INTRODUCTION
The new coronavirus 2019-nCov or SARS-Cov-2 is responsible for the most important pandemic in the 21st century, the coronavirus disease (COVID-19). It was initially identified in December 2019 in Wuhan, China, and nowadays (updated in September, 2020) it is known to have affected 213 countries, with more than thirty-three million documented cases.
Spain has been one the most affected countries. As of May 14th, 2020, there are more than 200.000 confirmed cases and more than 26.000 deaths. There is no doubt that we are dealing with a very powerful virus and its transmission routes are still being studied. The most common clinical manifestation is bilateral pneumonia, but some patients develop multiple organ failure. Around 5% of the Spanish patients end up needing intensive care.1 Thromboembolic events are among the most severe complications in these patients, accounting for a significant number of deaths;2,3 so it is reasonable to say that the development of coagulopathy in these patients may be associated with poor prognosis.3
This report describes the case of a 43-year-old patient infected by SARS-Cov-2, admitted to our intensive care unit (ICU) in Hospital Ramón y Cajal, Madrid, Spain. The patient had been on mechanical ventilation for 20 days and developed acute pulmonary thromboembolism with hemodynamic instability requiring systemic fibrinolysis.
CASE REPORT
This case discusses a 43-year-old male patient, 83 kg of body weight, BMI 26,5 (obesity class I), with an otherwise unremarkable medical history. The patient was admitted to the Hospital on March 19th, 2020, with a 7-day history of fever (39°C) in addition to diarrhea, epigastric pain and asthenia. A polymerase chain reaction (PCR) test was performed which was positive for the new SARS-CoV-2 virus. The patient was diagnosed with respiratory infection, COVID-19 bilateral pneumonia, CURB 65 2 points, NEWS10 points, was established as the most likely diagnosis due to the prevalence of the disease, and based on symptoms and on the presence of diffuse bilateral lung opacities, as shown on the chest X-ray. After 8 days on the medical ward, the patient progressively developed respiratory failure, and the physical examination revealed confusion, tachypnea, shortness of breath and sweating. Oxygen saturation was 81%, with 100% inspired oxygen fraction (FiO2) with an oxygen mask and additional reservoir. The patient required intubation and was admitted to the ICU. Due to the high demand for ICU-beds at the first Hospital, the patient was transferred to our ICU at Ramón y Cajal University Hospital, Madrid. There is no additional available information about laboratory results or oxygenation parameters at admission to our hospital.
The patient had no personal or family history of coagulopathy. As shown in Table 1, at ICU admission the D-Dimer (DD) levels were high, at 22.426 ng/mL.
TABLE 1 Patient's evolutive data: diagnostic images, ventilation modes and most remarkable laboratory results.
| Transferred to ICU in Hospital Ramón y Cajal ↓ |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Date | 3/4/20 | 7/4/20 | 10/4/20 | 12/4/20 | 13/4/20 | 14/4/20 | 15/4/20 | 16/4/20 | 17/4/20 | 19/4/20 | 21/4/20 | 25/4/20 | 26 -30/4 | 1-4/5 |
| IMAGING | X-Ray | X-Ray | X-Ray | X-Ray | X-Ray | X-Ray | X-Ray | X-Ray | ||||||
| TTE | ||||||||||||||
| Lung-US | Lung-US | Angio-CT | ||||||||||||
| TTE | TTE | TTE | ||||||||||||
| Ventilation | IOT | TRACHEOSTOMY | T-HF | COT 3-4lpm | ||||||||||
| 3a PRONE | 4a PRONE | |||||||||||||
| NITRIC OXIDE | ||||||||||||||
| Hemodynamics | Adrenaline + Noradrenaline | |||||||||||||
| FiO2 (%) | 65 | 60 | 60 | 50 | 65 | 100 | 85 | 85 | 70 | 55 | 50 | 55 | [40-45] | [28-40] |
| PaO2 (mmHg) | 117 | 84 | 72 | 63 | 75 | 72 | 70 | 87 | 64 | 79 | 105 | 82 | [64-71] | [66-105] |
| Pa/Fi | 180 | 140 | 120 | 130 | 115 | 72 | 82 | 102 | 91 | 143 | 200 | 150 | [158-232] | [160-375 ] |
| SIC score | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | [1-2] |
| SOFA | 4 | 3 | 3 | 3 | 7 | 7 | 7 | 7 | 5 | 3 | 3 | 3 | [2-3] | [1-2] |
| DD (ng/mL) | 22426 | 11142 | 19406 | 15477 | 19933 | >35000 | 6918 | 4948 | 4241 | 4288 | 12935 | 9749 | [6809-8720] | [1649-6964] |
| Hb (g/dL) | 10.3 | 8.8 | 9.4 | 8.2 | 8.1 | 8.2 | 8.5 | 8.3 | 9.8 | 11 | 12.8 | 11.2 | 11.3 | [12.19.2] |
| Platelets (/μL) | 287000 | 215000 | 201000 | 184000 | 171000 | 186000 | 239000 | 269000 | 314000 | 369000 | 356000 | 232000 | [22.5-25]x103 | [26.5-17.8] x103 |
| INR | 1.05 | 0.94 | 0.95 | 1.06 | 1.09 | 1.14 | 1.14 | 1.05 | 0.9 | 0.94 | 1.03 | 0.97 | [0.97-1.01] | [0.97-1.03] |
| Troponin (ng/ mL) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Creatinine (mg/ dL) | 0.65 | 0.55 | 0.58 | 0.51 | 0.52 | 0.49 | 0.59 | 0.52 | 0.49 | 0.52 | 0.53 | 0.49 | [0.53-0.55] | [0.54-0.57] |
| GFR (mL/min) | 133.4 | 161.8 | 152 | 176 | 172.6 | 184 | 149 | 172 | 184 | 172 | 168 | 184 | [162-168] | [165-127] |
| Bilirubin (mg/dL) | 1.57 | 0.46 | 0.19 | 0.47 | 0.46 | 0.41 | 0.47 | 0.38 | 0.33 | 0.38 | 0.76 | 0.37 | [0.55-0.63] | [0.63-0.39] |
| IL-6 (pg/mL) | 985.53 | 87.66 | 1097 | 287.41 | ||||||||||
COT: conventional oxygen therapy, DD: D-Dimer, Hb: hemoglobin, FiO2: inspired oxygen fraction, GFR: Glomerular Filtration Rate, IL-6: 6 interleukin, INR: International Normalized Ratio, Lung-US: lung ultrasound, Pa/Fi: fraction of inspired oxygen ratio, PaO2: partial pressure of oxygen, T-HF: high flow oxygen via tracheostomy, TTE: transthoracic echocardiography, SIC: Sepsis-Induced Coagulopathy, SOFA: Sequential Organ Failure Assessment.
SOURCE: Authors, from patient data while staying in Hospital Ramón y Cajal.
While in the ICU, the patient received alternating therapy with controlled ventilation and pressure support ventilation (PS/CPAP); our COVID-19 protocol was initiated which consisted of: lopinavir/ritonavir (80mg/20mg, 8 days) + Chloroquine (200mg, 8 days) + Azithromycin (500mg, 8 days) + Methylprednisolone (1mg/kg/day) + Remdesivir (100mg, 10 days) + Tocilizumab (400mg, 2 doses).
As shown on Table 1, our patient met the Berlin criteria for moderate Acute Respiratory Distress Syndrome (ARDS) with a PaO2/FiO2 of 180; lung ultrasonography ruled out hydrostatic edema. The patient had three long prone position sessions (2448 hours each) and on his fifth day after admission a tracheostomy was performed to facilitate weaning from mechanical ventilation.
After admission the patient was hemodynamically stable, and did not require any vasoactive drugs. Additionally, the patient received targeted antibiotic therapy with meropenem + ampicillin + anidulafungin to treat a documented enterococcal bacteremia and fungal colonization with Candida Glabrata identified in a bronchial aspirate culture. Notwithstanding antibiotic therapy, the patient continued with fever and rising C-reactive-protein (CRP) levels.
April 13th, 2020 - the day of the event
Earlier that morning, increasing DD levels and repeated positive blood cultures to enterococcus faecalis were documented. Anticoagulation therapy with 40mg enoxaparin/12 hours was initiated two days before, once again due to rising DD levels from 11.142 ng/mL to 19.406 ng/ mL. Platelets and coagulation parameters remained within normal ranges at all times.
A transthoracic echocardiography was performed to rule out active endocarditis; no signs of endocarditis, right heart failure or abnormal cardiac pathology were observed.
After a few hours, the patient experienced progressive and maintained desaturation with poor response to alveolar recruitment maneuvers requiring increased FiO2 up to 100%, to maintain a PaO2/FiO2 of 80-85. Due to hemodynamic instability, continuous noradrenalin and adrenaline perfusion were initiated.
Suspecting acute pulmonary thromboembolism, an angio-CT of the pulmonary arteries was performed (Image 1). The diagnosis was: central filling defects in the main pulmonary arteries; multiple thrombi in the lobar pulmonary arteries, as well as radiological signs suggesting hemodynamic consequences in the chambers of the right heart.
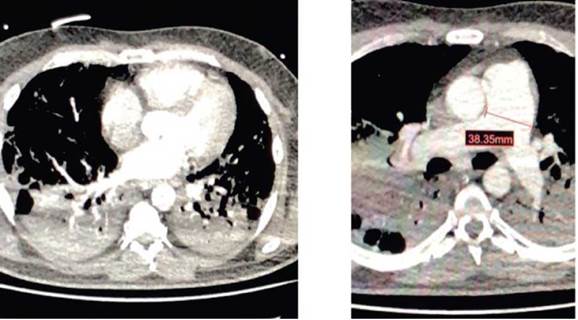
Central filling defects in both main pulmonary arteries, and presence of thrombi. Radiological signs suggestive of hemodynamic consequences in the right heart chambers: pulmonary artery caliper up to 38mm, rectification of the intraventricular septum. SOURCE: Authors.
IMAGE 1 Angio-CT scan: Acute pulmonary thromboembolism.
A subsequent bedside transthoracic echocardiography (TTE) showed: non dilated or hypertrophic left ventricle, preserved systolic function, with no segment disruptions. Dilated right ventricle (RV) with diminished function (TAPSE 12mm, S' 7,5). Moderate hypokinesia of the lateral wall or the RV (basal and middle segments) compatible with McConnell sign. Intraventricular image compatible with a 2x3,5 cm thrombus near the moderator band of the right ventricle. No thrombi were found in the pulmonary artery or its branches, and there was no significant valve dysfunction or pericardial effusion. Evidence of partial collapse and dilated inferior vena cava.
Due to constant hemodynamic instability and refractory hypoxemia, notwithstanding controlled mechanical ventilation with 100% FiO2, systemic fibrinolysis was performed with Alteplase 100mg (2-hour perfusion) followed by an Unfractionated-Heparin perfusion (UFH, in perfusion: 1000 UI per hour). Heparin was discontinued after two hours because the patient developed active bleeding through the tracheostomy wound. Once the bleeding was controlled, the perfusion was reinitiated maintaining a Partial Thromboplastin Time (PTT) 2.5-fold the normal value.
Soon after fibrinolysis the patient experienced a significant clinical hemodynamic and respiratory improvement. Saturation increased to 98% so FiO2 was lowered to 90% and adrenaline perfusion to 0.02 mcg/kg/min. Two hours later the patient desaturated again down to 90% with 100% FiO2 and became hypotensive; the adrenaline dose was increased to 0.05 mcg/kg/min. Because of refractory hypoxemia with 78% oxygen saturation and 60 PaFi value, a few hours later Nitric Oxide (NO) was administered at a concentration of 20 parts per million (ppm).
A transthoracic echocardiography (TTE) was performed ruling out the presence of intracavitary and proximal pulmonary thrombi. The right ventricle was dilated with mild-moderate disfunction (TAPSE 17), therefore Milrinone was started. An ultrasound of the lower extremities ruled out superficial and deep vein thrombosis. Meanwhile treatment with UFH was continued and DD value continued increasing up to >35.000 (ng/mL).
The patient's respiratory function continued to deteriorate and the following day the patient was pronated again. After 36 hours of pronation, the patient showed remarkable oxygenation and hemodynamic improvement, leading to a reduction of the NO dose, and to the withdrawal of adrenaline and noradrenaline. A new TTE showed improved right-ventricular function (TAPSE 23). Anticoagulation therapy was switched over to enoxaparin 80mg/12h and DD levels decreased progressively. DD values and other remarkable parameters are showed in Table 1.
Over the course of the following days, respiratory support and NO were gradually reduced until NO was finally removed after 8 days of treatment. Thirteen days after the event, twenty-two days after admission to our ICU, weaning was achieved to high flow oxygen (50% FiO2 and 50 liters flow) through tracheostomy. Finally, the patient was decannulated on April, 25th.
After 30 days in our ICU, the patient was discharged to the Internal Medicine ward with 3 liters of supplementary oxygen with a conventional nasal cannula (COT 3L), with adequate gasometric values. Anticoagulant treatment with enoxaparin 80mg/12hours was maintained.
Currently, the patient continues hospitalized, with no focal neurological signs and is hemodynamically stable; eupneic with 2 liters COT, receiving motor physical therapy because of the development of critical patient polyneuropathy and psychiatric care for opioid dependence.
DISCUSSION
2019-nCov infection leads to the development of a hyper-coagulable state, and worse outcomes in these patients. The necropsies performed on COVID-19 patients in our hospital have identified scattered microthrombi in the pulmonary vasculature. The pathophysiology of the exaggerated coagulation activation in these patients is still of uncertain origin, and it probably involves multiple mechanisms, different from those present in sepsis-associated coagulopathy. No specific data are yet available on the global incidence of thrombosis in patients with SARS-CoV-2 infection.4 The case herein reported discusses a patient with massive acute pulmonary thromboembolism in addition to an acute right ventricular thrombus formation, resulting in hemodynamic instability that required systemic fibrinolysis, with successful outcome. Other factors such as systemic infection, prolonged bed rest, and the use of a central venous catheter may have contributed to the development of the event.
There is little good quality data on the incidence of thrombotic events in ICU-COVID-19-patients. An observational study by Klok et al, conducted in Dutch hospitals estimated the incidence of these thrombotic events at around 31%. 5 Asian studies estimate the incidence of coagulopathy at around 19% 6, excluding the information about thrombotic events. However, they claim that these events are less common in Asian populations, either because of genetic factors or because they follow different antithrombotic prophylactic guidelines. 7
In absence of any contraindications, the prophylactic use of LMWH for allhospitalized-COVID-19-patients, including those that are not in the ICU, is widely accepted by the ISTH and the American Hematology Society. There is a strong debate about the therapeutic anticoagulation dose and timing in these patients. Klok et al, recommend using a "high prophylactic dose" in every patient.5
The current guidelines in most hospitals suggest using a prophylactic dose of LMWH in all patients. "High prophylactic doses" (greater than enoxaparin 40mg/24h, between 0,5 and 1mg/kg/h) and therapeutic dose (enoxaparin 1mg/kg/24h), should be reserved for high risk patients, which is consistent with Tang et al, who reported lower mortality after 28 days of hospital admission in a subpopulation of COVID-19 patients and sepsis (40% vs 64.2%; P = 0.02) or six-fold-D-Dimer levels (32.8% vs 52.4%; P = 0.01).8 Furthermore, a recent study at Mount Sinai Hospital by Paranjpe et al concluded that longer anticoagulation therapy in hospitalized COVID-19 patients, was associated with lower mortality (hazard ratio 0,86 per day; 95% CI, 0,82-0,89; P<0.001).9
Neither of these two studies, Tang and Paranjpe, reported any significant anticoagulation-associated events. The incidence did not rise 9 and were described as rare and generally mild.8
It is clear that a proper thrombotic risk stratification is needed in order to determine the optimal anticoagulation regimen. Nowadays, most of the guidelines include SIC score >4 (including platelet count, INR and SOFA scale) and D-Dimer increased levels (three or four fold). 7-9 Other factors considered for thrombotic risk are: age over 65 years old, overweight, underlying chronic disease, cancer, sepsis, personal or family history of venous-thromboembolic disease, high cytokine levels, or special ICU-associated risk factors (sedation, immobilization or venous central catheters). 8-11
Among all of the above-mentioned factors, our patient only fulfilled: immobilization, having a central venous catheter and markedly raised DD levels and cytokines (IL-6).
An interesting discussion could be whether the venous central catheter manipulation in these patients, who are more likely to have a hypercoagulable state, could be related to the genesis, or to the mobilization of the intracardiac thrombi. Our patient suddenly developed this impressive clinical presentation, despite a normal echocardiography just a few hours earlier and being anticoagulated with LWMH: 40mg/12hours, notwithstanding a SIC value < 4.
In our center, all patients admitted to the intensive care unit receive prophylactic doses according to the following protocol - provided a normal renal function - : <80 Kg of bodyweight: enoxaparin 40 mg/day; 80-100 kg: enoxaparin 60mg/day; >100kg: enoxaparin 40mg/12 hours. The therapeutic doses (1 mg/kg every 24 hours) are reserved for patients with risk factors (SIC> 4 or a history of venous thromboembolism).
Serial measurements of DD values, prothrombin time and platelet count are recommended (in descending order), for all COVID-19 patients, considering that as their condition deteriorates, more aggressive therapies are required.12 The DD level has been considered an independent predictor of mortality,3,6 and could be valuable, not only for early detection of patients with high thrombotic risk, but also to predict their outcome.9
We see patients without any risk factors, except for immobilization and high DD levels, that develop life-threatening thromboembolic events. Further studies are needed to correctly identify which patients could benefit from therapeutic anticoagulation in order to prevent thromboembolic events. Moreover, there have reports of patients with ARDS due to COVID-19 who develop more complications, and of higher severity, than the thrombotic complications related to non-COVID-19 ARDS patients.13
We do believe that this case report is a valuable contribution, since it discusses the clinical evolution of the first COVID-19 patient successfully treated with fibrinolysis. This may pave the way for new hypothesis for future research on the need to administer early anticoagulation therapy to critical patients with SARS-Cov-2 infection.
Nonetheless, this is a purely descriptive case and therefore no statistical associations can be made. Prospective, randomized, double blind, studies are needed, in line with the studies conducted by Tang et al and Paranjpe et al, to assess the association between early anticoagulation and mortality.
CONCLUSION
COVID-19 involves a state of hypercoagulability, probably cytokine-mediated, which leads to micro and macrovascular injury. This case highlights the need to pursue further studies for improved thrombotic risk stratification in SARS-Cov-2 infected patients, in order to assess the potential and the benefits of early anticoagulation.
A sudden decline in the respiratory function, with hypoxemia refractory to alveolar recruitment maneuvers in a SARS-CoV-2 infected patients, should always lead us to think about acute pulmonary thromboembolism, as the underlying cause. Echocardiography and Angio-CT should be useful to establish the diagnosis and guide the indication of early systemic fibrinolysis, in the presence of hemodynamic instability.
ETHICAL DISCLOSURES
Protection of human and animal subjects The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of data The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent The authors declare that no patient data appear in this article. The authors have obtained the informed consent of the patient and/or subject referred to in the article. This document work in the power of the correspondence author.











 text in
text in 



