BACKGROUND
Silicon injections of unknown purity have been around since the 60's. 1 They were initially considered for medical use, but unfortunately their use has generated a growing negative impact on healthcare. 2,3 In 2017, The US Food and Drug Administration issued a warning to both patients and physicians against the use of injectable silicone for body contouring procedures. 4 Notwithstanding this caution, liquid silicone or biopolymers, are widely used both in females and males for buttocks and lips augmentation, or to correct facial furrows and wrinkles. 3,4
The use of smaller amounts for body contouring has been observed in women, in contrast to extreme cases of transgender males with volumes between 4 to 15 liters in an attempt to feminize their bodies.3 The incidence varies from country to country, though it is underestimated because these procedures are usually performed in an illicit fashion, leading to under-registration. There is also a close association with tourism for cosmetic procedures. 2,3 This practice, and its growing complications have been considered an emerging epidemic. 2
The gradual increase of a wide variety of injectable substances in different areas of the body has shown multiple potential complications. Some of the most well-known complications include migration, inflammation, edema, development of granulomas and infections. Severe health problems have been documented in some cases such as pulmonary embolism, renal failure, tissue necrosis, and even fatalities. 1,5,6
Its wide use in cosmetic procedures is due to its low cost, durability and thermal stability. Contrary to what was expected, and despite the fact that injected solutions are chemically inert, they give rise to different levels of local inflammatory reactions and necrosis. 3,7 Recently, they have been identified as a cause for systemic response associated with rheumatological involvement such as the autoimmune/ inflammatory syndrome induced by adjuvants (ASIA). 8 The persistence of the material and the negative health impact, developing sequelae even 25 or 30 years after their injection 2, has generated the need for multiple continuous treatments, including surgical resection as an essential part of timely management for control purposes. 8
THE CURRENT SITUATION
The analysis of patients undergoing biopolymer resection procedures has shown migration - even cephalic migration into the lumbar region (Image 1). The literature describes how unpredictable this migration is 6, considering the reports of some cases of migration to very distant areas from the initial injection site, such as the retroperitoneal space 9, the back of the thighs 10,11, the popliteal fascia, the perineum and the genital area 12, inter alia. The intraoperative findings include inflammatory tissue, fibrosis and multiple clusters of encapsulated silicone that in most cases still have fluid contents, despite the fact that the injections were administered years and even decades before.
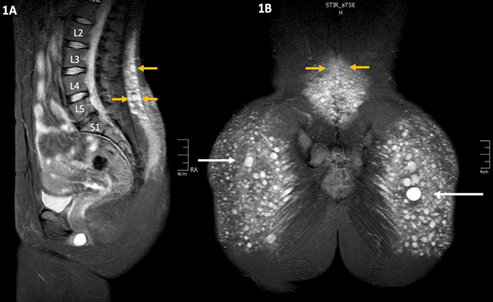
SOURCE: Authors.
IMAGE 1 MRI showing the migration of liquid silicone (white arrows) into the lumbar region, with multiple lesions or varying sizes (yellow arrows) and local inflammatory reaction.
Magnetic resonance image (MRI) is the most frequently used imaging study for diagnosis because of its ability to identify the lesions, even when it is small particles that have migrated into the lumbar region. Unfortunately, it is usually not possible during the physical examination to properly palpate the clusters of encapsulated silicone (siliconomas). When assessing the tissue with ultrasound (Image 2), the image obtained only allows for the identification of some of the lesions, mostly the larger ones. Unfortunately, ultrasound does not deliver a clear and reliable assessment of the amount of migration and liquid silicone present, which is a limitation of this diagnostic tool for immediate bedside assessment.
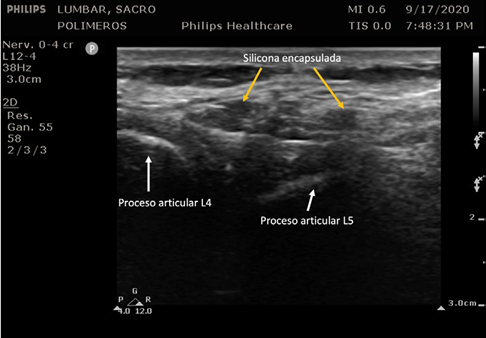
SOURCE: Authors.
IMAGE 2 Ultrasonographic assessment of the lumbar region, with evidence of a few liquid silicon capsules (siliconomas).
The potential risk of perforation of the siliconomas that migrated and remain in the areas of intervention of anesthetic procedures (caudal, epidural, spinal), give rise to a potential displacement of different substances injected and contamination of the spine and the nervous system. Since these substances are clearly associated with inflammation, edema and necrosis, the motor and sensory nerves are exposed to a high risk of direct injury from toxicity and inflammatory response. The anesthesia team should then be aware of this potential issue when approaching the neural axis.
Considering the well acknowledged multiple benefits of neuraxial anesthesia, this procedure may be safely used in patients with a history of liquid silicone injections or biopolymers, having and MRI available to rule out any migration or local complications. Due to the potential toxicity and keeping in mind the safety of the patient during neuraxial procedures, these procedures should be avoided in patients with a history of polymer injection and the identification of migration into the lumbar region.
In conclusion, the indiscriminate use of liquid silicone or biopolymers for cosmetic procedures, the denial of the patient of its use when taking his/her medical history, or the lack of awareness about the lesions caused by such injections, make it necessary to create awareness among patients and healthcare practitioners about such potential risks. Being cognizant of the complications associated with the migration and dissemination of these potentially toxic substances for the neural axis, there is a need to revisit the considerations prior to the administration of neuraxial anesthesia, based on the potential edema, inflammation or necrosis that these substances may elicit in the human body.
In patients with a history of silicon or polymer injection, diagnostic imaging studies such as MRI may contribute with valuable information about the condition of the anatomical structures to complement the puncture site assessment before administering epidural, spinal or even caudal anesthesia.
ACKNOWLEDGEMENTS
Contribution by the authors
WAZ: Original design of the article, structural planning, data collection, image interpretation and final drafting of the manuscript.
VMM: Planning of article structure, data collection, initial drafting of the manuscript.
ISG and JAJ: Collection of images, drafting and final approval of the manuscript.











 text in
text in 



