following actions are recommended: maintaining normal temperature, chlorhexidine body wash the night before surgery, methodical skin preparation for a specifically determined period of time in the operating room with chlorhexidine, and changing gloves after completing certain steps of the procedure that may cause contamination (i.e., following intestinal anastomoses).
Minimally invasive technique
The use of minimally invasive techniques such as laparoscopy, has proven to reduce inflammation, shorten the length of stay, and lower perioperative morbidity; hence, it is an independent predictor of early tolerance for liquids and solids, faster intestinal transit, and shorter time to first bowel movement.30
Use of gastric tubes and drains
Gastric tubes are used to decrease abdominal distention due to air and secretions during the postoperative period, and are intended to prevent nausea, vomiting, pulmonary complications, development of fistulae, wound complications, and to shorten length of stay. However, recent publications have not shown the benefits described.11,4,31-33 Studies in colon resection surgery avoiding the use of catheters and drains report earlier start of oral feeding, increased patient comfort, and shorter length of stay.34
Gastric tubes and urinary catheters may be removed in the immediate postoperative period, or maximum 24 hours after surgery, without patient safety concerns and additionally with improved patient comfort. With regard to the use of abdominal drains in colorectal surgery, there are no specific studies showing better results, with or without them. However, all research projects using ERAS as the protocol in pediatric surgery, removed those drains within a maximum of 48hours, with no associated complications.
Analgesia: use of regional techniques and opioids
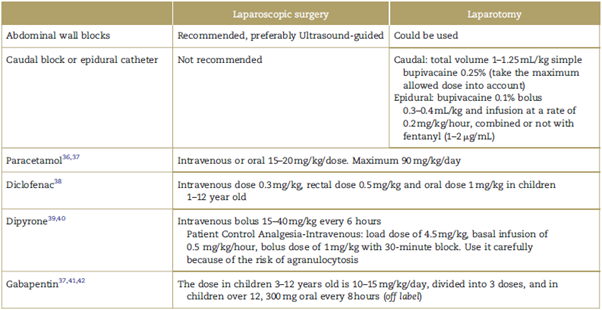
Opioids have secondary effects that prolong postsurgical recovery and delay hospital discharge and return to the baseline condition. We, as anesthesiologists, can influence the success of the ERAS protocols with adequate pain control, using a multimodal analgesia strategy to reduce the use of opioids3,35-42 (Table 2).
Normothermia
Perioperative hypothermia as a preventable event, represents a risk factor for increased bleeding, and for the need to administer blood transfusions, the development of hydroelectrolytic disorders, cardiovascular complications due to increased concentrations of serum catecholamines, and increased SSI, inter alia. The use of various heating techniques, both passive and active, is recommended, as well as intraoperative temperature monitoring.43
Use of fluids during the perioperative period
The purpose of using electrolyte solutions is to maintain tissue perfusion without causing edema. Any excess or deficit of fluids has deleterious effects on the patient's homeostasis. Pre-operative dehydration should be avoided and the intake of clear fluids should be encouraged, as previously stated. Baseline needs (BN) should be met in accordance with the 4-2-1-Holliday-Segar formula, using isotonic solutions (IS). In neonates. Between 1% and 2.5% dextrose should be added to meet the BN and prevent hypoglycemia (3 mg/kg/min). The use of electrolytes in dextrose should be avoided due to the risk of hyponatremia.44-46
Any deficits in intraoperative fluids should be replaced with IS, preferably using balanced solutions. Fluid loads should be administered over an interval of 20 minutes, since a rapid administration results in more fluid distribution into the interstitial space, failure to achieve intravascular volume expansion, damage of the glycocalyx, and the development of edema. In surgery, excessive base, CO2 delta, and lactate are helpful to guide fluid replacement. Pulse wave variability measurements and the elevation of the lower extremities test are of little use in children under 5 years old. In children, the best parameter to predict who will be volume-responders is the variation in aortic flow peak velocity.47,48
Fluids should be administered through infusion pumps and one catheter should be used for BN and a different one for administering boluses.
An increase of more than 10% of the body weight due to water overload (WO) is associated with longer Intensive Care Unit stay, more days on mechanical ventilation, surgical site infection, and additional medical care costs. Therefore, the recommendation is to do a daily follow-up of the total fluids administered (FA) and eliminated (FE), in accordance with the following formula:
During the postoperative period, intravenous fluids should be prescribed every 12 hours instead of every 24 hours, as is traditionally the case, to constantly adjust the dose in accordance with the patient's condition.(49 The recommendation during this period is to administer only 70% of the BN of fluids and discontinue them as soon as oral feeding is reinitiated.
Nausea and vomiting prevention
The Eberhart scale is recommended to determine the risk of postoperative nausea and vomiting, as well as the use of pharmacological strategies in accordance with the risk and characteristics of the patients.50
Conclusion
The ERAS protocols have shown a positive impact on the recovery of adult patients. There is limited evidence on the value of these protocols among the pediatric population undergoing major abdominal surgery, since they have been developed based on the results obtained with ERAS in adults, rather than based on the fundamental question leading to its development which was originally asked by Henrik Kehlet: Why is the patient still in hospital today?
In addition, there is a big disparity between the number of trials that evaluate the effectiveness of the ERAS protocols in adults and in children. There are studies showing the effectiveness of some individual strategies, but these have not been evaluated when integrated to other strategies in 1 same protocol. It is also unclear whether those protocols are applicable to all age groups in which there are marked differences; for example, in terms of the physiological response to stress, in the risk of venous thromboembolism, and in the ability to ambulate.
There are also controversies about the use of insulin to correct intraoperative hyperglycemia among this group of patients, because it is not clear whether the benefit justifies the risk. Furthermore, medicines such as liposomal bupivacaine have not been integrated yet into these protocols and therefore their impact is unknown.
Notwithstanding the above, the evidence points to achieving better results in children when the strategies of the ERAS protocols are adopted.
It is essential for institutions to promote the establishment of surgical groups that develop, adopt, and apply these types of protocols in all pediatric units in the country. The experience acquired should be followed-up regularly, assessing the outcomes within a strategy of continuous improvement and evaluation.











 texto em
texto em