Introduction
Malignant hyperthermia (MH) is an acute hypermetabolic syndrome triggered by certain drugs, mainly succinylcholine, and inhaled anesthetics.1 In less than 1 hour, a MH crisis may progress toward an irreversible metabolic disorder which is usually lethal.2 The incidence of MH may vary by age, gender, race, and geographic region.3-6
Dantrolene is the only drug available clinically for the specific treatment of MH crises.7,8 Guidelines recommend keeping a permanent stock of dantrolene in the operating theater, ensuring availability within 10 minutes of making the diagnosis.9 Before the introduction of dantrolene as standard treatment for MH, in the first description, lethality identified in 1 family was 41%,10 whereas the figure increased to close to 65% in later descriptions.11 It is currently accepted that lethality without the use of dantrolene may be as high as 80%.8,12 Although dantrolene is a specific effective treatment for MH crises, its availability in Colombian operating rooms is not mandatory or universal.13
There are no national data to quantify the problem of dantrolene supply shortages. The unavailability of the drug has been described as a common factor in Latin America,14 and incomplete supplies have been documented even in countries like Germany and the United States.15,16
In Colombia, dantrolene shortage is multifactorial, although the main alleged reason is the unfavorable cost-effectiveness relationship. Low availability of dantrolene may result in absent or delayed treatment of patients with MH crises, with the increased risk of morbidity and mortality. Given that dantrolene is part of the effective treatment, as supported by national and international guidelines, healthcare institutions that cannot provide treatment to a patient due to the unavailability of the drug may be liable to lawsuits and administrative proceedings and, consequently, increased costs. A determination of the cost-effectiveness relation-ship of dantrolene supplies from the perspective of healthcare institutions might show that it is financially wiser to cover the cost of the medication than to have to cover the cost of a life lost. On the other hand, in the event the cost of keeping dantrolene in stock is higher that the benefits, resources could be invested in other initiatives that could have a higher impact on patient safety.12,17
Direct extrapolations of financial assessments to different economic contexts are not possible given the differences in terms of costs and effectiveness.18,19 Understanding the cost-benefit relationship of keeping complete stocks of dantrolene on a permanent basis may have important political implications. The objective of this pharmacoeconomic assessment is to determine, from the perspective of a healthcare institution, the cost-effectiveness relationship of keeping stocks of dantrolene (complete or partial) permanently in the operating theater, based on the calculation of the financial consequences threshold.
Methods
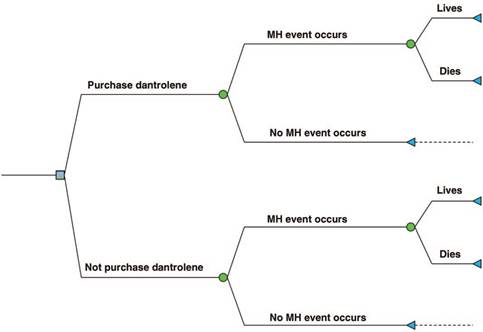
A cost-effectiveness economic analysis was conducted, taking the following independent variables into consideration: incidence of MH, probability of occurrence in the institution, cost of dantrolene supplies, and effectiveness of dantrolene in terms of mortality reduction. A decision tree was built as a tool to determine the expected value of 2 mutually exclusive alternatives: purchasing versus not purchasing dantrolene supplies (Fig. 1).
Source: Authors.
Figure 1 Decision tree. The blue square represents the decision. The green circles represent an event beyond the control of the decision-maker. MH = malignant hyperthermia.
Incidence of MH
It is difficult to estimate the exact incidence of MH.20 It is known to vary between 0.3 and 2 for every 100,000 general anesthesias administered.3,5,12,21-23 For the purpose of this assessment, an intermediate incidence of 1 case for every 100,000 general anesthesias with trigger drugs was used.
Determination of the probability of MH occurrence
The probability of at least 1 MH event occurring in a healthcare institution in a 1-year period was determined by means of the application of the binomial distribution. The binomial is the discrete probability distribution of the number of successes in a sequence of independent experiments, each of which results in a binary outcome (success or failure), where an experiment is defined as 1 general anesthesia using trigger drugs and a "success" refers to a MH attack.24,25
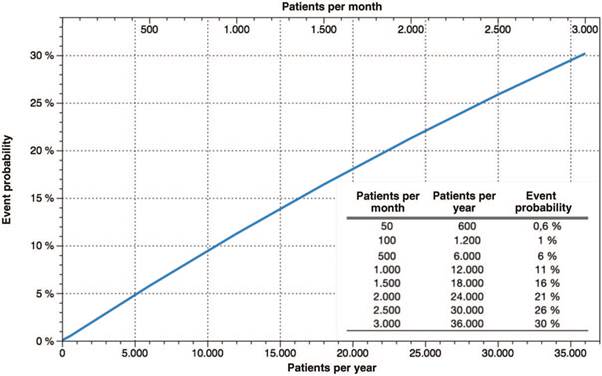
The probability of at least 1 event occurring in 1 year was calculated using the DISTR.BINOM.SERIE function in Microsoft Excel for Mac version 16.25 (19051201) Redmond (Washington), USA (Fig. 2).
Source: Authors.
Figure 2 Probability of occurrence of at least 1 MH event in 1 year. The lower horizontal axis (X) is the number of patients exposed to trigger drugs in 1 year. The upper horizontal axis is the equivalent of the number of patients exposed to trigger drugs in 1 month. The vertical axis (Y) is the probability of occurrence of at least 1 MH event. MH=malignant hyperthermia.
Dantrolene cost
The MHAUS (Malignant Hyperthermia Association of the United States) guidelines recommend availability of at least 36 20 mg vials (a total dose of 720 mg) or 3 250 mg vials (total dose of 750 mg) in the operating theater.9 The European Malignant Hyperthermia Group recommends a minimum stock of 720mg but warns that up to 1000mg might be needed for the treatment of an adult patient.26 The Spanish Malignant Hyperthermia Group recommends a lower minimum supply of 240 mg of dantrolene, with the idea of giving the initial dose, provided prompt access can be gained to the remaining dose to complete the treatment within the first 24hours.27 This recommendation is similar to the 1 set forth by the Colombian Society of Anesthesia and Resuscitation (S.C.A.R.E.),28 and other recommendations proposed by national regulatory agencies like the Bogota District Health Secretariat.13
In Colombia, dantrolene is imported as a vital unavailable drug because it does not have the approval of the National Drug and Food Oversight Agency (Invima).29 Economic assessments carried out in the past led to the pricing of dantrolene at USD 84 for every 20 mg vial.12 For purposes of this economic assessment, a price of USD 125 was established, at an exchange rate of Colombian pesos (COP) 3300 for USD 1, after accounting for importation and intermediation costs.
Dantrolene effectiveness
The lethality of a crisis of MH without the use of dantrolene during treatment is not accurately clear. There is general consensus that MH lethality before the introduction of dantrolene as standard treatment could have been as high as 80%.8,11 The effectiveness of dantrolene is determined by the timing of the initiation of treatment, patient comorbidities and the implementation of other support measures, among others.30 The figure cited most often is lower than 5%. However, lethality rates between 1% and 12% have been reported in the literature.3,21,31,32
Scenario simulations
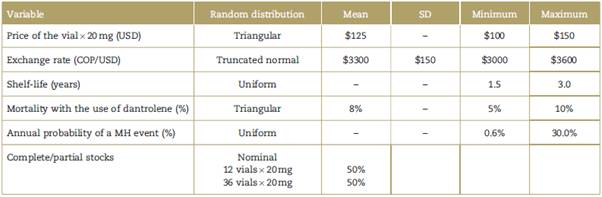
Simulations of 10,000 scenarios were carried out using the Monte Carlo technique, implementing a random modification of the variables that influence the price of dantrolene supplies (price of the vial in USD, COP/USD exchange rate, shelf-life, and number of vials in stock).
The probability of occurrence of the event as well as mortality from a MH crisi with and without dantrolene treatment were also modified. Random distribution and specific limits were established for each variable to circumscribe the simulation behavior to scenarios with a reasonable probability of occurring in real life. Table 1 summarizes the specific criteria used to frame the simulation.
Construction of indifference curves
Given the absence of data on the cost that needs to be paid by a healthcare institution in the event of death of a patient as a result of a MH attack and no availability of dantrolene supplies, a hypothetical threshold was estimated with equal expected values attached to both alternatives in the decision tree. If, for the decision-maker, the real financial consequences exceed that threshold, the option of purchasing dantrolene supplies has the best cost-effectiveness relationship. Theoretically, economic consequences for a healthcare service provider institution when a patient dies may include costs such as legal representation, third-party and administrative liability, and loss of reputation.
Sensitivity analysis
Sensitivity analyses were conducted to determine whether the results obtained were robust to changes in the predefined assumptions for the model. The analyses were carried out for the same variables used in the Monte Carlo simulation, and the values for each variable were selected so as to represent plausible ranges in the clinical context.
Descriptive statistics and information technology applications
The categorical variables used in the economic assessment were described as proportions, while quantitative variables were described in terms of medians and interquartile ranges (IQR). The Monte Carlo simulation and the decision trees were run in TreePlan 2.05 for Macintosh Excel (Copyright 2018 Michael R. Middleton dba TreePlan Software, San Francisco, California, USA). Descriptive statistics were run in Wizard for Mac Versión 1.9.32 (251) (Copyright 2014-2016, Evan Miller).
The final manuscript was written in accordance with the ISPOR CHEERS Task Force Report (Consolidated Health Economic Evaluation Reporting Standards).33
Results
The probabilities of at least 1 MH event occurring in 1 year and the monthly equivalents were obtained in accordance with the number of patients seen per year (Fig. 2). The lowest probability of occurrence of the event was 0.6% for 50 patients exposed per month (600 patients per year) to procedures under general anesthesia with the use of trigger drugs.
Medians and IQRs of the independent variables associated with the cost of annual dantrolene supplies were derived from the results of the Monte Carlo simulation (Table 2).
Table 2 Result of the Monte Carlo simulation.
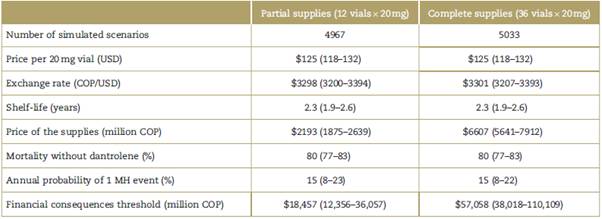
Values are expressed as medians (interquartile range). COP=Colombian pesos, MH=malignant hyperthermia, USD=United States dollars.
Source: Authors.
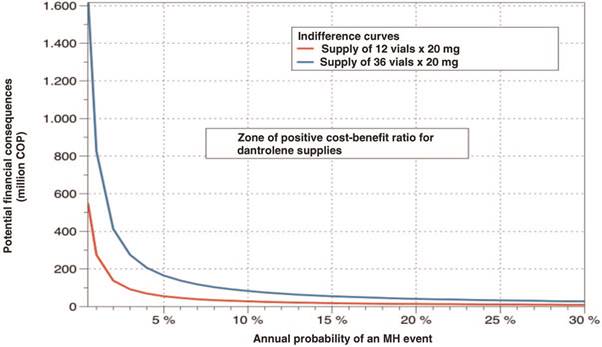
The cutoff point for a change in decision for the combined values of the annual probability of a MH event and the financial consequences of 1 death was determined based on the results of the simulated scenarios and using the decision tree. In other words, the value amount at which the financial consequences that would lead to a change in the decision of purchasing dantrolene was determined for each event probability. In Fig. 3, the right upper region of the curve is the area in which the purchase of dantrolene is financially justified, and the lower left is the region where it is not. Two analyses were carried out for the purchase of 36 vials (upper curve) and 12 vials (lower curve). The results of the sensitivity analysis are visually displayed in a tornado plot (Fig. 4).
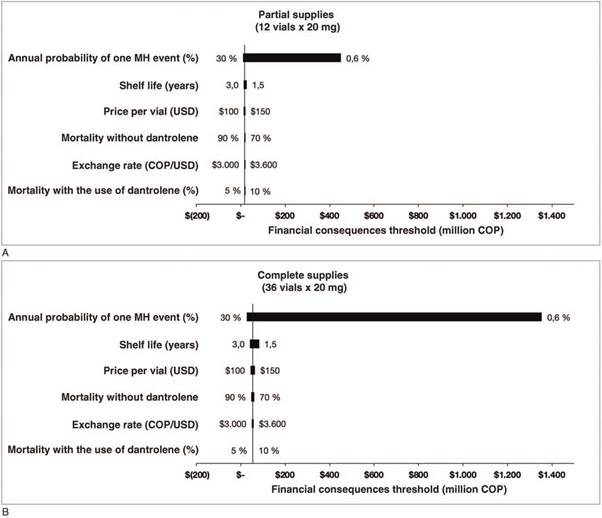
Source: Authors.
Figure 4 Sensitivity analysis. Tornado plot showing the impact of each of the variables on the financial consequences threshold variation in million COP. (A) Partial supplies (12 vials x 20 mg). (B) Complete supplies (36 vials x 20 mg). COP = Colombian pesos, MH = malignant hyperthermia, USD=United States dollar.
Post hoc analysis
The value of a statistical life has been estimated to range between USD 4 and 10 million.34,35 The lowest value in this range was used for the purposes of this economic assessment, and it was estimated that in a case of fatality, 5% of the value of the statistical life, COP 660 million, could be attributed to the healthcare institution, at an exchange rate of 3300 COP/USD. Based on these assumptions, 100% of the scenarios simulated with the purchase of 12 vials x 20 mg show a positive cost-effectiveness ratio in favor of the purchase of dantrolene supplies. For those scenarios simulating the purchase of a stock of 36 vials x 20mg, the favorable cost-effectiveness ratio was achieved in 97.1% of cases. For the 144 cases (2.9%) with no cost-effectiveness, it was found that they were all in the scenarios of the purchase of 36 vials and, moreover, they represented a median probability of an annual event of 1% (IQR 0.8%-1.2%).
Discussion
According to the results of this economic assessment, if the financial consequences related to the death of a patient due to the unavailability of dantrolene are higher for a healthcare institution that the threshold defined by the indifference curve, the purchase of dantrolene supplies is favorable given its cost-effectiveness ratio. The approach used in this research shows a strong association between the threshold of financial consequences and the probability of the event occurring. Moreover, the annual probability of a MH event occurring depends on the estimated incidence of the crisis and the volume of patients exposed to trigger drugs.
For example, if the annual probability of the MH event is 5%, at a death value amount lower than COP 55 million, not even the purchase of 12 vials would be justified; between COP 55 and 165 million, the purchase of 12 vials would be justified but not so of 36 vials; and above COP 165 million, the purchase of 36 vials would be justified.
Aderibigbe et al22 found that keeping dantrolene stocks in outpatient surgery centers as per the MHAUS recommendation is profitable as compared with the estimated statistical life values used by the regulatory agencies in the United States. Keeping 36 vials x 20 mg of dantrolene in stock in each outpatient surgery center in the United States would save 33 lives every year, with an incremental cost-benefit ratio of approximately USD 200,000 (2010 monetary value) for every life saved, which indicates that the recommended guideline is very profitable.
On the other hand, Ho et al assessed the cost-benefit relationship of keeping dantrolene stocks in maternity units. The analysis showed that costs associated with keeping a MH cart with a full supply of dantrolene within 10 minutes of a maternity unit exceeded the benefits. Models suggest that a more profitable approach would be to keep just one initial dantrolene dose in the maternity unit, with partial supplies available within the first 30 minutes.12 The latter is similar to the proposal set forth in this economic assessment.
The binomial function analysis appears to show that the number of MH crises should be greater than formally reported for Colombia.36 In fact, it should be sufficiently high so as to prompt hospital leadership to promote the availability of dantrolene in the operating theater given the overwhelming financial burden resulting from its unavailability. However, dantrolene is not in stock in all operating theaters and it might be that the number of MH crises is lower than shown by the binomial distribution results.
This could be construed in several ways: first, the real incidence of MH is much lower in our setting than assumed in this paper (1:100,000); second, there is under registration (or under reporting) of cases; third, mortality and complications are less frequent, or the cases are less severe than described. Consequently, there is a need to determine which of these or other causes may be influencing the incidence of MH in Colombia, and there is also a need to conduct further studies to gain deeper local knowledge about this topic. However, considering that mandatory reporting of MH is not required, national statistics may suffer from registration bias. Despite the above, there is no evidence that the incidence of MH in Colombia might be different than in other parts of the world.37
According to the information available, this is the first economic assessment of the cost-benefit relationship of keeping dantrolene stocks in the Colombian health system. However, the external validity of the results is completely dependent on the consistency between MH incidence data and what is truly observed in Colombia. It would be desirable for this determination to bring about at least 1 of 2 actions: first, the autonomous decision by the leaders of each institution where drugs that trigger MH are used to keep dantrolene stocks in their operating rooms; and second (perhaps complementary), the decision by the national regulatory agencies to require the availability of dantrolene stocks as a prerequisite for licensure of surgical services at all levels of complexity.
It would be reasonable to believe that increased demand for the medication would encourage new vendors to come on the market and, as a result, increase the supply. Submission by a producer or importer of dantrolene for approval by Invima would be another useful step which could greatly facilitate production or importation processes. It would also reduce barriers to entry into the domestic market of new medications for orphan diseases.
To conclude, given the convenience of the cost-effectiveness relationship from the perspective of healthcare institutions, the purchase of a complete supply of dantrolene (36 vials x 20 mg) would be justified, based on the fact that 1 MH death due to the unavailability of the drug results in financial consequences that exceed the threshold of COP 57 million. Seen from a more conservative approach, if the financial consequences exceed COP 18.5 million, the cost-effectiveness relationship favors the purchase of partial supplies (12 vials x 20mg) of dantrolene.











 texto en
texto en