What do we know about this issue?
Systematic reviews of the literature within the clinical setting aim to synthesize evidence focused on the effectiveness / efficiency of treatments, diagnosis and prognosis, to support health decision making. Although there is variability in methodological appreciation criteria and tools that guide this type of study, AMSTAR is a validated instrument that allows us to critically appreciate quality across global domains.
¿What is this study's contribution?
Although there are reports on the validation and structure of AMSTAR, this manuscript is a guide that presents a historical, theoretical, and practical approach that guides clinicians and methodologists on its use within evidence-based practice, specifically in quality assessment of systematic reviews. This contributes to the resolution of questions in the clinical setting and to support evidence-based decision making.
INTRODUCTION
Systematic reviews of the literature (SRLs) and expanded syntheses have played a very relevant role in healthcare and decision-making in evidence based medicine (EBM) 1,2. This methodological strategy has helped support the development of evidence-based clinical practice guideline recommendations, which are the second best available source of scientific literature when SRLs are based on randomized clinical trials, according to the evidence pyramid 1. This secondary or integrative research design allows to answer a research question according to population, intervention, comparator and outcomes 3, given that it analyzes the results of a set of original research and provides an answer in a short period of time and at a lower cost than that of a primary study 3.
The concept of EBM has evolved over time, becoming a worldwide method to communicate clinical decisions through biomedical literature consumption 4. It develops from a health problem translated into a clinical question which guides the search of a body of evidence 4. Once the biomedical literature is identified, the following questions need to be asked about the results of the studies found: are the results of the article valid, what were the results, and are the results of this study applicable to the population in which the clinical case was identified? 5 The answers to these questions will serve as the basis for decision-making regarding the health problem.
In view of the importance of making responsible evidence-based decisions, tools supported by checklists have been designed and validated to support the critical appraisal of the literature according to the type of methodological design 6. The suggestion is that the reader should select one of the resources available to support the critical appraisal of the evidence, depending on the type of research.
Several tools have been developed for SRL-based evidence, one of which has been validated and updated: A Measurement Tool for Assessment of Multiple Systematic Reviews (AMSTAR). It is one of the tools with the highest validity and reliability for quality reporting, with satisfactory SRL results; and because appraisal may vary substantially among clinicians, it is critical to understand and interpret each item so that it can be implemented. The objective of this study is to describe and guide the use of the AMSTAR tool for assessing the quality of the evidence derived from a systematic review of the literature.
ABOUT SECONDARY OR INTEGRATIVE STUDIES
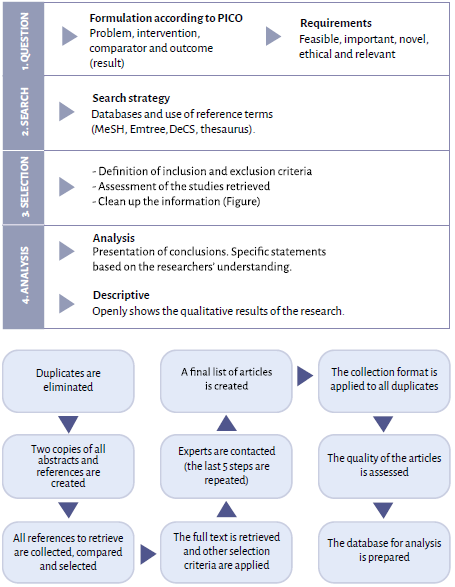
The purpose of systematic reviews is to identify, assess and summarize findings from all relevant individual studies on a given health-related topic, and to bring the available evidence closer within the reach of decision-makers 7. Unlike narrative reviews, the starting point of systematic reviews of the literature is a research question [PICO format: P (Patient, Population or Problem) I (Intervention) C (Comparison) O (Outcome] that drives the search for primary articles based on an understandable search strategy, in different databases 8. The article selection stage in a systematic review of the literature involves applying specific criteria and assessing the quality of the primary studies included. Finally, in a narrative review, the summary of studies is usually qualitative in contrast with the systematic synthesis (qualitative or quantitative, meta-analysis) which is carried out in the SRL 8,9(Figure 1).
CRITICAL APPRAISAL OF THE EVIDENCE IN SYSTEMATIC REVIEWS OF THE LITERATURE
Evidence-based practice requires the deliberate, explicit and judicious use of the best available evidence for decision-making 10. So how can the best be identified among all the available evidence? Critical appraisal requires knowledge of the type of methodological design used to answer the research question 10.
Critical appraisal of SRLs: tools for quality assessment
In view of the growing number of healthcare professionals who practice evidence-based medicine, and considering the responsibility involved in this process, several tools that condense criteria for assessing the quality of the evidence have been developed and validated 11. One of the first tools is the "Checklist for Review Articles," developed by McMaster University Department of Clinical Epidemiology and Biostatistics and published in 1994 in the British Medical Journal. It was designed to identify relevant studies to approach the clinical/research question and to generate validity of the chosen design 12. It was initially rated as rudimentary because of its open-ended questions and the lack of an objective rating system 12.
In 1996, Oxman and Guyatt developed the "Overview Quality Assessment Questionnaire" (OQAQ), a validated tool for assessing methodological quality in SRLs of intervention studies 13.
Later, in 1999, the "Quality of Reporting Of Meta-Analyses" (QUOROM) was described as a result of a conference of epidemiologists, clinicians and statisticians of the United Kingdom and North America who met with the aim of finding an interdisciplinary consensus for SRL reporting 14. The output of this conference was a structured check-list of 18 items which the authors of meta-analyses and journal editors should consider at the time of publishing their work in the form of an article in a medical journal. It also includes a flow chart describing the entire process, from the initial identification of potentially relevant studies to the final selection. The goal of QUOROM is to encourage authors to gather all the information that could be essential for the interpretation and adequate use of the results of a meta-analysis 14.
At the time of its publication, the QUOROM work group determined the need for a regular review and update of the tool in accordance with new published evidence. Accordingly, in July 2009 came the publication of the PRISMA ("Preferred Reporting Items for Systematic Reviews and Meta-Analyses") declaration, developed by a group of 29 reviewers, methodologists, physicians, medical editors and consumers 15. An evidence-based consensus process was used to develop a 27-item check-list, conceived as a tool to enhance reporting quality and transparency, as well as SRL publications. PRISMA has not been validated as a tool to assess SRL quality and should not be used for that purpose. It focuses on the way authors may ensure submittal of complete and transparent reports on systematic reviews and meta-analyses 15.
It does not deal directly or in detail with how to conduct systematic reviews, for which other guidelines are available. It was considered to include the relevant elements that must be present when it comes to reporting systematic reviews of non-randomized studies that assess the benefit and harm derived from interventions 16.
Based on previous models, empirical evidence and expert consensuses, AMSTAR saw the light in 2007 at the Bruyère Research Institute in Ottawa, Ontario, Canada. This tool combines items of the Overview Quality Assessment Questionnaire 17, and its advantages include good inter-reviewer correlation, great reliability for systematic review analyses, and the fact that it is easy to use and understand 18. Moreover, it has been endorsed by the Canadian Agency for Drugs and Technologies in Health (CADTH), and it has been cited approximately 200 times over the past three years. Another important consideration is the excellent reliability of the score obtained with AMSTAR, not to mention its practicality, simplicity and ease of interpretation 18. There is currently a growing body of nonrandomized scientific evidence, creating the need to synthesize the evidence under the same methodology used for SRLs. Hence the development of the second version, the AMSTAR 2, which will help decision-makers with the identification of high quality systematic reviews, including those based on non-randomized healthcare intervention studies 19.
The aim of this tool is to produce valid, reliable and thorough assessments that can help users with quality distinctions between the various systematic reviews by focusing on their methodological quality, thus paving the way for the development of high quality reviews 20. The tool must be interpreted individually by each reviewer, based on the premise that there are no good or bad results, but rather a wide range of intermediate results on the quality of SRLs, which need to be correlated with the objectives and conclusions of each article in order to gain a real and concrete perspective of the context.
"A MEASUREMENT TOOL FOR ASSESSMENT OF MULTIPLE SYSTEMATIC REVIEWS" (AMSTAR)
In the clinical realm, systematic reviews of the literature are designed to synthesize evidence focused on the effectiveness/ efficiency of treatments, diagnosis and prognosis. Although the methodological appraisal criteria vary, there are certain internationally accepted standards to determine whether the SRL is of good quality or not 21. Because of their variability, the tools developed for the critical appraisal of systematic reviews of the literature and meta-analyses (Table 1) can each lead to different conclusions when a single SRL is assessed, depending on the reviewer and the weight of the tools, making interpretation difficult for the readers.
Table 1 Tools for assessing the validity of scientific studies.
| Study type | Tool | Source |
|---|---|---|
| Randomized clinical trials | Cochrane tool for bias risk. | https://training.cochrane.org/es/resource/evaluaci%c3%b3n-del-riesgo-de-sesgo-de-los-estudios-incluidos |
| Oxford University Evidence Based Medicine (EBM) center tool for critical appraisal | https://www.cebm.net/2014/06/critical-appraisal/ | |
| Scottish Intercollegiate Guidelines Network (SIGN) check-list for critical appraisal of the literature | https://www.cebm.net/2014/06/critical-appraisal/ | |
| Critical Appraisal Skills Program (CASP): Randomised Controlled Trial Appraisal Tool | https://casp-uk.net/casp-tools-checklists/ | |
| Physiotherapy Evidence Database (PEDro) Scale Physiotherapy Evidence Database Scale | https://www.pedro.org.au/ | |
| The Jadad Scale | http://onlinelibrary.wiley.com/doi/10.1002/9780470988343.app1/pdf | |
| Graphic Approach To Evidence based practice (GATE)-Critically Appraised Topic (CAT)- Intervention Randomised Controlled Trials (RCT) Studies. Centre for Evidence Based Medicine, Oxford University. | https://www.fmhs.auckland.ac.nz/assets/fmhs/soph/epi/epiq/docs/GATE%20CAT%20Intervention%20Studies%20May%202014%20V8.docx | |
| Joanna Briggs Institute (JBI) Checklist for Randomised Controlled Trials. | http://joannabriggs.org/research/critical-appraisal-tools.htm | |
| Analytical cohort observational studies | Critical Appraisal Skills Program (CASP): Cohort Studies | https://casp-uk.net/casp-tools-checklists/ |
| Graphic Approach To Evidence based practice (GATE)-Critically Appraised Topic (CAT) - Intervention Cohort Studies. | https://www.fmhs.auckland.ac.nz/assets/fmhs/soph/epi/epiq/docs/GATE%20CAT%20Intervention%20Studies%20May%202014%20V8.docx | |
| Joanna Briggs Institute (JBI) checklist for Cohort Studies | https://joannabriggs.org/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Cohort_Studies2017_0.pdf | |
| Analytical observational case control studies | Critical Appraisal Skills Program (CASP): Case Control Studies | https://casp-uk.net/casp-tools-checklists/ |
| Graphic Approach To Evidence based practice (GATE)-Critically Appraised Topic (CAT) - Case Control Studies | https://www.fmhs.auckland.ac.nz/assets/fmhs/soph/epi/epiq/docs/GATE%20CAT%20Case%20Control%20Studies%20May%2 | |
| Scottish Intercollegiate Guidelines Network (SIGN) Methodology Checklist 4: Case Control Studies. | http://www.sign.ac.uk/checklists-and-notes.html | |
| Joanna Briggs Institute (JBI) Checklist for Case Control Studies. | http://joannabriggs.org/research/critical-appraisal-tools.html | |
| Diagnostic tests | Critical Appraisal Skills Program (CASP) Diagnostic Critically Appraised Topic (CAT) | https://casp-uk.net/casp-tools-checklists/ |
| Graphic Approach To Evidence based practice (GATE)-Critically Appraised Topic (CAT) for Diagnostic Test Accuracy Studies. | https://www.fmhs.auckland.ac.nz/assets/fmhs/soph/epi/epiq/docs/GATE%20CAT%20Diagnostic%20Studies%20May%202014%202014%20V5.docx | |
| Joanna Briggs Institute (JBI) Checklist for Diagnostic Accuracy Studies. | http://joannabriggs.org/research/critical-appraisal-tools.html | |
| Critical Appraisal Skills Program (CASP) Diagnostic Critically Appraised Topic (CAT) | https://casp-uk.net/wp-content/uploads/2018/03/CASP-Diagnostic-Checklist-2018_fillable_form.pdf | |
| Health economic evaluations | Critical Appraisal Skills Program (CASP):Economic Evaluation Studies. | https://casp-uk.net/wp-content/uploads/2018/01/CASP-Economic-Evaluation-Checklist-2018.pdf |
| Scottish Intercollegiate. Guidelines Network (SIGN). Methodology Checklist 6: Economic studies. | http://www.sign.ac.uk/checklists-and-notes.html | |
| Joanna Briggs Institute (JBI)Checklist for Economic Evaluations. | http://joannabriggs.org/research/critical-appraisal-tools.html | |
| Systematic reviews of the literature and meta-analysis | Critical Appraisal Skills Program (CASP): Systematic Reviews. | https://casp-uk.net/wp-content/uploads/2018/01/CASP-Economic-Evaluation-Checklist-2018.pdf |
| A Measurement Tool for Assessment of Multiple Systematic Reviews (AMSTAR) | https://amstar.ca/Amstar_Checklist.php | |
| Scottish Intercollegiate. Guidelines Network (SIGN). Methodology Checklist 1: Systematic Reviews. | http://www.sign.ac.uk/checklists-and-notes.html | |
| Joanna Briggs Institute (JBI) Checklist for Systematic Reviews. | http://joannabriggs.org/research/critical-appraisal-tools.html | |
| Centre for Evidence Based Medicine, Oxford University. | https://www.cebm.net/2014/06/critical-appraisal/ |
Source: Authors.
AMSTAR is a tool used to estimate the methodological quality of reviews. It began as a tool to assess SRLs of intervention studies; however, as a result of its evaluation and methodological evolution, AMSTAR 2 was created, which includes the appraisal of SRLs of experimental and non-experimental studies 20. In 2007, B. J. Shea et al., of the University of Ottawa, set out to work on achieving consistency in the assessment of systematic reviews by focusing the appraisal on two quality criteria of these studies with the first AMSTAR version, namely, the methodological quality of the review and the quality of the information with which the methodology and the results were reported. Their external validation prospective study showed the reliability of the tool when compared with the reference criteria for the assessment of systematic reviews at the time, yielding consistent results in the evaluation of 42 systematic reviews 22. Three years later, the same authors conducted another study to measure consistency, reliability, validity and feasibility of AMSTAR. This time they worked on 30 systematic reviews of the literature using the "Overview of Quality Assessment Questionnaire" (OQAQ) 10, the "Rating Scale of Sacks et al.," and the AMSTAR tool. The study showed that AMSTAR met good quality for the assessment of the items mentioned above, and yielded better results than the other tools. 23
DOMAINS ASSESSED BY AMSTAR 2
AMSTAR was developed on the basis of a quality assessment questionnaire. The check-list consists of 16 items that guide the reviewer to systematically consider each of the factors that may compromise the validity and reliability of an SRL. Moreover, this is one of the reasons why the AMSTAR may also be used as a guide during SRL development and reporting 18(Table 2).
TABLE 2. AMSTAR domains 1. Did the research questions and inclusion criteria for the review include the components of PICO?
| Check if “yes” | Optional (recommended) | Where to look in the article? | Answer |
|---|---|---|---|
|
√ Population √ √ Intervention √ √ Comparator √ √ Outcome |
√ Follow-up time period. | The research question is rarely described in question format; it is identified in the Title or the Objective. |
√ □ Yes. √ □ No |
This condition is met if it is determined that the authors considered a research question in which they identify population, intervention, comparator group and outcome.
2 Did the report of the review contain an explicit statement that the review methods were established prior to the conduct of the review and did the report justify any significant deviations from the protocol?
| Check if partial “yes” | Check if “yes” | Where to look in the article? | Answer |
|---|---|---|---|
|
Authors state that they had a protocol that includes: √ Review question √ Search strategy √ Inclusion/exclusion criteria √ Risk bias evaluation |
Apart from the above, the protocol is documented and the following is specified √ The meta-analysis plan (if applicable) and √ the plan to look into the causes of Heterogeneity √ Justification for any deviation from the protocol. |
In Background or in the study Methods section |
□ Yes □ partial Yes □ No |
This item assesses whether a design was provided before the review in which the research question and the inclusion and exclusion criteria are defined. It guides and asks about the existence of an SRL protocol, and is important for picking up potential deviations from the protocol during the execution of the study.
3 Did the review authors explain their selection of the study designs for inclusion in the review?
| Check if “yes” | Where to look in the article? | Answer |
|---|---|---|
|
The review must meet ONE of the following: □ Explanation for including only randomized clinical trials (RCTs). □ Or only non-randomized studies of interventions (NRSI). □ Or explanation for including RCTs and NRSIs. |
In the Methods section. |
□ Yes □ No |
4 Did the review authors use a comprehensive literature search strategy?
| Check if partial “yes” | Check if “yes” | Where to look in the article? | Answer |
|---|---|---|---|
|
The authors: √ Conducted the search in at least two databases. √ Provided keywords or search strategy. √ Added justified publication restrictions (language, year of publication). |
Aside from the above, the authors: √ Conducted a search in the references of the studies included. √ Inquired into the registries of trials and studies. √ Consulted with experts in the field. √ Used grey literature (if relevant). √ Conducted a search within the 24 months following the completion of the review. |
In the Methods section or in article supplements. |
□ Yes □ Yes partial □ No |
Asks about the databases included, the time period, keywords; MeSH and EMTREE terms must be documented and the search strategy used should preferably be described. Moreover, all searches must be supplemented with queries in specialized registries or consultation with experts in the study field, review of the references listed in the studies retrieved, and review of the grey literature.
5 Did the review authors perform study selection in duplicate?
| Check if “yes” | Where to look in the article? | Answer |
|---|---|---|
|
The review must meet at least ONE of the following: □ Selection of eligible studies and consensus on which studies to include. Or □ Two reviewers selected a sample of eligible studies and achieved good agreement (at least 80%), and the rest was selected by one reviewer. |
In the Methods section. |
□ Yes □ No |
The reviewer is guided to appraise the methods of the study.
6 Did the review authors perform data extraction in duplicate?
| Check if “yes” | Where to look in the article? | Answer |
|---|---|---|
|
The review must meet at least ONE of the following: □ At least two reviewers achieved consensus on which data to extract from the studies included O □ Two reviewers extracted data from a sample of eligible studies and reached good agreement (at least 80%), with the rest extracted by one reviewer. |
In the Methods section. |
□ Yes □ No |
7 Did the review authors provide a list of excluded studies and justify the exclusions?
| Check if Partial yes | Check if “yes” | Where to look in the article? | Answer |
|---|---|---|---|
|
The authors: √ Provided a list of all the relevant studies read in their full text but which were excluded from the review. |
Apart from the above, the authors: √ Justified exclusion from the review of each potentially relevant study. |
In the Methods section or in the article supplements. |
□ Yes □ Partial Yes □ No |
The process of selecting studies in a SRL is carried out at two different times: when assessing the articles in accordance with the selection criteria by title and abstract, and when assessing the full article. This process guarantees reproducibility and transparency because it reveals why each article was excluded.
8 Did the review authors describe the included studies in adequate detail?
| Check if Partial yes | Check if “yes” | Where to look in the article? | Answer |
|---|---|---|---|
|
The following are found: √ Described interventions √ Described comparators √ Described results √ Described research design s |
√ Population described in detail √ Intervention described in detail (dose, if relevant √ Comparator described in detail (including the dose, if relevant) √ Setting described √ Follow-up calendar |
In the Results section, or in annexes or supplements. |
□ Yes □ Partial Yes □ No |
9 Did the review authors use a satisfactory technique for assessing the risk of bias (RoB) in individual studies that were included in the review?
| Check if Partial yes | Check if “yes” | Where to look in the article? | Answer |
|---|---|---|---|
|
Must have assessed ROB of: √ Occult assignment, and √ Lack of patient and reviewer blinding when assessing the results. |
Apart from the above, must have assessed ROB of: √ Assignment sequence which was not really random, and √ Selection of the reported result among multiple measurements or analysis of a specific result. |
In the Methods, Results section, annexes or supplements. |
□ Yes □ Partial Yes □ No |
Given that biases may be introduced at several stages of the study design, planning, conduct and analysis, this item needs to be assessed for every primary article of the SRL.
10 Did the review authors report on the sources of funding for the studies included in the review?
| Check if “yes” | Where to look in the article? | Answer |
|---|---|---|
| √ Must have reported the sources of funding for the individual studies included in the review. | In considerations, notes or acknowledgements. |
□ Yes □ No |
It is important to appraise the sources of funding of the studies due to publication bias and/or information bias.
11 If meta-analysis was performed, did the review authors use appropriate methods for statistical combination of results?
| Check if “yes” | Where to look in the article? | Answer |
|---|---|---|
|
The authors: √ Justified combination of data in a meta-analysis. √ Used a weighted technique suitable for combining results of the study and adjusting for heterogeneity. √ Inquired about the causes of any heterogeneity. |
In the Methods section |
□ Yes □ No □ No meta-analysis was performed. |
This assesses if the authors justified the combination of data in a meta-analysis and if they used an appropriate weighted technique for combining study results (adjusting for heterogeneity and statistically combining the estimated effects of non-randomized studies that were adjusted for confusion), instead of combining raw data or unprocessed data, when the adjusted effect estimates were not available and abstract estimates for RCT and NRSI were reported separately (when they were both included in the review).
12 If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis?
| Check if “yes” | Where to look in the article? | Answer |
|---|---|---|
|
The authors: √ Included a single RCT with low risk of bias, or √ if the grouped estimate was based on RCT and/or NRSI on variable ROB, did the authors carry out an analysis to determined the potential ROB impact on the summarized estimates of the effect. |
In the Methods section. |
□ Yes □ No □ No meta-analysis was performed. |
This item requires reviewer to examine how results vary with inclusion or exclusion of primary studies considered as having a high risk of bias.
13 Did the review authors account for RoB in primary studies when interpreting/discussing the results of the review?
| Check if “yes” | Where to look in the article? | Answer |
|---|---|---|
|
The authors: √ Included only one RCT with low risk of bias, or √ if RCTs with moderate or high ROB or NRSI were included, did the review prompt a discussion about the probable impact of the ROB on the results. |
In the Results section |
□ Yes □ No |
Reviewers are expected to refer explicitly to the potential impacts of the risk of bias when interpreting or discussing the results of their review, and when arriving at conclusions or making recommendations.
14 Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the results of the review?
| Check if “yes” | Where to look in the article? | Answer |
|---|---|---|
|
√ There was no significant heterogeneity in the results. √ Or if there was, the authors researched the sources of any heterogeneity in the results and discussed the impact thereof on the results of the review. |
In the Results or Discussion section. |
□ Yes □ No |
It is important to look into the possible causes of heterogeneity, variation of the items included in the framework of the PICO question (item 1) and of those associated with methodological and design considerations (item 9). With the inclusion of non-randomized studies, variations in design and analysis may contribute to heterogeneity.
15 If they performed quantitative synthesis did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the results of the review?
| Check if “yes” | Where to look in the article? | Answer |
|---|---|---|
| √ Graphic or statistical tests were carried out for publication bias and the probability and magnitude of the impact of publication bias was discussed. | In the methods section,supplements, annexes. |
□ Yes □ No □ No metaanalysis Was performed |
Publication bias is a significant problem that occurs when the result of a trial or study influences the decision to publish or distribute. Publishing only results that show a significant finding disrupts the balance of findings and introduces bias in favor of positive results17.
16 Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review?
| Check if “yes” | Where to look in the article? | Answer |
|---|---|---|
|
√ The authors report there are no conflicting interests, or √ the authors describe their sources of funding and how they dealt withpotential conflicts of interest. |
In the methods section,supplements, annexes. |
□ Yes □ No □ No metaanalysis was performed |
Like with primary studies, the review authors must report their sources of funding for the SRL. Source: Authors.
For an effective use of the tool, the reviewer must have as much information as possible on the SRL and the meta-analysis (if applicable): access to the full text of the SRL article, annexes, tables, figures, supplementary material. Reading the article initially is recommended, followed by the application of the checklist with the options to answer "yes" if the item assessed meets the condition on the question; "no" if the item assessed does not meet the condition being evaluated, and "cannot be answered" or "not applicable."
Once the 16 items of the checklist have been reviewed online, the tool adds the points automatically: every "yes" gets 1 point and every "no"/"cannot be answered" gets a 0. At the end, it rates the article as low, medium or high quality 18.
This version of AMSTAR came about from a meeting of the original authors and members of the Bruyère Research Institute and Ottawa Hospital with experience in the development of non-randomized studies, during which different studies and surveys on the use of AMSTAR were discussed 18. The main changes highlighted by the group of researchers were the following: simplified answer categories; alignment between research question definition and the PICO question framework; more details about the reasons for exclusion of studies from the review; determination of whether the authors of the SRL conducted a sufficiently detailed assessment of the risk of bias for the included studies (either in a random or non-random way); determination of whether the risk of bias with the studies included was appropriately considered during the statistical combination of the results (whether it was done); and determination of whether the risk of bias with the included studies was appropriately considered when interpreting and discussing the review findings 24.
In terms of similarity with the previous tool, ten of the original domains were retained. Two domains were given more detailed coverage in the AMSTAR 2 than in the original instrument: selection of duplicate studies and data extraction now have their own items (previously combined in the original tool). Moreover, the potential influence of the sources of funding is now considered separately for the individual studies included in the review and for the review itself. One domain was removed: consideration of the grey literature which was previously a separate item is now part of the bibliographic search item. Four domains were added in total, two of them taken from the ROBINS-I tool 11: the PICO elaboration and the way in which the risk of bias was managed during evidence synthesis. One of the other new items, the discussion of potential causes and the significance of heterogeneity, is a content elaboration of the original AMSTAR tool. Another new rationale for the study design selection item was part of the AMSTAR adaptation to deal with non-random designs. The domain questions in the AMSTAR 2 are framed in such a way that a "yes" answer indicates a positive result; the "not applicable" and "cannot answer" options of the original AMSTAR were removed, in such a way that if information to rate an item is not provided, the review authors should not be given the benefit of the doubt and the item must be rated as "no". Finally, a "partial yes" answer has been provided in some cases in which it is worthwhile to identify partial compliance with the standard 24.
CONCLUSIONS
Because of its efficacy, AMSTAR is a tool commonly used for assessing the internal validity of systematic reviews of the literature. It consists of 16 items that provide a global assessment of the methodological quality of a review, and it is currently validated. It is expected of every reviewer to apply the checklist carefully, judiciously and responsibly in order to determine the methodological quality of the review and avoid classification bias when under or overestimating. Although it is the preferred tool and it is used indiscriminately, it is not devoid of limitations and it may be subject of future updates based on new reproducibility and validation studies.
ACKNOWLEDGMENTS:
Authors' contribution
AB-L: Idea conception as well as the design and structure of the manuscript.
ABP and AB-L: Searching and interpretation strategy of the selected literature.
ABP and SC: Studies selection and data extraction.
AB-L, SC and ABP: Suggestions and critical comments during the writing of the manuscript.











 text in
text in