What do we know about this problem?
Conventional coagulation tests are not very useful in patients with Glanzmann thromboasthenia.
What is the contribution of this study?
Thromboelastic testing may be used to guide perioperative hemostatic management in these patients.
INTRODUCTION
Glanzmann thromboasthenia (GT) is a rare (1/1,000,000) autosomal recessive disorder 1 caused by a mutation in platelet glycoproteins αϋ b encoding genes (ITGA2B; 607759 and ITGB3; 173470) in chromosomes 17q21.31 and 17q21.32, respectively. This gives rise to a qualitative or quantitative alteration of the platelet integrin αIIbβ3 (glycoprotein IIb/IIIa) receptor that acts as fibrinogen receptor on the platelet surface 2. GT is classified as type I when <5% of glycoprotein αIIbβ3 is expressed, or as type II when >5% is expressed. Bleeding manifestations appear in early childhood, and more than 75% of the patients require blood product transfusions for the rest of their lives. Prothrombin time (PT), partial thromboplastin time (PTT) and platelet count (PC) are generally normal and cannot be used to assess hemostasis or the effectiveness of the treatments administered to these patients.
The following is a description of a case managed successfully with thromboelastography-guided prophylactic use of tranexamic acid (TA) and platelet transfusion (PTr) in a pediatric patient taken to a surgical procedure associated with a high risk of bleeding.
CASE DESCRIPTION
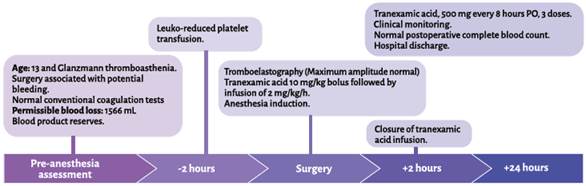
A 13-year-old caucasian female patient, attending secondary school and coming from the urban area of Manizales, Colombia, with a diagnosis of GT, assessed in the pre-anesthesia consult before undergoing endoscopic anterior bilateral ethmoidectomy plus turbinoplasty due to chronic pansinusitis and turbinate hypertrophy. The patient was diagnosed at 11 years of age by the hematologist due to episodes of epistaxis, spontaneous bruising, dental exfoliation with bleeding and hemorrhage following cauterization of septal varices. No genetic variant was found on clinical exome sequencing. She had no history of menarche, allergies, toxic substances, drugs or prior transfusions. Findings on clinical examination were normal, with no difficult airway predictors. Weight 41.7 kg, height 150 cm. Results of preoperative testing: hemoglobin 13.5 g/ dL-1, hematocrit 41.6%, leukocytes 6,190 μL-1, PC 335.000 μL-1, PT 13.2 seconds, PTT 30 seconds, INR 0.97; blood group O-positive. Permissible blood loss of 1,556 mL. Red blood cells, fresh frozen plasma and platelet apheresis.
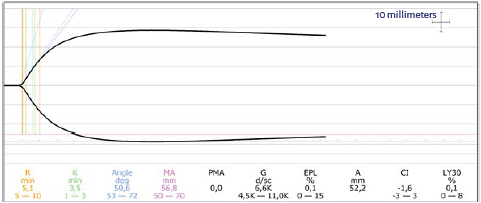
The hematologist prescribed transfusion of one unit of leukocyte-reduced apheresis-derived platelets before the surgery, and hemostasis was assessed two hours later using tromboelastography (TEG), with the following results: response time ®, 5.1 min; coagulation time (K), 3.5 min; α angle, 50.6°; maximum amplitude (MA), 56.8 mm (reference values: 5-10 minutes, 1-3 minutes, 53-72° and 50-70 mm, respectively) (Figure 1). The decision was to take the patient to surgery.
TA was used as an initial bolus of 10 mg/ kg followed by an infusion of 2 mg/kg/hour during the surgery. Thermal protection and standard monitoring were used. Remifentanil, propofol and rocuronium were used for general anesthesia induction, and sevoflurane and remifentanil were used for maintenance. An Oral RAE No. 6.5 tube was used for tracheal intubation. Dexamethasone and ondansetron were administered for antiemetic prophylaxis. Analgesic management consisted of paracetamol and hydromorphone. The surgical procedure lasted 60 minutes and blood loss was minimum. TA was continued during the next two hours after surgery, followed by oral administration of 500 mg every eight hours until three doses were completed. The results of the postoperative complete blood count were hemoglobin 13.1 g/dL-1, hematocrit 39.1% and PC 360,000 g/dL-1. There were no postoperative complications or evidence of bleeding, and the patient was discharged the following day. There were no bleeding episodes during the first 30-day follow-up period (Figure 2).
DISCUSSION
Perioperative management of pediatric patients with GT poses several challenges: high risk of bleeding, low permissible blood losses, use of hemostatic agents that entail the risk of complications, and little use for conventional coagulation tests. Therapeutic options include TA, PTr and recombinant factor VIIa (rFVIIa). TA has been shown to be useful in association with other therapies in patients with GT 1. It is an anti-fibrinolytic agent that inhibits plasminogen and reduces plasmin production, preventing clot lysis. Recombinant factor VIIa is an option in patients with anti-integrin αIIbβ3 and/or HLA antibodies, and refractory to PTr 3-5. It is a preferred option in women in child-bearing age in order to avoid PTr-related antibodies which may cross the placenta and produce fetal thrombocytopenia (associated with intrauterine demise and intracranial bleeding)1. PTr is the best option for bleeding prevention or treatment in patients with GT 6; however, its effectiveness may be impaired by several factors. Al-Battat et al. 7 found that conventional platelet doses may not be sufficient for adequate hemostasis given that endogenous platelets are bigger in number than transfused platelets and adhere in a higher proportion at the site of injury. Moreover, reactive isoantibodies against integrin αIIbβ3 and/or major histocompatibility complex class I molecules in patients with prior transfusions may block platelet aggregation and lead to a rapid clearance of transfused platelets 8. This situation is difficult to ascertain in the surgical setting because it requires antibody measurements which take time and are not always available.
The patient had bleeding complications during a previous surgery before she was diagnosed. For this reason, the hematologist ordered prophylactic use of leuko-reduced PTr before the surgery. PTr prophylaxis is effective for the prevention of perioperative bleeding in patients with GT, preferably with HLA-compatible platelets or, if not, leuko-reduced platelets to avoid alloimmunization 1. Although the patient had not received prior transfusions that could put her at risk of having anti-thrombocyte or anti-platelet antibodies, it is reasonable to use more objective measurements of the quality of hemostasis before surgery, according to the findings by Al-Battat et al. 7. For this reason, the surgical team decided to use TEG before the procedure in order to assess the effect of the treatment. Some authors have described the usefulness of viscoelastic tests for the determination of hemostatic status during the surgical procedure and as guidance for therapeutic measures in patients with GT 9,10. MA, which assesses maximum clot stiffness (depending on platelet function and number and, to a lesser degree, fibrin formation) was normal, as were all the other measured parameters. Consequently, it was considered safe to perform the procedure. In this context, TEG vas a valuable tool for making a quick determination of hemostatic status, favoring more objective decisions, and improving patient safety.
Finally, surgical planning and teamwork were crucial for the success of the procedure.
CONCLUSIONS
Pediatric patients with GT are at a high risk of bleeding during and after surgery. PTr is the best prophylactic and therapeutic option; however, even in the absence of anti-platelet antibodies, its effectiveness may be compromised. Therefore, viscoelastic tests are useful for determining treatment efficacy and enhancing patient safety during the surgical procedure.
ETHICAL CONSIDERATIONS
Human and animal protection
The authors declare that no experiments in humans or animals were carried out for this research.
Data confidentiality
The authors declare that they have followed the protocols on patient information disclosure implemented by their institution.











 texto en
texto en