BRIEF HISTORY OF CANNABIS AS A MEDICINE
The cannabis plant has been known since ancient times, as it grows in many climate zones and for millennia has been used mainly as a source of fiber in the manufacturing of textiles and ropes 1. Given that environmental factors alter the composition of substances that could have pharmacological properties, only in areas of the world where the content of these compounds was sufficiently high to spark human beings' curiosity regarding the properties that different parts of the plant could have 1. The most ancient evidence of cannabis farming dates back to 4000 BC in Pan-p'o village, China, in pollen deposits 2. The therapeutic use of cannabis was documented in China in the oldest pharmacopoeia, the Pen-ts'ao ching, attributed to the emperor Shennung, who ruled around 27003. Recent research reports discoveries of cannabis plant material in tombs in Siberia dating between 2800 and 2400 years 4. The Pen-ts'ao ching describes cannabis use for the treatment of rheumatic pain and malaria, as well as the plants' psychoactive effects, attributing the ability to facilitate "communication with the spirits and the alleviation of the body " to the cannabis fruits 2,3. The cannabis plant also spread to India, where it was used extensively for medical and recreational purposes 3. Herodotus, the ancient Greek historian documented, in 450 BC, the use of cannabis seeds that were incinerated as part of funeral rituals in order to obtain psychoactive effects 3. The Persian physician Avicenna, author of the Canon of Medicine, documented the usefulness of cannabis for the treatment of severe headache, infected wounds, rheumatic ailments and edema 5. Knowledge on the effects of cannabis was introduced in Europe in the 19th century, where medical and non-medical use followed different paths; the French were more interested in the psychoactive effects, whereas in England, interest on the medical properties prevailed 1. The English physician William O'Shaughnessy, through his observations of the traditional use of cannabis in India, described effective treatments for tetanus and seizures, leading to the inclusion of cannabis in the British Pharmacopoeia and, subsequently, in the United States Pharmacopeia 6.
In the United States, cannabis was widely used as a patent medicine during the 19th and early 20th centuries, said uses first described in the Pharmacopoeia in 1850. However, the federal restriction on the use of cannabis occurred in 1937 with the Marijuana Tax Law 7. Cannabis was subsequently removed from the United States Pharmacopoeia in 1942; legal penalties for possession increased in the 1950s and led to a federal ban under the Controlled Substances Act of 1970 8. These laws created limitations on research by restricting the acquisition of cannabis for academic and scientific purposes. In 1970, through the Controlled Substances Act, marijuana was designated as "Category 1" or a substance that has a high potential for abuse, with no currently accepted medical use in the United States, and which lacks safety data to be used under medical supervision 9.
Despite regulations that significantly hindered the scientific study and analysis of the cannabis plant, there was a breakthrough in cannabinoid science in the 60s with the discovery of the delta-9-tetrahydrocannabinol (THC) molecule by Israeli scientist Raphael Mechoulam in 1964 10. Subsequently, type 1 and type 2 endocannabinoid receptors were identified in 1990 and 1993, respectively 11,12, whereby the phytocannabinoids exert their effects.
In Colombia, there were signs of early cannabis crops in the early 1920s, however, the plant would not be deemed as problematic until the 30s, around the same time cannabis started to face rejection in the United States. Marihuana crops were fully outlawed in 1939, through a ruling that mandated the destruction of all existing crops and established penalties for those not abiding by this new law 13.
Notwithstanding this law, farming and use were growing; reports from the 40s and 50s associated its use with the lower social classes, and later with the outbreak of rural violence during the 60s. 13
The changing historical connotations given to cannabis have influenced the perception by the population and the authorities about the use of the plant and at the same time have influenced the willingness of the healthcare authorities to allocate resources for research. 14 Likewise, the perceptions of healthcare practitioners have also been influenced by the social context of cannabis during the 20th century. 15
MEDICAL CANNABIS IN COLOMBIA
Similar to the experience in other jurisdictions around the world, the movement to legalize cannabis for medical and scientific use in our country was spurred by patient testimonials that report clinical benefits. Senator Juan Manuel Galan, author of the draft bill that would give rise to the current law 1787 of 2016, became interested in the therapeutic effects of cannabis after listening to testimonials of experts from Israel, Canada, Colorado and Chile and after listening to the experiences of patients like Charlotte Figi in Colorado 16 as well as local experiences 17. After assessing the existing scientific evidence, Galán's team, with the support from the Ministry of Health, in 2014, set out to create the medical cannabis legislation, based on access, quality, fair price and safety, embracing the principles of social justice, seeking to ensure technology transfer for the production of material, raw materials and processing, bringing benefits for both the small growers wanting to become part of the legal industry, as well as large agro-pharmaceutical companies 18.
The discussions that took place in Congress before the regulation in 2016 focused not only on the plant's medical potential, but on the potential legal implications of the regulation of cannabis for these purposes, as some members of Congress saw this movement as a legitimation of illicit substances, which could pave the way to further drug trafficking 19. During the course of the debates, the fear held by some members of the legislature that the approval of the medical cannabis regulatory framework could result in the plant being used for "other purposes" became apparent 20. Despite the contradictory viewpoints, the regulation was passed with the support from a vast majority of house of representatives members in May 2016 and was signed into law on July 6th of that same year.
Presently, under the legislative framework for cannabis use, the Ministry of Justice is the body in charge of affording cultivation licenses for psychoactive cannabis - with a THC composition greater than 1% - and non-psychoactive cannabis 21 . As of this writing, this entity has granted 656 licenses: 98 for the use of seed for sowing, 164 for the cultivation of psychoactive cannabis and 394 for the cultivation of non-cannabis psychoactive 22 . Additionally, the Ministry of Justice is in charge of maintaining a registry of natural persons considered small and medium-sized growers, those which hold a total cultivation area which does not exceed 0.5 ha; To August 2020, there were 4217 individuals registered under this modality 23 . The Ministry of Health is in charge of cannabis derivatives manufacturing and export licenses, and, to date, it has granted 171 licenses for these purposes 24. Of these, 74 companies are licensed to conduct scientific research.
The companies that currently hold the required licenses are diverse, with different funding sources, but the country has recently witnessed a major inflow of Canadian companies seeking to buy the rights of local companies 25-28. Some of these companies have expressed interest in establishing operations in Colombia given its excellent climate conditions, low production costs, as well as the existence of cannabis varieties that have been in the territory for several decades - which are considered "native" - and which may have a unique chemical profile. Additionally, as Latin American countries begin to regulate cannabis for medical purposes, the marketplace in these region could expand: several Colombian companies have designed their cultivation and manufacturing processes to be able to export to countries like Peru and Brazil.
One of the most expeditious routes through which medical cannabis licensed producers can introduce their products to the market is through manufacturing of "compounded preparations". The regulation recognizes compounded preparations as those products "prepared by a pharmaceutical establishment to meet a medical prescription for an individual patient, who requires some type of intervention of varying complexity "29. These preparations should be formulated specifically for a patient, with a cannabinoid ratio indicated for the clinical condition that requires treatment, and should be dispensed in establishments that have the relevant sanitary permits 30. The commercialization of compounded preparations began in March 2020, therefore, the majority of Colombian patients do not have real access to cannabinoid-based medicines, despite the fact that Law 1787 has been in effect for 4 years.
Compounded preparations present a challenge for prescribing physicians, who have trained under a paradigm of predetermined doses for the different pharmaceuticals. The use of compounding preparations requires some flexibility, in-depth knowledge of the different cannabinoids available - THC and cannabidiol (CBD) being the most relevant-, their therapeutic actions, the different concentrations or proportion of cannabinoids depending on the clinical condition and patient characteristics, comorbidities, tolerance and previous exposure of the patients to cannabis 31. Prescription and dosing are two of the aspects of cannabinoid use that clinicians consistently identify as challenging when considering recommending cannabis-based treatments, and these generate uncertainty regarding the use of these medicines 32-35. Currently, both general practitioners and specialist physicians can prescribe compounded preparations of medical cannabis for conditions with sufficient clinical evidence, and these would be dispensed exclusively by pharmacies that have met the Standards of Best Practice (SOBP) as stipulated by the Institute of Food and Drug Surveillance (Invima) 36
MEDICAL CANNABIS AROUND THE WORLD
According to Forbes magazine 37, within the next 10 years, the legal cannabis industry will experience global growth. It is expected that spending on legal cannabis in the world will reach $ 57 billion in 2027: the Adult Use Market (Recreational) will represent 67% of spending, and medical cannabis the remaining 33%. The largest group of cannabis buyers will be in North America, escalating from $ 9,2 billion in 2017 to $ 47.3 billion 10 years later. However, the largest differential growth is expected in markets in the rest of the world, going from $ 52 million spent in 2017 to a projection of $ 2.5 billion in 2027.
Several countries have implemented different medical cannabis regulatory models, and more countries join this list each year.
Canada is considered a pioneer country in terms of legislation which allows for the use of cannabis with medical purposes. In 2001 the Canadian government issued the first set of rules for the use of cannabis for medical purposes, allowing patients with terminal illnesses or serious conditions access to the dry plant material when they cultivated it themselves. The legislation has undergone several changes over the years, and in 2016 the Access to Cannabis for Medical Purposes Regulations (ACMPR) was created, expanding the number of licenses granted to companies to grow, process and market dry flower and cannabinoid oils 38.
Under the medical cannabis regulatory framework, patients can access cannabinoid-based medicines through a recommendation - not a prescription -from a family physician or specialist; after completing a medical document, signed by the responsible physician, the patient must present it to the licensed producer so it can dispense the product, either in the form of the dried flower, oils or topical products with different cannabinoid and terpene concentrations and which are derived from specific strains. Medical cannabis orders are placed online through the website of each licensed producer, and are home-delivered to patients by mail.
On October 17 201839 Canada became the first G-7 country to legalize cannabis for adult use. Law C-45, The Cannabis Act, legalized and regulated the access to cannabis in Canada, aimed at providing a public health framework that reduces the likelihood of negative health outcomes and the potential consequences resulting from criminalization. 40
Uruguay, another leader country in the regulation of both adult-use and medical cannabis, legalized both its uses in 2013. The Uruguayan Government allows residents or senior citizens 18 years old, who have previously registered, - the acquirers-to obtain cannabis from authorized pharmacies, to grow up to six plants and grow in membership clubs 41. Similarly, medical cannabis is sold in pharmacies and supplied to people over 18 years of age who hold a medical prescription, and will be automatically enrolled in the official registry 42.
In the countries of the European Union, there are different levels and types of legislation. No country has legislation that allows the use of cannabis for recreational purposes, however Austria, Croatia, Czech Republic, Denmark, Greece, Germany, Italy, Malta, Holland, Norway, Poland, Slovenia and Switzerland allow the prescription of cannabis-based compounded preparations, and in most countries, cannabinoidbased medicines (such as nabiximols, or tetrahydrocannabinol synthetic dronabinol) are permitted 43.
In the United States, 32 of the 51 states have already authorized the use of medical cannabis 44. All states, with the exception of the District of Columbia, limit the use of medical cannabis to certain clinical conditions 45. Most states require physicians to be registered to be able to recommend medical cannabis, and to have an existing relationship with the patient before giving their recommendation. To this date, nine states have legalized cannabis for recreational purposes 46
CANNABIS AND CANNABINOIDS THERAPEUTIC EFFECTS
Over the past three decades, two types of synthetic cannabinoids have been approved: dronabinol and nabilone. Dronabinol, a synthetic delta-9-tetrahydrocannabinol (THC) preparation, was approved for use in the United States by the FDA in 1986 for the treatment of chemotherapy-induced nausea and vomiting which was refractory to conventional treatment, and anorexia related to HIV / AIDS 47,48. In some European countries, it is also authorized for the treatment of these conditions, as well as for cancer-associated chronic pain and palliative care 49. Nabilone, another synthetic THC molecule, has been used in Canada since 2000, for the treatment of chemotherapy-associated nausea and vomiting 50 and has the same indication in several EU countries. 49 However, given the low tolerability profile evidenced in a number of systematic reviews 51-54, its use is usually relegated to a second or third line therapy for managing the above-mentioned conditions 55.
Figure 1 illustrates the structures of six Phyto cannabinoids.
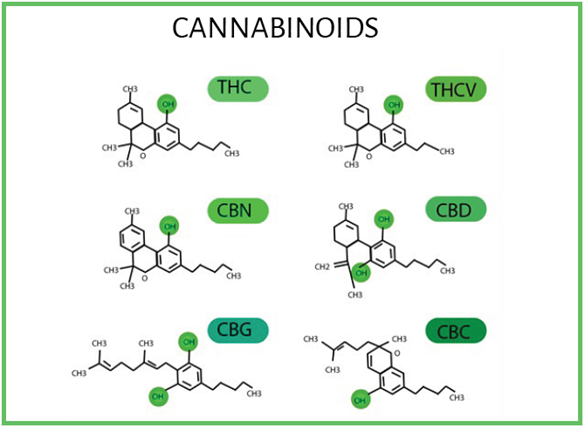
SOURCE: Elsohly and Slade 56.
FIGURE 1 Δ9-tetrahidrocannabinol (Δ9-THC), cannabinol (CBN), cannabigerol (CBG), tetrahydrocannavivarin (THCV) cannabidiol (CBD) and cannabichromene (CBC) structures.
The epidemiological and anecdotal evidence has shown the plant-derived cannabinoids, also called phytocannabinoids, could have therapeutic potential. Given the challenges in the treatment of chronic pain 57,58, insomnia 59, Crohn's disease and Alzheimer's, people have turned to the use of cannabis to improve their symptoms, which has driven the revival of cannabis as a medicine 60.
One of the most complete compilations on the health effects of cannabis was conducted by the National Academy of Sciences, Engineering and Medicine of the United States (NASEM), that rigorously reviewed the existing relevant literature published from 1999 to 2016. 61 This report represents the most comprehensive review of the evidence about cannabis for medicinal purposes, and rates the evidence found as "conclusive", "moderate" or "limited" according the quality of the studies analyzed. It also made an analysis of the studies intended to clarify the risks associated with the use of cannabis, allowing for a better understanding of the really significant risks.
The authors of the aforementioned study warn that the vast majority of findings cannot be considered as definitive, since much of existing research isn't considered to be of good quality and the evidence is derived from studies with low methodological quality. The report also points out to the lack of good quality research given the legal barriers that exist due to the classification of cannabis as a highly restricted substance - "Schedule I" in the DEA (Drug Enforcement Administration) classification - hindering research with this compound.
The strongest or most conclusive evidence has been found for the following conditions: Chronic pain management in adult patients, as antiemetics for patients with chemotherapy-induced nausea-vomiting and for the treatment of spasticity related to multiple sclerosis. That conclusive evidence was found to support the role of cannabinoids in the management of chronic pain confirms what some authors have suggested as one of the most important potential benefits of these compounds: as alternatives to opiates 62-65.
According to the report, there is moderate evidence for the treatment of short term- sleep disorders associated with sleep apnea, fibromyalgia, and chronic pain from multiple sclerosis.
Contrary to many of the empirical and anecdotal medical cannabis uses, analysis of research results found limited evidence regarding treatment of Tourette syndrome, anxiety symptoms, treatment of HIV/AIDS -related poor appetite and weight loss, and symptoms of post-traumatic stress disorder, despite the fact that cannabis has become one of the most common treatments for veterans suffering from this disorder 66.
This report also mentions that there is "insufficient evidence" or "no evidence" to support or refute the use of cannabis or cannabinoids in the treatment of neurological diseases, such as epilepsy, paralysis due to spinal cord injury, and motor symptoms of Parkinson's disease. The lack of effectiveness mentioned in this research with respect to epilepsy contrasts with the findings from recent studies examining cannabidiol for treating refractory epilepsy syndromes 67,68. This type of evidence led, for the first time in history, to the FDA approval of cannabidiol, a Cannabis sativa-derived cannabinoid which lacks psychotropic properties, for the treatment of pediatric refractory epilepsy- Dravet and Lennox Gastault syndromes 69. Cannabidiol is currently marketed in Colombia for these same indications.
Given the existing clinical evidence on the effect of cannabinoids on the treatment of non-cancer chronic pain, several clinical guidelines from different countries or scientific societies include the use of medicinal cannabis or cannabinoids. For instance, the position statement of the European Pain Federation on the appropriate use of cannabis-based medications and medical cannabis for the management of chronic pain from 201870 claims that "The therapy with cannabis-based medications should only be considered by experienced physicians as part of a multidisciplinary treatment and preferably as complementary medication, if the therapies recommended by this guideline as first and second line have not provided sufficient efficacy or tolerability". The 2017 Australian guidelines for the use of medicinal cannabis for the treatment of chronic pain 71 recommend clinicians to consider adding medicinal cannabis for the treatment of chronic noncancer pain as adjuvant therapy and never to replace first or second line treatments. The simplified guidelines for the prescription of medical cannabinoids in primary care in Canada 72 states that clinicians may consider medical cannabinoids for refractory neuropathic pain-weak recommendation- having previously met a set of conditions such as a comprehensive informed consent process, having failed other pharmacological therapies, and their use as adjuvant therapy. The consensus statement by the Canadian Pain Society 73 includes cannabinoids as third line therapy in its pain management algorithm.
HARMFUL EFFECTS OF CANNABIS
A large part of the already-mentioned NASEM study 61 focused on discussing the harmful effects of cannabis use, differentiating between the risks with better-quality evidence and those effects that are less established based on existing studies.
One of the effects strongly associated with the use of inhaled cannabis products is chronic cough and bronchitis. While this association was conclusive, it was not possible for the study authors to elucidate whether smoking cannabis entails a higher risk for other respiratory issues, such as asthma, chronic lung disease, or decreased lung function based on existing evidence. Likewise, the report concluded that smoking cannabis does not increase the risk of lung cancer or head and neck cancer in adults, which generally are associated with smoking tobacco cigarettes. One of the effects strongly associated with the use of inhaled cannabis products is coughing and bronchitis. While this association was conclusive, it was impossible to clarify whether smoking cannabis entails a higher risk of other respiratory problems such as asthma, chronic pulmonary disease, or decreased lung function. Similarly, the report concluded that cannabis smoking does not increase the risk of lung or head and neck cancer in adults, which are usually associated with tobacco cigarette-smoking.
The effects of cannabis use during pregnancy were also studied, and the authors in the report concluded that smoking cannabis during pregnancy was associated with low birth-weight infants. Despite these findings, recent studies have ascertained that the number of women who smoke cannabis during pregnancy has increased, primarily to control nausea 74.
As mentioned during public discussions about the legalization of cannabis for recreational purposes, one of the major concerns is its use by teenagers and minors, the consequences cannabis use could have on the developing nervous system, as well as the danger of encouraging the use of other drugs with higher addictive potential 75. The report found that cannabis use by adolescents is related to por academic performance, in education attainment and in future employment and wages, as well as social relationships; this evidence was rated as "limited". It has also been identified that the risk for problematic cannabis use increases with frequent use of the substance and early-age onset.
Another link that has been established for several years is the relationship between cannabis use and the risk for psychosis or schizophrenia episodes 76-78. Indeed, the report showed that the risk for the general population increases with cannabis use. With respect to mental health, the study authors consider that substance use may be a potential risk factor for the development of mental illness, while mental illness could also be a risk factor for developing substance use disorder.
One of the observations made by the study authors is that a correlation between cannabis use and other mental health conditions does not necessarily establish cannabis as the causative agent. However, findings from this report, together with existing evidence, shed more light about the populations that are at higher risk for harmful outcomes from cannabis use: pregnant women, people at risk from suffering mental illness, smokers and people with respiratory conditions, children and adolescents. The report by the National Academy of Science, Engineering and Medicine ends by making four recommendations regarding cannabis research: 1) Address research gaps, 2) improve the quality of research through development of a set of standards, 3) improve surveillance capacity to ensure that there is sufficient population data to study effects on people's health and, 4) address barriers that prevent cannabis and cannabinoid research 79.
CURRENT RESEARCH
As more countries legislate and regulate the use of cannabis for medical purposes, more research results will be seen, either confirming or disproving the therapeutic benefits that have been attributed so far. To date there are 186 studies on cannabis registered as active in the clinical trials registry from the United States clinicaltrials.gov. Although a large proportion still focus on assessing adverse effects and / or treatments for problematic cannabis use, around 100 of the registered trials use some form of medical cannabis as a therapeutic intervention for diverse diseases such as post-traumatic stress disorder, pancreatic cancer, glioblastoma multiforme, psoriasis, among others, and a significant proportion analyze the role of cannabis as a sole therapy for the management of chronic pain 80. The vast majority of these clinical studies are conducted in the United States, Canada and Europe.
CONCLUSION
Despite the complex and controversial history of cannabis as a medicine, many countries have adopted laws that permit its use, and great part of this adoption has been driven by the patients themselves, seeking alternatives to treat ailments for which conventional drugs haven't been deemed effective enough. Colombia has positioned itself as a potential leader for the production of cannabis derivatives for medical and scientific purposes due to its optimal climate for the cannabis plants, low production costs and its geographic location, which facilitates access to South and North America, in addition to having one of the most robust and complete legislations in the world. A significant amount of incoming foreign investment seeks to take advantage of these conditions with the aim to boost the industry's reach into a potential multi-million dollar market.
Although much of the global cannabis market economic boom focuses on the recreational market's potential, the enthusiasm around the use of the plant for medical purposes has clinical and scientific grounds. The effect of cannabis has been extensively studied at the epidemiological and preclinical level and, recently, through randomized controlled studies, the clinical possibilities that medical cannabis holds have been demonstrated. Although a large gap in knowledge with respect to many therapeutic applications still exists, cannabis has been recognized as a suitable therapeutic option for the treatment of patients with difficult-to-manage epilepsy, patients with certain neurological ailments and chronic pain. However, despite these clinical effects, many health professionals around the world do not consider that they have enough information on dosing systems or creating effective therapeutic plans using cannabis.
As healthcare professionals, it is our duty to inform and educate ourselves, from unbiased sources, with respect to the potential uses of cannabis derived compounds, to be able to ethically, competently and in an evidence-based fashion, respond to our patients' needs. Colombia, now the epicenter of this new agro-pharmaceutical industry, has the potential to become an important landmark for medical cannabis research.











 text in
text in 


