Introduction
Myotoxicity induced by the use of local anesthetics (LA) injected directly into or adjacent to the skeletal muscle in humans is a recognized condition in ophthalmic surgery, but it is seldom reported or studied in the postoperative period following major surgery.1 However, with the introduction of new techniques and the higher frequency of procedures conducted under regional anesthesia, there has been a growing interest in this-until now-unusual entity, since occasionally it may have significant con sequences on the recovery of functionality in the patient.
Regional anesthesia is widely used as postoperative analgesia in total knee arthroplasty (TKA). Frequently, the adductor canal block or femoral nerve block are used, both with similar efficacy of analgesia and the presence of adverse effects.2 Following is a description of a case of local anesthetic-induced myotoxicity associated with continuous femoral nerve block for the management of postoperative pain in TKA surgery. This article discusses the prevalence, the clinical manifestations, and the strategies to reduce the risks of the disease.
Case description
A 54-year-old male, 82 kg, American Society of Anesthesi ologists (ASA) II, with a history of cervical myelopathy a few months earlier, which was managed with steroids, and dome shaped right proximal tibial osteotomy for varus correction conducted 2 years ago. The patient was diagnosed with right primary gonarthrosis Grade IV, with significant functional impairment (Oxford scale 15/48), and underwent TKA. The analgesic technique used was an ultrasound and neurostimulation guided insertion of a perineural femoral catheter (PFC) using a high-frequency lineal transducer, via an 18 g Touhy needle out of plane, after hydrodissection of the perineural sheath with 15-mL bupivacaine 0.5%, plus epinephrine 5 µg/mL. In addition, a single-dose sciatic nerve block was performed through a US-guided subgluteal approach with bupivacaine 0.5% 20 mL without epinephrine, and neurostimulation with a curved, low-frequency transducer using the in plane technique with a 100 mm stimulation needle. No compli cations were reported during the anesthetic procedure.
The surgery was completed uneventfully after 70 min under general anesthesia. No tourniquet was used on the thigh of the lower extremity during surgery.
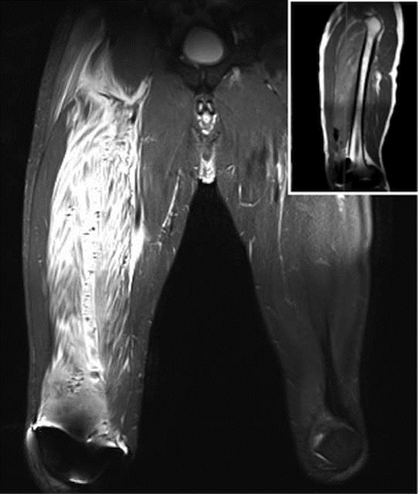
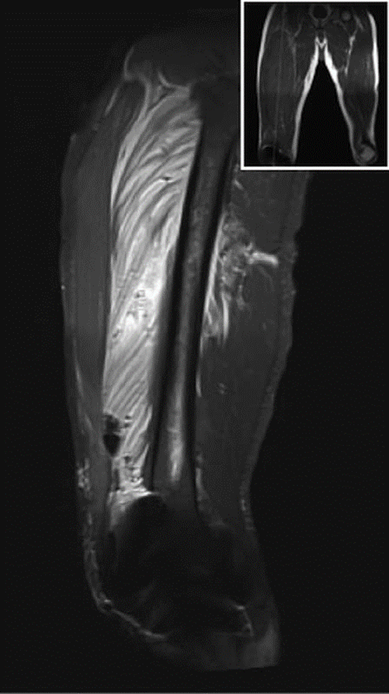
At the postanesthesia care unit, the patient received a bupivacaine 0.125% infusion at a rate of 6ml/h plus a 4mL bolus, through the PFC, with 30-min block on demand by the patient using a PCA (patient controlled analgesia) device. The immediate postoperative evolution was adequate. During the assessment the morning of the first postoperative day, the maximum pain intensity was 4/10 in the Verbal Rating Scale (VRS); the patient used 118 mL of the PCA analgesic mix. In the afternoon that same day-24 hours after surgery-the patient experienced increased proximal thigh pain in the operated extremity, associated with progressive edema and weakness that failed to respond to the PCA. The physical assessment evidenced proximal motor block (1/5 muscle strength and 3/5 in the calf [5-point scale: 0 weakest, 5 strongest]), associated with induration and tenderness of the proximal and medial anterior region of the thigh. There was no evidence of distal neurovascular anomalies in the operated limb. In view of the severity of the situation, a nuclear magnetic resonance imaging (MRI) was conducted, identifying an extensive inflammatory and blood infiltration into the vastus intermedius muscle and air bubbles dissecting the muscle bundles-findings compatible with myositis (Figs. 1 and 2).
Source: Authors.
Figure 1 Coronal view MRI STIR T2, increased signal over the VIM. The vastus lateralis and the adductor muscles are not compromised. The blurred image seen on the right knee is due to magnetic interference. MRI = magnetic resonance imaging, VIM = vastus intermedius muscle.
Source: Authors.
Figure 2 Sagittal view MRI STIR T2, specific intense signal for VIM from its origin through the quadriceps/patellar tendon. Air bubbles and infiltration dissect muscle bundles. MRI = magnetic resonance imaging, VIM = vastus intermedius muscle.
In view of the suspicion of LA-induced myotoxicity, the PFC was removed. The management of analgesia was continued with strong opioids. After 24hours the patient improved his initial symptoms by 80%, experiencing less motor block and pain, although the induration and the functional limitation persisted. At 48 hours, the serum creatine-phosphokinase (CPK) levels were elevated at 423 UI/L with no renal failure. There was a progressive decrease in the pain intensity and in the use of opioids. The strength of the quadriceps muscle gradually im proved. The patient was discharged 5 days after surgery, with assisted gait. 15 days later the patient came to his control visit and was pain-free; the knee range of movement was preserved, there were no inflammatory changes and no gait limitations. A control MRI was conducted 1 year after surgery, showing no evidence of myocytic changes or any other musculo-tendinous alter ations.
Discussion
The femoral nerve block and the adductor channel block are analgesic techniques frequently used in TKA because they are considered safe procedures with a low rate of complications. However, local anesthetic-induced myotoxicity in humans has become an increasingly significant condition over the past few years. The systematic review by Hussain et al3, found that the LA used at therapeutic concentrations induce myotoxic injury in in vitro, ex vivo, in vivo, and in human studies. This condition was acknowledged and described by Brun back in 1959, when he managed to demonstrate in experimental animal models that the local infiltration of lidocaine at concen trations between 0.25% and 2% in the cutaneous maximus muscle in mice and rabbits, generally resulted in inflam mation and tissue necrosis.4 Benoit et al5 identified that the models of tissue injury are qualitatively similar in animals and humans, and that the necrosis pattern caused by the intramuscular injection is directly correlat ed with the effective concentration of the anesthetic agent to which the muscle is exposed and is simultaneously dependent on factors such as volume and concentration of the local anesthetic agent, the presence of vasoconstrictor and the size of the muscle receiving the injection.
Documented histological changes in subsequent stud ies include the lytic degeneration of the sarcoplasmic reticulum and the mitochondria of the striate muscle cells, followed by tissue necrosis and phagocytosis.6 Mitochondrial bioenergetic disorders, oxidative stress, and mitophagy play the most important role in the pathophysiology of the disease.7 Functional and structur al alterations of the mitochondria present in the muscle tissue are closely related with the interaction of local anesthetic agents.
Studies conducted in rats found that the injections of high concentrations of bupivacaine (16mg/kg) into the muscle resulted in disjointed fibers, interstitial edema, and infiltrating cells.8 More recent studies have shown that with lower doses injected through a femoral nerve catheter (2.5 mg/kg), bupivacaine-induced edematous subsarcolemmal mitochondrial aggregates and partial loss of interfibrillar mitochondria, leading to a significant inhibition of the production of adenosine tri-phosphate (ATP). With regard to mitophagy, this same study found that the intact or degraded mitochondria are surrounded by membranes considered autophagosomes, hence sug gesting bupivacaine-induced mitophagy.9
Local anesthetic agents disrupt the calcium homeostasis, affecting calcium inflow and intracellular mobiliza tion.10 First, they inhibit early Ca2+ (calcium) release from the sarcoplasmic reticulum, regulated by the ryanodine receptor, hindering the actin-myosin binding; second, they drive the delayed release of calcium from the sarcoplasmic reticulum into cytosol11,12; finally, LAs inhibit the gradient-dependent Na+(sodium)/Ca2+ ex changer and the Na+/K+ (potassium) ATPase pump, resulting in increased intracellular calcium.3,7 This leads to continuous muscle contraction, cell damage, disjointed mitochondria, and apoptotic cell death, regardless of the local anesthetic concentration11,12, possibly due to alter ation of the phosphorylation chain and ATP production.
The level of toxicity of local anesthetic agents from milder to stronger is: lidocaine, ropivacaine y bupivacaine.13 The 2 most important factors potentiating the myotoxic effects are increased concentration and exposure duration.14 The severity risk factors for the disease include: prior alterations of the mitochondrial metabolism that compromises energy production-that is, patients with chronic hypoxia, diabetes mellitus type 2, anemia, obesity, obliterating arthropathy of the lower extremities, and statins therapy.15,16
LA-associated myotoxicity in humans was initially described in ophthalmic surgery with the use of several regional anesthesia techniques,17 with postoperative diplopia as the most frequent symptom.18 A recent systematic review identified 234 of 44,478 patients (0.53%) with diplopia following ophthalmic surgery. Similarly, in orthopedic surgery, 9 of 6121 (0.14%) were identified with weakness and pain as the most frequent symptoms of myotoxicity following regional anesthesia.3 The clinical manifestations of the disease are non-specific and present 1 to 2 days following the injection of the local anesthetic agent.
The diagnosis may be difficult since in many of the patients presenting with a certain level of myotoxicity, the symptoms may be masked by the weakness and pain expected from the procedure2; however, monitoring, suspicion and clinical judgement are necessary to identify those cases in which muscle weakness and pain extends beyond the surgical recovery timeline. This may trigger the use of diagnostic aids, including serum CPK, which rises after myotoxic damage. However, its sensitivity and specificity have yet to be established.6 With regard to the use of images, the MRI without contrast is considered helpful for the diagnosis. The findings range from localized edema, inflammatory changes and increased T2-weighted image signal intensity.19 However, the final diagnosis is made with a muscle biopsy.3
The treatment of LA myotoxicity may be watchful waiting, accompanied by directed physical therapy, since the muscle regeneration may be complete in 3 to 4 weeks,2 though there are some cases in which recovery is not total. According to Hussain et al3, out of 206 patients, 80 (38%) had a total recovery, while 126 (62%) had partial or no recovery, requiring surgical or mechanical interventions. These recent studies suggest that some antioxidant medications reduce myotoxicity. Nouette-Gaulain et al found that prior therapy with 5000 UI/kg of erythropoietin, partially avoided myotoxicity, both in rats and cultured human myoblasts. The dose used was higher than the doses used in clinical practice, and hence requires strict follow-up and on-going evaluation that limit its use.12 The administration of N-acetyl cysteine with bupivacaine has also been studied, particularly in the inhibition of the production of oxygen reactive species. However, its impact has not yet been measured in clinical practice. These medications are still being researched and further studies are needed before they are released for clinical use.20
The prevention of LA-induced toxicity is aimed at reducing the incidence and the severity of the disease. Some of the main measures include: using the minimum LA concentration required to achieve the desired effect; limiting the exposure duration; using other options to extend the duration of the block; making a judicious and individualized assessment of patients with risk factors for myotoxicity; and, limit the use of bupivacaine in these patients.14 Likewise, the use of us-guided peripheral nerve block contributes to reducing the volumes of local anesthetic administered.21,22 These measures have to be adapted to the postoperative analgesia protocols to prevent the disease.
Going back to the case herein discussed, the clinical presentation of the patient was consistent with LA-induced myotoxicity, due to the satisfactory evolution the first postoperative day, followed by weakness, tender ness in the proximal third of the thigh, and edema. Due to the high suspicion of this condition, the CPK levels were measured and the result was elevated. In addition, an MRI was compatible with myositis that led to the decision to remove the catheter. The muscle biopsy was ruled out because the clinical evolution of the patient was satisfac tory. 12 hours after removing the catheter, the symptoms improved by about 80%. The patient was followed at 15 days, 1 month and 1 year after surgery, with total recovery as a result of timely diagnosis. This presentation was compatible with the report of a case series1 following the use of continuous adductor canal block in TKA, evidencing similar changes in the MRI. Strikingly, only 1 of the 3 cases reported achieved a complete recovery over the first 30 days.
The recent identification of these cases leads to the suspicion of a potential association with the use of the adductor canal block in the last few years, notwithstand ing the many previous decades during which regional anesthesia with high doses of LA was used in lower limb surgery.3 In contrast, this case discusses the use of femoral nerve block with continuous infusion, with significant involvement of the vastus intermedius muscle and a significant impact on the patient's recovery.
A timely identification of the condition allows for a specific approach to reducing any noxa and facilitating functional recovery. As a differential diagnosis, a postop erative compartment syndrome23 shares clinical charac teristics such as the presence of paralysis, pain, and edema. However, disproportionate pain, particularly with passive stretching, is the primary characteristic for the diagnostic suspicion, which in this case was different because the symptoms were primarily located at the catheter insertion area and the proximal region of the thigh, and not in the distal region, proximal to the surgical wound. In addition, the absence of distal neurovascular involvement dismissed such possibility. The identification of the compartment syndrome and its later management is indispensable, since notwithstanding its low incidence, it is a major complication that may potentially be life-threatening and jeopardize the limb,24 because of the absolute need for surgical decompression in the first 6 hours, to offset the ischemic muscle injury and reduce the severity of the lesion.
Conclusion
Local anesthetic-induced myotoxicity has been shown in studies in animal and human cells. The extent of the injury depends mostly on the concentration of the local anesthetic agent and the exposure duration, as well as on the presence of individual risk factors. Notwithstanding the fact that this is an unusual pathology, it should be kept in mind as a potential complication following regional blocks for TKA, because of its negative impact on patient's recovery and satisfaction. Consequently, protocols that ensure an effective block with the lowest possible concentration and volume of the medication should be adopted. The use of antioxidant agents during the anesthesia protocol shall be studied in clinical practice.











 texto en
texto en 


