INTRODUCTION
A cardiac implantable electrical device (CIED) is an electronic system that generates intermittent electrical impulses to activate the cardiac tissue and deliver an effective contraction. In addition to the conventional cardiac pacing, some of these devices are capable of identifying ventricular tachyarrhythmias and treat them with defibrillation.
CIED insertion rates increase with age. It is estimated that between 70 % to 80 % of all pacemakers are implanted in patients aged 65 or older.1 Aging of the population worldwide and the longer life expectancy have increased the demographic rate estimates of CIEDs in most countries. 2 The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators was published in 2011, reporting 1.002,664 pacemakers in 2009. The United States had the largest number of implants. 3 Practically every country showed increases in the number of implants from 2005 to 2009. Since the approval of the use of cardiac resynchronization devices by the FDA in 2001, the use of these devices has been constantly on the rise, whether alone or in combination with a defibrillator. The report of the US National ICD Registry indicates that there were 263,284 procedures conducted between 2010 and 2011 4. Currently, the resynchronization devices account for up to 40 % of the devices implanted in the United States. 5
According to the healthcare situation analysis (ASIS) conducted in Colombia in 2016, there are 120 electrophysiology, pacemaker and arrhythmia services in the country. 6 However, there are no Colombian statistics to know the exact number of patients with CIEDs or how many devices are implanted per year. Some data can be gathered from reviews reporting the use of CIEDs, such as the article by Clara I. Saldarriaga et al., which claims that in just 10 months, of the 511 patients admitted to the heart failure program at a cardiology center in Medellín, 29.3 % had implantable cardioverter-defibrillators (ICD). 7 A review by Fernando Rosso et al., includes a 9-year follow-up of cardiovascular devices-associated infection, at a cardiology center in Cali, reporting 281 implantable cardioverter-defibrillators placed. 8
Consequently, the number of patients with CIEDs is estimated to be significant and continues to increase, in addition to the probability for these patients to undergo cardiac or non-cardiac surgery at some point in time. Furthermore, considering that CIEDs are becoming increasingly complex in their operation, the specialist is required to learn the essentials about how these devices work, the indications for implanting them and how the operation of the device may be affected intraoperatively. A sound knowledge of all these variables will result in safer perioperative care.
THE MOST FREQUENT CIEDS IN CLINICAL PRACTICE AND THEIR INDICATIONS
Akey aspect for the preoperative assessment of these patients is establishing whether the patient has a CIED, what type and which was the indication for implantation. An electrical cardiac stimulation device refers to any of the following permanent devices: pacemakers (PM), implantable cardioverter-defibrillator (ICD), or cardiac resynchronization therapy (CRT) device. 9
Pacemaker
Pacemakers are designed to identify and correct bradycardia through cardiac stimulation, generating an electrical impulse to regularly re-establish myocardial contraction. Hence, pacemakers are firstly able to stimulate the tissue. The minimum voltage needed from the impulse generated to elicit myocardial conduction is called the stimulation threshold and is measured in volts (a normal threshold should be less than 1.5v). Secondly, peacemakers have the ability to detect the intrinsic cardiac activity- or the patient's heart activity - depending on the chamber where the lead is placed. In other words, it detects the presence of P waves as an indicator of atrial activity and/ or the presence of R waves as an indicator of ventricular activity (measured in millivolts- a normal P wave is at least 1 mV , while a normal R wave is at least 5 mV -).
Depending on the number of leads, PM may be single chamber (1 lead) localized in the right atrium or the right ventricle, or dual chamber (2 leads) localized in the right atrium and ventricle.
The most frequent indications for permanent PM implantation are 10:
Symptomatic bradycardia caused by a second or third degree atrial-ventricular block UAVB)
Sinus node dysfunction
Chronic atrial fibrillation, when atrioventricular synchrony is not needed.
There are temporary pacemakers with external generators used for cardiac pacing. These may be transcutaneous, when delivering stimulation energy through the chest via patches, or transvenous based on a stimulation lead to pace the heart through a central venous access.
Cardiac Resynchronization Therapy Device
This is a three-chamber device with a lead placed in the right atrium and two right and left ventricular leads. The atrial lead recognizes the atrial depolarization and then elicits a biventricular stimulation, two ventricular leads optimize the synchronization during contraction, which does not happen when there is only right ventricular pacing. Hence this device is called cardiac resynchronization therapy (CRT). 10 CRT devices have other roles in addition to resynchronization, such as a defibrillator function for the treatment of malignant ventricular arrhythmias (CRT-D or cardiac resynchronization device), or the role of a pacemaker (CRT-P) when the patient requires pacing. 11 CRT devices are indicated for patients with chronic heart failure that meet the following requirements 12:
Implantable Cardioverter-defibrillator (ICD)
This device is able to monitor and analyze the heart's electrical activity and hence treat malignant ventricular tachyarrhythmias in two ways: with anti-tachycardia stimulation or with shock therapy to defibrillate through a coil placed in the right ventricle (RV) lead and occasionally in the superior vena cava. Anti-tachycardia therapy is usually delivered with lower frequency arrhythmias (ventricular tachycardia) and uses less energy. This decreases battery depletion and is less painful, hence better tolerated by patients. Moreover, these devices have a pacemaker function which may be needed either because of the patient's baseline rhythm or to protect him/her in case of bradycardia or asystole following an electrical discharge. 11,13 The most frequent indications for implanting an ICD are 12:
WHAT SHOULD WE KNOW ABOUT CIEDS?
When speaking about CIEDs we must keep in mind that the system comprises a generator and 1, 2 or 3 leads. The generator has one battery and electric circuit and is typically implanted in the chest area (right or left). The generator is responsible for eliciting electrical impulses that depolarize the heart (Image 1). The leads are inserted via percutaneous puncture and then advance towards the heart chambers -right atrium (RA), right ventricle (RV), left ventricle (LV) - through the cephalic, subclavian or axillary vein. When one of the leads needs to be placed in the left ventricle, access is through the coronary sinus. In patients with congenital heart disease, the leads are implanted through the pericardium. 14
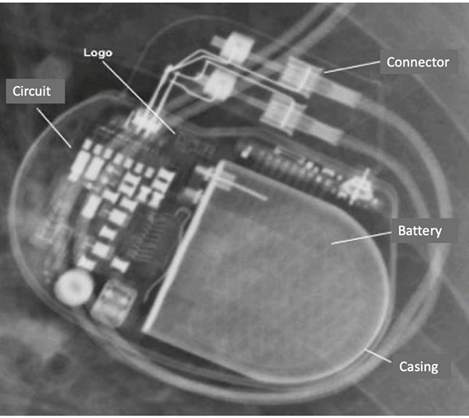
SOURCE: Authors.
Image 1 The impulses generator has a titanium casing housing a lithium battery and an electronic circuit. The manufacturer's logo is visible and the connecting ports for the leads.
With regards to children, the indications for a CIED and the preoperative assessment are similar to the adult's. The placement of the generator in younger children may be in the abdomen and the vascular access for the insertion of the leads should take into consideration the diameter of the leads to be implanted.
Modern devices can detect whether the heart has intrinsic or independent activity (sensing function). If the CIED does not detect cardiac activity or depolarization, i.e., does not detect QRS complex or P wave, an electrical impulse is elicited to generate myocardial depolarization (capture or stimulus function).
The ECG tracing will reflect such capture as a wide QRS complex, predominantly negative and preceded by a pacing spike. The same happens with atrial stimulation where the spike precedes the P wave.
Due to the complexity of the devices and their programming, there is an internationally accepted standard nomenclature that facilitates our understanding about how the CIED is programmed. 15 A 5-letter code indicates the programming mode: The letter in position I indicates the paced chamber (A: Atrium, V: Ventricle, D: Dual). Position II indicates the sensed chamber (A, V, D, O: None). The letter in position III indicates the device's response versus what was sensed (I: Inhibits, T: Trigger, D: Dual, O: None). Position IV indicates the presence or absence of frequency modulation; this function is available in devices with the ability to increase the heart rate, depending on the physiological needs of the patient. The letter in position V indicates the ability for multisite pacing, which involves more than one lead in the same cardiac chamber, or that the device stimulates the right and left atrial or ventricular chambers, as is the case with the CRT that provides biventricular pacing. When positions II and III (sensed chamber and response to what was sensed) are in "O" mode, it is called asynchronous mode. A CIED in asynchronous mode (AOO, VOO, DOO) is generated when the electrophysiologist reprograms the device in this mode or when a magnet is used. In both cases, the pacing of the chamber in position I will be elicited, regardless of whether there is any intrinsic activity in the patient.
In the emergent perioperative context, what is most important is to know how to interpret and understand the first 3 positions (Table 1).
Table 1 CIEDs nomenclature.
| I | II | III | IV | V |
|---|---|---|---|---|
| Chamber paced | Chamber sensed | Response to sensing | Frequency modulation | Multisite pacing |
| A: atrium | O:none | O:none | O:none | O:none |
| V: ventricle | A: atrium | T: triggered | R: Frequency modulation | A: atrium |
| D: dual (A+V) | V: ventricle | I: inhibited | V: ventricle | |
| D: dual (A+V) | D: dual (T+I) | D: dual (A+V) |
SOURCE. Adapted from Tracy, et al. 12.
Image 2 illustrates different EKC tracings and the nomenclature for the programming mode of each device.
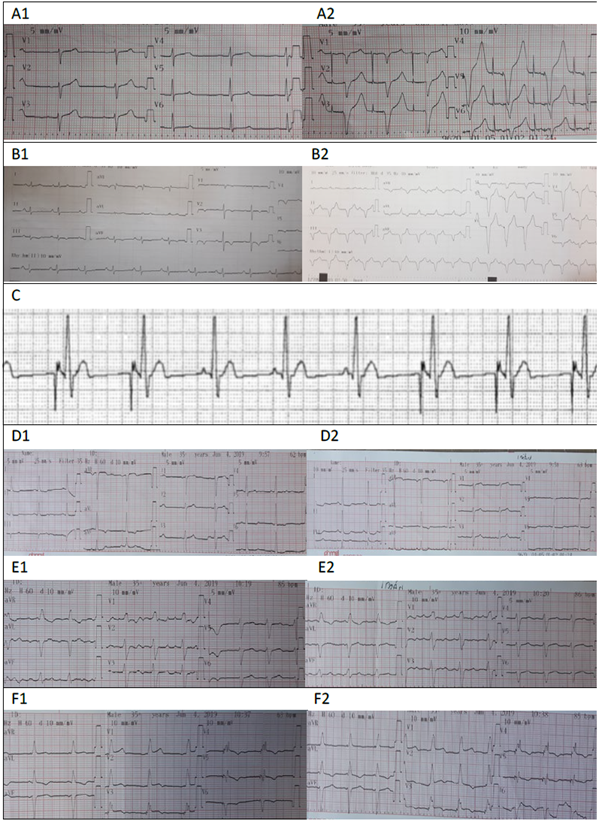
A1: Sinuous rhythm with intrinsic patient activity, A2: EKG with magnet: atrial and ventricular pacing spike is observed. These findings correspond to a dual chamber PM programmed in DDD mode: Paced chambers (A+V= Dual), Sensed chambers (A+V=Dual), PM response versus the sensing information (Dual = Trigger + Inhibits). The PM senses intrinsic atrial and ventricular activity and inhibits from delivering an impulse; when the magnet is applied, the trigger or pacing response is elicited, both in the atrium and the ventricle. B1: Patient’s own sinuous rhythm. B2: EKG with magnet evidencing ventricular pacing at a rate of 100 beats/minute. This suggests the presence of a single chamber pacemaker with VVI programming. : The atrial pacing spike and the P waves typical of the patient can be seen. The PM inhibits when sensing intrinsic atrial activity. This suggests the presence of a single chamber pacemaker programmed in AAI mode. D: EKG of a patient with a CIED. The patient’s intrinsic sinuous rhythm is evidenced in D1. D2: EKG with magnet, showing no changes in the QRS morphology or in the heart rate. E: EKG of a patient with a CIED, E1: tracing without magnet and E2 tracing with magnet; no changes are visible. F: EKG of a patient with a CRT-P; F1: shows the device pacing at 63 beats per minute. F2: shows response to the presence of a magnet, when increasing the heart rate to 85 BPM. SOURCE: Authors.
Image 2 Tracings.
IT IS IMPORTANT TO KNOW WHAT TYPE OF LEADS DOES THE PATIENT HAVE
Leads are made from an alloy of one or several conductor metals covered with an insulating silicon or polyurethane sheath; the tips are specialized to optimize the capture and sensing functions and come in different designs to allow for anchorage in the heart. 16
In the traditional insertion mode, the leads are aimed through the vein towards the endocardium. Depending on the tissue fixation mode, the leads may be fixed passively or actively 17, (Image 3A).
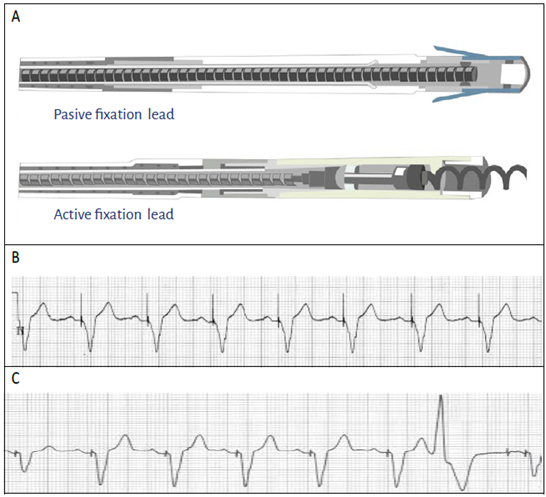
SOURCE: Authors.
Image 3 A. The passive fixation lead can be identified because of the cone-shaped structure of the tip, and the active fixation lead has a screw tip. B. EKG showing a ventricular pacing spike with unipolar configuration. Notice that the spike is wide or high-voltage. C. EKG showing a ventricular pacing spike with bipolar configuration. Notice the narrower spike or lower voltage.
Based on the pacing mode configuration, the leads may be unipolar or bipolar.
Unipolar lead. In the unipolar configuration the stimuli travel between the proximal end of the generator (the anode) and the tip of the lead where the cathode is located. This configuration is identified in the surface EKG as a wide stimulation spike or higher voltage (Image 3B).
Bipolar lead. In the bipolar configuration the stimulus is generated between the tip of the lead (cathode) and the anode, located at 1 or 3 centimeters from the tip. This type of leads are currently the most common and can be configured to pace and sense both in monopolar as in bipolar mode. In a surface EKG, the bipolar configuration is identified as a low voltage pacing spike, and occasionally it is difficult to identify 18, (Image 3C).
There are epicardial localization leads, that may be preferred in patients with congenital heart disease and marked anatomical differences, with persistence of intracardial shunts and with potential thromboembolism risk due to the intravascular device. The epicardial implant requires a thoracotomy. 19
There are transitory stimulation leads which are bipolar and in most cases passive fixation. Such leads go through conventional venous introducers and are connected to external generators of transitory pacemakers.
WHAT IS THE ROLE OF CHEST X-RAYS IN THE PATIENT WITH CIED?
Traditionally we consider that the EKG is the most important test to obtain CIED information; however, the chest X-ray is equally important for the anesthesiologist because it helps in identifying the type of CIED, its chest location in the patient, the number, type, localization and integrity of the leads. It also allows for the identification of any potential complications associated with the device implant. 20
Image 4. shows the different CIEDs and characteristics in a chest X-ray.
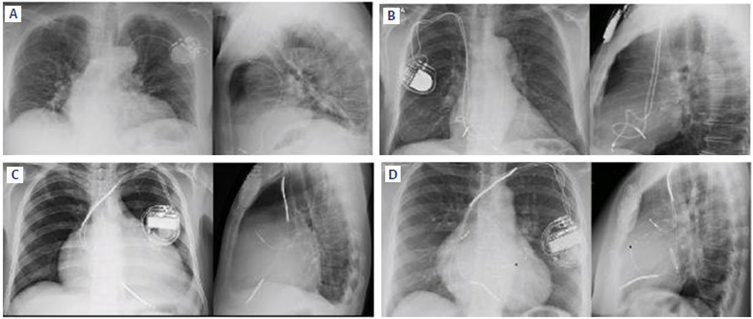
A: Unicameral pacemaker with a single lead RV localization. B:Bicameral PM with leads in RA and RV.C: ICD with leads in RA and RV. Notice two radio-opaque thickened areas along the right ventricular lead, corresponding to the high voltage shock coils. D: CRT, tricameral device with leads in RA, RV, and LV. The shock coil of the right ventricular coil delivers the defibrillation ability of the device (CRT-D, also known as cardio-resynchronization device). Notice an annular radio-opaque image corresponding to a stent structure in the mitral position. A non-functional lead from an old device that could not be removed is marked with a black star. Source: Authors.
Image 4 Different CIEDs and characteristics in a chest X-ray.
HOW DOES THE MAGNET AFFECT THE OPERATION OF A CIED?
The decision to place a magnet over the CIED depends on several considerations: the type of device (whether it is a pacemaker or an ICD), how is it programmed, the patient's baseline rhythm and the likelihood of intraoperative electromagnetic interference (EMI). 13 Currently, the use of the magnet is only justified when it is not possible to do a formal CIED assessment by the electrophysiology service and when the patient depends on the device and will undergo emergent surgery. 21 It is also acceptable to use the magnet when a quick sensing assessment and pacemaker capture is needed.
The EKG is a required test because it allows to determine whether the patient with a CIED is under intrinsic rhythm or is being partially or totally paced by the device. However, in order to get complete information about the type of device and how it is programmed, there is a need to see the CIED's response to a magnet. Such response may be variable and depends on the brand, type of device and condition of the battery. Once the magnet is placed on the chest and just on top of the device, the following reactions may be expected 22,13:
The PM configuration changes to asynchronous mode: AOO, VOO o DOO. In practical terms, when positioning the magnet, the sensing function of the CIED in inhibited (like blindfolding the pacemaker) and subsequently the device generates stimuli at a fixed rate (usually between 85 and 100 beats per minute), either transiently or during the time the magnet is in place. Keep in mind that such response varies depending on the brand of the device and the condition of the battery, as explained hereunder.
- Medtronic: When the magnet is placed, the device becomes asynchronous at 85 beats per minute (BPM). If the device is in ERI, the response to the magnet will notbe 85but65BMR
- St. Jude: When the magnet is placed, the device becomes asynchronous at 100 BPM. If it is in ERI, the response will be 85 BPM.
- Boston Scientific: the same as the above.
- Biotronik: When pacing the magnet, the device will become asynchronous at 90 BPM. Ifit is in ERI, the response will be 80 BPM. ERI: elective replacement indicator, which means that the battery is low and the recommendation is to change the device in the next 1 to 3 months.
In the case of ICD and CRT-D there is no conversion to asynchronous mode, but the anti-tachycardia pacing is inhibited. Defibrillators with a pacemaker function, this function shall not be affected by the magnet placement. 23
In a CRT-P there is temporary conversion to asynchronous, according to the brand of the device.
It should be kept in mind that improper use of the magnet may involve risks and complications. In an ICD it may inhibit the anti-tachycardia pacing, and also generate inappropriate bursts. A pacemaker configuration may be altered or even damaged, with life-threatening consequences for the patient. 24
WHAT DOES IT HAPPEN WITH ELECTROMAGNETIC INTERFERENCE (EMI)?
The anesthesiologist is required to understand any situations that may result in problems with the CIEDs, and how to reduce the risks. Anything that emits waves with a radiofrequency between 0 and 109 Hz may generate EMI and interfere with the proper operation of the device. 23
Electrical interference refers to the CIED sensing external signals as intrinsic cardiac signals or patient signals and may generate inappropriate bursts, pacing inhibition or resetting back to the factory mode (VVI, VOO). 25 For example, in a dependent patient, if the pacemaker senses EMI in one of its leads, it may interpret that as intrinsic cardiac events, discontinue pacing and result in bradycardia or asystole. In an ICD, the EMI may be sensed as tachyarrhythmia and in turn the device generates rapid ventricular pacing (anti-tachycardia therapy). 26
Some of the factors generating intraoperative EMI include: the use of electrocautery, peripheral nerve stimulator, evoked potential monitors, tremors, fasciculations, procedures such as extracorporeal lithotripsy, MRI imaging, electroconvulsive therapy, radiofrequency ablation and a high tidal flow in the ventilator.
The monopolar electrocautery is one of the main sources of EMI, delivering an electric current of 100 kHz to 4 MHz for cutting or clotting biological tissue. The risk of EMI with this equipment depends on the surgical site and the location of the dispersive pad; the ideal is that neither the current path or the electric current of the electrosurgical unit, passes through or near the generator and the device leads. 9 Hence, the higher risk surgeries of EMI are: cardiac, chest, head and neck, shoulder, abdomen, and to lesser degree, pelvis. With regards to infra-umbilical surgeries (for instance, surgeries of the pelvis and lower extremities) the possibility of interference will be much lower. The larger the distance between the CIED and the surgical site and the dispersive pad, the lower the risk of EMI.
In case of supraumbilical surgery the recommendation is that PM-dependent patients be reprogrammed to asynchronous mode. Additionally, that the device of the ICD patient undergoing electrosurgery (both supra and infra-umbilical) be reprogrammed to avoid that the EMI inappropriately generates anti-tachycardia therapy. 9
At this point it is important to recall that the modern CIEDs are less vulnerable to EMI, since the vast majority have bipolar leads.
KEY PERIOPERATIVE ASPECTS
It would be ideal for patients with CIED to be adequately assessed preoperatively by the electrophysiology service. The key element in that assessment is the programmer, which is a computer connected to the CIED via radiofrequency that collects information, evaluates and makes changes in programming. Each device brand has a specific programmer.
This assessment enables the anesthesiologist to fully and reliably identify the implanted device, know the indications for the implantation, determine the condition of the leads and of the battery, reprogram the asynchronous pacemaker function when there is a high probability of EMI and discontinue the anti-tachycardia therapy of the ICD when considered necessary. 27
The literature is unclear in defining the ideal time interval to assess the CIEDs. However, most experts suggest it should not be more than 3 to 6 months. 9 If the device needs to be reprogrammed to the asynchronous mode before surgery, this should be done as close as possible to the time of the procedure.
During the intraoperative period, the anesthesiologist should keep in mind three pillars: patient monitoring and CIED operation, prevention of potential events that cause malfunction of the device, and be knowledgeable to perform cardioversion, defibrillation and provide heart rate support in an emergent situation. 24
Monitoring. In addition to basic ASA monitoring, continuous cardioscope monitoring and deactivating the artifacts filter in order to clearly identify the stimuli or bursts delivered by the device. Continuous monitoring of peripheral pulse is required, so that as much as possible, an arterial line be available to allow for closer and accurate pulse and rapid identification of cardiac problems. 28
A temporary pacemaker should always be available for patients with a pacemaker. The recommendation is to have transcutaneous pacemaker patches that have the added advantage of being immediately available and easy to use. The limitation is that the capture threshold is usually high and erratic; so in case the need for temporary pacing persists, a central vascular access should be promptly secured to place a temporary transvenous pacemaker. Patients with ICD in whom the anti-tachycardia therapy has been deactivated should have a defibrillation equipment available immediately. The adhesive external defibrillation plates should be used whenever possible (some surgeries such as thoracic surgeries may limit its preventive perioperative use). The recommendation is to do it with an anteroposterior approach to avoid having direct contact with the CIED and minimize the current flowing through the generator and leads. 9
Reduce the sources of EMI. The risk of EMI is higher in supraumbilical surgeries using the monopolar electrocautery. The recommendation is to use the ultrasonic scalpel (harmonic) or bipolar cautery; if only the bipolar is available, the pulses should be short and intermittent (duration < 5 seconds, interval between bursts of 5 seconds) using the least possible energy. 28 An electric scalpel and a dispersive pad should be available so that the current path and the electrical field do not pass through or close to the generator or CIED leads, and avoid moving the activated lead over the generator. These measures lower the risk of EMI.9
Based on the above considerations, the authors suggest the following assessment approach for the patient with a CIED, who is programmed for emergency surgery:
During the physical examination and questioning - Check the side of implantation and the size of the device. Most ICDs, CRT-D, CRT-P are implanted in the left infraclavicular region and are larger than a PM. Ask how often the device is checked. PM are usually checked every 12 months, and the other devices every 6 months. Ask the patient about the condition of the battery. Ask for the device ID card to identify the type, indication and programming mode.
Ask about the symptoms that prompted the indication for the implant. In the presence of symptomatic bradycardia, need for intravenous PM, recurrent syncope or AV node ablation, consider that the patient is PM-dependent.
Get a PA and lateral chest X-ray. In order to determine the type of device, position and integrity of the leads, and define any limitations to vascular access.
Take a 12-leads EKG, with and without magnet. In order to establish the device type, get some guidance about the programming mode, try to define how dependent the patient is, and quickly assess the sensing function, capture and battery condition. If the EKG with no magnet shows pacing spikes most of the time during the tracing, this is another reason to consider that the patient is device-dependent.
Basic ASA monitoring. Whenever the cardioscope continuous monitoring has 5 leads, add an arterial line and defibrillation paddles.
Assess the risk of EMI and the limit the sources or origin.
Define the need to use the magnet over the device during the surgical procedure. Keep in mind the algorithms designed by the authors (Figure 1).
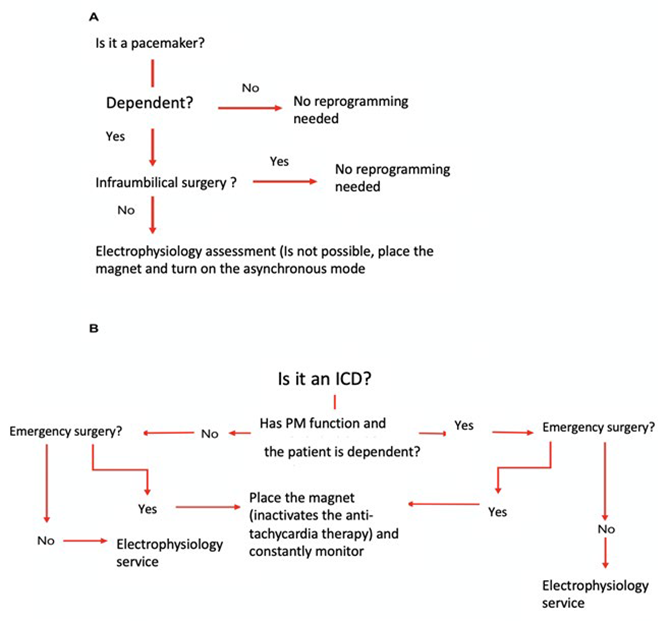
A: ln a pacemaker-dependent patient with a high risk of EMI, the asynchronous mode should be programmed to constantly pace, regardless of sensing. In emergency surgery this may be achieved with the magnet. B: In a patient with ICD and surgery with high risk of EMI, discontinue the anti-tachycardia therapy to avoid sensing interference that may result in inappropriate bursts. In elective surgery, the electrophysiology service should be in charge of deactivating the anti-tachycardia therapy (this may even be required in infraumbilical surgery), since in obese patients and with some devices, the response to the magnet may fail or be inactive. Only in case of emergency surgery use the magnet to discontinue anti-tachycardia therapy. SOURCE: Authors.
Figure 1 Algorithms for the assessment approach for the patient with a CIED, who is programmed for emergency surgery.
Once the surgery is over - Have the electrophysiology service do a postoperative assessment to survey the device and restore the normal function required by the patient. This assessment is recommended even when a preoperative assessment could not be conducted, since significant EMI or the use of the magnet may disrupt the normal CIED functioning.
CONCLUSION
Any patient with a CIED during surgery is at risk of device-associated adverse outcomes (unable to pace, unable to elicit bursts, programming changes, and inappropriate cardio-defibrillator therapies) in addition to adverse clinical outcomes (hypotension, bradyarrythmias, tachyarrhythmias, or any type of myocardial injury). 9,29,30 Although the frequency of these adverse outcomes is not clearly established in the literature, the risk of occurrence demands a comprehensive and multidisciplinary assessment of the patient before surgery. Emergent situations may limit a proper assessment. The surgical team, under the leadership of the anesthesiologist, should have a practical and organized knowledge, not only of the devices, but also of the comprehensive management in an emergency situation. This allows for an adequate approach to the type of device, close monitoring and surveillance during the intraoperative period, and the implementation of strategies to reduce the risk of electromagnetic interference that may disrupt the CIED operation, so as to avoid a life-threatening situation for the patient.
ETHICAL DISCLOSURES
This article is the result of a non-systematic literature review and, pursuant to the Nuremberg Code, it does not jeopardize the health of any patient. From its inception, and throughout its development, all the required ethical aspects were considered to deliver a scientific publication that is socially, scientifically and clinically relevant, and with minimum biases, considering that the data reported are based on scientific evidence and publications and are endorsed by the social responsibility of the authors.
Contribution by the authors
FAS: Design and planning of the article, collection of bibliography, procurement of the images in the article, development of the figures, drafting of the manuscript.
LMJ: Article planning, selection of bibliography, development of the figures, drafting of the manuscript.
JFA and JAC: Article planning, interpretation of images and charts, drafting and final approval of the manuscript.











 text in
text in 



