INTRODUCTION
The definition of adult in-hospital cardiac arrest (IHCA) is the loss of circulation in inpatients. 1 This physiological collapse is a relatively frequent occurrence, with informed incidence of up to 1.6 cases per 1,000 patients admitted, in developed countries 2, and a higher frequency in intensive care units (ICU). Notwithstanding the efforts to optimize the management of IHCA, these events exhibit high mortality rates. 3 In the United States, for instance, approximately 200,000 cases of IHCA are reported. Furthermore, a systematic review in 2018, including more than one million events and 39 studies between 1992 and 2016, found an overall survival rate of 13% at one year. 4,5 Moreover, there is a significant decline in quality of life, functional dependency and increased healthcare costs from rehabilitation and the management of sequelae from the event. 6-8 Consequently, adequate prevention and identification, as well as the updating and adoption of institutional protocols for a successful resuscitation, are all key factors for the survival and long-term prognosis of these patients. 9
Following timely and high quality cardiopulmonary resuscitation (CPR), it is sometimes possible to achieve a return of spontaneous circulation (ROSC) leading to the post-cardiac arrest syndrome (PCAS), as defined back in 2008 by the International Liaison Committee on Resuscitation (ILCOR). This syndrome corresponds to a series of pathophysiological events, including brain injury, myocardial dysfunction, a systemic response to molecular dysfunction secondary to ischemia/reperfusion, and the functional compromise of the comorbidity caused by the cardiac arrest. 10,11
The Utstein guidelines have facilitated the publication and search of statistical data on adult cardiac arrest worldwide. 12 However, a review was conducted in Colombia searching for available data or records in Colombia, which failed to produce any results. 13 Notwithstanding the above, those guidelines are extremely important for studying the etiology and epidemiology of adult cardiac arrest, because of its direct implications, both in terms of the optimal management of the patient with IHCA and in terms of defining survival and neurologic prognosis. However, there is still the hurdle of a consensus to publish epidemiological data which has led to the emergence of new classification systems that have both advantages and disadvantages with respect to the Utstein approach. 1 Now then, since the development of this concept, the significant progress in technology and diagnostic methods, in addition to a better understanding of the different intra- and inter-cellular messengers, have rekindled the academic interest in the area, as a determining factor in cardiac arrest-associated morbidity and mortality; this is reflected in the growing number of related studies published in PubMed, which increased from 5 in 2008 to 31 in 2017. These studies open the door to new therapies such as those involving the relationship between IL-6 and TNF a and targeted vasopressor therapy; endothelial damage and endothelial activation markers have shown the potential to be markers of the intensity of the process. 14
The deleterious effects in the evolution of PCAS are the result of a non-resolved underlying pathology and of the ischemia/ reperfusion injury which translates into brain tissue damage, myocardial dysfunction and liver, kidney and other organs involvement. In 2010, The American Heart Association (AHA) added a fifth link to the survival chain, so that once the spontaneous cardiac circulation recovers, all efforts should be focused on preventing the disastrous consequences of reperfusion, using strategies such as adequate oxygenation/ventilation, a systolic blood pressure > 90 mm Hg, and therapeutic hypothermia to ensure a reduction in metabolic oxygen and to prevent the injury resulting from the products of acidosis (myocardial and cerebral depressors). 15
Within the framework of the above-mentioned difficulties, this narrative review is intended to describe the epidemiological, pathophysiological and diagnostic and management aspects of this syndrome, based on the current evidence and the latest information. Hence, a bibliography search was conducted through a direct digital review of the scientific literature in the following databases: MedLine (accesses via PubMed), SciELO, Embase and Cochrane. The MESH English terms used were: "postcardiac arrest syndrome", "postcardiac arrest care" "postcardiac arrest management", "post cardiac arrest management guidelines"; the Spanish keywords used were: "síndrome postparada cardiaca", "síndrome postparo cardiaco".
Based on the development of search formulae, combining the above-mentioned terms, the scientific English literature published between 2014 and 2020 was reviewed. For Spanish publications, the search was expanded from 2008, so as to include regional literature; however, only management protocols were found but no Latin American epidemiological data could be identified. The initial search found 248 articles; any grey literature articles, duplicate articles, unpublished accepted reviews, documents with no abstract or with insufficient information and opinion articles were excluded. Finally, among the categories of original articles, systematic reviews, meta-analysis, and clinical practice guidelines, a total of 56 documents were reviewed.
INPATIENT CARDIAC ARREST
Overall, the in-hospital cardiac arrest etiologies may be divided into cardiac and non-cardiac, representing around 56 % and 44 %, respectively. Table 1 lists the most frequent causes of in-hospital cardiac arrest in order of importance. With regards to the non-cardiac causes, acute respiratory failure (ARF) was described as the most relevant, with a variable proportion between 11.6 %-38 % of the cases. 1,3,16 However, over the last few months, this relationship tended to be reversed, as a result of the SARS-CoV-2/COVID-19 pandemic, which is a cause of increased mortality around the world. 17 Likewise, the significance of AIHCA secondary to septic shock should not be overlooked, given the increased incidence of severe infections accounting for up to 27% of the adult hospitalized population. 11,18,19.
TABLE 1 Etiologies of adult cardiac arrest.
| Cardiac causes | Non-cardiac causes | Others - múltiple or unknown causes |
|---|---|---|
| Acute coronary syndrome | Respiratory failure | 2 probable causes |
| Arrythmia-induced cardiomyopathy | Toxicological | Not studied |
| Right heart failure | Upper airway obstruction | Other identified cause |
| Left heart failure | Metabolic disorders | 3 probable causes |
| Structural cardiopathy | Distributive shock | 4 or more probable causes |
| Congenital arrhythmia | Non-traumatic exsanguination | Extensive and inconclusive study |
| Trauma | ||
| Neurologic catastrophe |
SQURCE: Adapted from Chen et al. 1.
The distribution by services is variable; the 2013 GWTG-Resuscitation registry reported that 80% of IHCAs in the United States occur in hospitalization wards and in the ICU, while the remaining 20% occur in surgical patients. 11. According to the data published by the United Kingdom NCAA for 2011-2013, they have a rate of 1.6 per every 1,000, and specific rates of 1.516.28 per every 1,000 patients admitted to US and European hospitals. 3,20-22 The probable cause of this clear fluctuation among the institutional rates should be the lack of a consensus when publishing data and the proportion of ICU beds, coronary care unit (CCU) and Catheterization Laboratories (cath-lab) versus the general hospitalization wards. 20
The statistical analysis of epidemiological studies indicates that cardiac arrest is slightly more frequent in males, with 58.3 %-66.2 % of all IHCA cases. 1,3,10 Its incidence is proportional to the age of the patient, with a peak between 52 and 77 years. 20,21 Likewise, there is a lineal and inversely proportional relationship between age and in-hospital survival of patients experiencing an IHCA, although no differences have been described among the age groups and the neurologic prognosis following ROSC. 3,21
With regards to the cardiac arrest rhythm identified at the start of resuscitation, in contrast with out-of-hospital cardiac arrest, the non-shockable rhythms are more frequent among inpatients; in other words, pulseless electrical activity (PEA) and asystole which are found in 62 %-82.6 % of the cases 1,20. This may be due to the difference between a witnessed cardiac arrest and a cardiac arrest accidentally identified during rounds of the nursing staff, since the patient could have collapsed hours before being detected. Therefore, some studies have shown a correlation between the cardiac arrest detection peak during the day and the first morning rounds. 21 In contrast, cardiac rhythms susceptible to defibrillation, pulseless ventricular tachycardia (pVT) and ventricular fibrillation (VF) are not only more frequent in males, but they are also associated with better outcomes and survival at hospital discharge. 3,12,21
HISTORICAL APPROACH TO THE CONCEPT OF POST-CARDIAC ARREST SYNDROME
Although the concept of cardiac arrest can be traced back to the literature around 17001800 and a number of guidelines have been written for reestablishing spontaneous circulation under this condition, it was only recently that a concept associated with the morbidity and mortality of patients who succeed in recovering spontaneous circulation was developed; this is the post-cardiac arrest syndrome. 22
This condition was initially described in 1972 by doctor Vladimir Negovsky in his article "The second step in resuscitation - the treatment of the post-resuscitation disease", published for the Academy of Medical Sciences of the USSR. Based on clinical outcomes studies and experimental physiology in animal studies, Negovsky was able to establish that following the recovery of spontaneous circulation using resuscitation strategies, a new nosological entity developed from total body ischemia, which compromised homeostasis and metabolism of different systems, triggered a constellation of pathological processes and Negovsky named it post-resuscitation disease. 23
Clinical studies were continued showing a high mortality; patients died, notwithstanding the recovery of spontaneous circulation. Several experimental studies showed a common pathophysiology involving target organ failure after successful resuscitation. However, it was not until 2008 that the International Liaison Committee on Resuscitation (ILCOR) redefined this new entity as post-cardiac arrest syndrome. 11
POST-CARDIAC ARREST SYNDROME
Definition
The post-cardiac arrest syndrome is defined as the group of systemic physiological responses developing as a result of ROSC. It is a complex combination of immune physiological and hematological alterations 1, including consequences such as post-cardiac arrest brain damage, post-cardiac arrest myocardial dysfunction, and the systemic response to the ischemia/ reperfusion process. 10 This situation is often further complicated by a fourth component: the persistence of the underlying pathological process that initially caused the cardiac arrest. 24
This syndrome not only involves the prolonged hypoperfusion experienced by the patient during the event, but also additional injuries caused during and after the reperfusion maneuvers, the patient's comorbidities, and the organ dysfunction that resulted in the cardiac arrest. 25
Pathophysiology
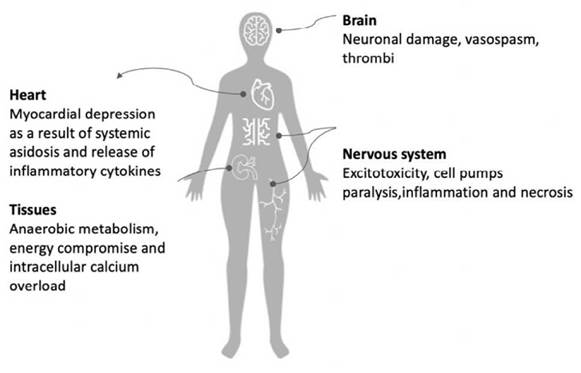
During cardiac arrest, hypoxia and generalized acidosis develop. 26 The extent of cellular dysfunction, injury and necrosis is affected by the magnitude and the duration of the ischemia, and by the particular tissue susceptibility. Moreover, although reperfusion is necessary to reestablish the oxygen and nutrient delivery to support cellular metabolism and reverse the condition, there may be pathophysiological responses that favor the expansion of an already established lesion caused by ischemia. This brings about the release of inflammatory mediators and free radicals into the blood flow, originating from the revascularized tissues and which then reach distant organs and translates into systemic lesions. 21 (Figure 1)
Cellular response
The prevalence of anaerobic metabolism during ischemia causes a reduction in intracellular pH. To dampen this accumulation of hydrogen ions, an excretion of these ions takes place via the inflow of Na+. With anaerobic metabolism there is less ATP availability, which leads to ATPase activation, reducing Ca++ outflow and limiting its reuptake by the endoplasmic reticulum, resulting in intracellular calcium overload. The above process has been suggested as a mechanism to encourage the opening of the mitochondrial permeability transition pore (MPT), which dissipates the mitochondrial membrane potential and further compromises ATP production. 27-29 In the heart, these cellular changes are accompanied by the activation of intracellular proteases (for example, calpains) that damage the myofibrils and lead to hypercontracture and necrosis. 30,31
Prompt reperfusion restores oxygen delivery and the necessary substrates for aerobic ATP production, and normalizes the extracellular pH by removing the accumulated hydrogen ions. Hence, reperfusion itself seems to be responsible for underlying multifactorial lesions involving: 1) reactive oxygen species (ROS) that increase with the reintroduction of molecular oxygen when the blood flow is reestablished; 2) calcium overload; 3) MPT pore opening; 4) endothelial dysfunction; 5) prothrombotic phenomena, and 6) pronounced inflammatory responses (10).
Post-cardiac arrest syndrome stages
A physiological approach for the management of patients in cardiac arrest and of the post-cardiac arrest period involves defining the stages of care, based on the time elapsed after the arrest, in addition to its classification as in-hospital or not. Neumar et al. suggest the outline depicted in Figure 2, which allows for a reliable approach to the patients prognosis, with improved ability to predict the potential side effects, while favoring goal-directed management 10.
Post-cardiac arrest syndrome components
Brain injury
Post-anoxic brain injury is the most dramatic cardiac arrest complication. Brain injury is the cause of death in around 68 % of the patients following an out-of-hospital cardiac arrest and in 23% of the patients after in-hospital cardiac arrest. 10 The unique vulnerability of the brain is attributed to its limited tolerance to ischemia, its high oxygen requirements (around 15 % of the cardiac output) and its unique response to reperfusion. 10,32 Histologically, the subpopulations of selectively vulnerable neurons are in the hippocampus, the cortex, the cerebellum, the corpus striatum and the thalamus, which degenerate in a matter of hours after the onset of ischemia and apoptosis. 10 The relatively long duration of a cascade of injuries and histological changes suggest a broad therapeutic window for neuroprotective strategies after cardiac arrest. 25
During cardiac arrest, the blood flow is cut-off and arterial blood oxygen pressure drops to 0 mm Hg. A prolonged cardiac arrest may also dynamically compromise reperfusion of the microcirculation of the brain, notwithstanding an adequate cerebral perfusion pressure (CPP). 32 Oxygen and available glucose decline, reduce the aerobic ATP production, leading to the above-mentioned alterations. When the neuronal ATP drops, the sodium-potassium-magnesium ATPase pumps stop, promoting cell apoptosis due to the inflow of sodium, calcium and chloride and the outflow of potassium. When the cell depolarizes, the kainate, quisqualate, and N-methyl-D-aspartate receptors are activated, resulting in opening of the sodium and chloride channels that release excitatory amino acids - primarily glutamate - which will further increase the inflow of calcium into the cell, allowing for the release of mitochondrial calcium and a subsequent rise in intracellular calcium. 33 All of these ion responses lead to cellular edema, proteolytic and lipase enzymes activation, and cell destruction as a consequence of cytosolic enzyme degradation and release of arachidonic acid, increased prostaglandins and oxygen free radicals and of other free fatty acids. 34
Additional injury develops after reperfusion. Initially there is hyperemia, followed by vasospasm-associated multifocal hypoperfusion, increased local tissue pressure, capillary congestion due to endothelial and perivascular edema, which may then promote prothrombotic conditions with the presence of a pool of cellular components that may occlude the blood vessels and impair the microcirculation under low-flow conditions, and subsequent failure to restore perfusion in certain areas. 32,35
During the post-cardiac arrest the neurologic injury will primarily define the functional prognosis, expressing a broad spectrum of outcomes ranging from a transient alteration of consciousness to a vegetative state after resuscitation. 7,8 Of the patients who survive a cardiac arrest, only 37 % recover their original function. 7 This represents a significant economic burden for the healthcare system, for patients and families, in addition to compromising quality of life. 6,8
Myocardial disfunction
Myocardial disfunction after experiencing a cardiac arrest is another contributing factor to the low survival rate following an inhospital or out-of-hospital cardiac arrest. 32) Immediately after the return of spontaneous circulation, the heart rate and blood pressure are extremely variable, probably due to a transient increase of the local and circulating catecholamines. Likewise, systolic contractility and diastolic relaxation are affected with ROSC; this condition has been called stunned myocardium, primarily in acute coronary syndromes 36 and its intensity depends on the extent of the ischemic injury, that may lead to a decline in cardiac output down to 22 % in animal models 10, with subsequent hemodynamic instability. These alterations are secondary to the etiology of cardiac arrest, but are also the result of some of the therapeutic interventions such as defibrillation and the administration of epinephrin. 10,32 Moreover, the arrest triggers the onset of the systemic inflammatory response syndrome, with leukocyte and complement system activation leading to increased levels of cytokines and endothelial dysfunction, similar to what happens in sepsis. 37
During the first few minutes of myocardial reperfusion, the sarcoplasmic reticulum experiences a significant Ca2+ overload and the mitochondrial activation of ATP synthesis which activates the sarcoplasmic reticulum Ca2+-ATPase (SERCA), responsible for cytosolic Ca2+ uptake, despite the persistent increased Ca2+ from the extracellular medium. The result is a significant accumulation of Ca2+ that exceeds its storage capacity and finally Ca2+ is extruded through the ryanodine receptors and for subsequent reuptake, giving rise to a pattern of rapid Ca2+ oscillations that spread throughout the cell and develop a mechanical force which may exceed the elastic capacity of the sarcolemma. 32
The alterations most frequently described in the literature include myocardial dysfunction expressed as tachycardia, elevated left ventricular end-diastolic pressure, hypotension and decreased cardiac output. This overall dysfunction is transient and may evolve towards full recovery. The ultrasound-assessed cardiovascular dysfunction reaches its nadir 8 hours after resuscitation and may significantly improve over the next 24 hours and potentially normalize after 72 hours in out-of-hospital cardiac arrest survivors. 38
Persistence of the precipitating pathology
The pathophysiology of PCAS is further complicated due to the persistence of the pathology that caused or contributed to the cardiac arrest. 10 The diagnosis and treatment of persistent precipitating pathologies - such as acute coronary syndrome (ACS), acid-base imbalance, hypoxia, bleeding, sepsis, and various toxidromes - are critical, since such disorders may have an additive effect to the ongoing pathophysiology in this clinical condition, that worsen the patient's prognosis. 25 When the cardiac arrest is due to respiratory failure, the lung pathology may exacerbate after the return of circulation. Blood redistribution in the lung vasculature may give rise to acute pulmonary edema, or at least to increased alveolar-arterial oxygen gradients; likewise, brain injuries such as edema, are more significant when the cardiac arrest is associated with ventilation disorders. 10 Sepsis on the other hand, is a cause of cardiac arrest, acute respiratory distress syndrome and multiple organ failure. Therefore, there is a predisposition to a flare-up of the post-cardiac arrest syndrome in the presence of sepsis. Multiple organ failure is the most frequent cause of death in the ICU after the initial resuscitation of an in-hospital cardiac arrest. Other precipitating causes of cardiac arrest may require specific treatment during the post-cardiac arrest period. For instance, poisoning should be treated with its specific antidotes, while environmental causes such as hypothermia, may require more active temperature control. The specific treatment for these underlying disorders after cardiac arrest should be coordinated with the neurologic and cardiovascular support teams. 10
MANAGEMENT OF THE POSTCARDIAC ARREST SYNDROME
Post-cardiac arrest care has the potential to improve early mortality resulting from hemodynamic instability and failure of multiple organs and systems, in addition to having an impact on the morbidity and late mortality resulting, among other causes, from neurologic involvement. The primary objectives of these interventions are to improve cardiopulmonary function and systemic perfusion, provide rapid transfer to a higher level healthcare institution or to the ICU, to identify the precipitating causes, and to prevent relapses. All of these measures are intended to improve the long-term prognosis of patients, including their neurologic function. 39
Cardiac arrest prevention
The first link in the hospital chain of survival is early identification of the patient's declining condition and the prevention of the cardiac arrest. In this regard, several sources make reference to changes in vital signs and behavior of patients 8 hours prior to the cardiac arrest in up to 84 % of the cases. Once the cardiac arrest develops, approximately 20 % of the patients experiencing an in-hospital cardiac arrest survive and recover until discharge. The European Resuscitation Council Guidelines for Resuscitation 2015 suggest an approach for preventing in-hospital cardiac arrest, including staff education, patient monitoring, identifying the worsening of the patient's condition, a system to ask for help, and an effective chain of survival response. 11 Moreover, Smith refers to the First International Conference on Medical Emergency Teams, which described the key characteristics of rapid response systems, using a similar concept to that of the neurologic reflex arc whereby the system should have an afferent mechanism (for the detection of events and activation of response) and an efferent mechanism (response to the identified crisis), in addition to other two components: (a) an assessment mechanism for patient safety and process improvement, and (b) an administrative structure. 40
Most adult in-hospital cardiac-arrest survivors develop ventricular fibrillation and are managed with immediate defibrillation, The underlying cause of the arrest among this group of patients is usually primary myocardial ischemia. In contrast, a cardiac arrest in non-monitored areas is usually a predictable event not caused by a primary illness. 41 In this latter group, the cardiac arrest usually follows a period of slow and progressive physiological decline involving unrecognized or inadequately treated hypoxemia and hypotension. The rhythm of the underlying cardiac arrest is usually asystole and the probability of survival at hospital discharge is extremely low. 42
Regular monitoring and effective early treatment of seriously ill patients, improve the clinical outcomes and prevent a number of cardiac arrests. Closer monitoring of patients with risk factors for cardiac arrest, or with evidence of hemodynamic instability, even if they do not require basic or advanced life support, may also improve the results, since one third of these patients die during their hospitalization. 42
Ventilatory support
Patients with PCAS require early administration of inspired oxygen through any of the airway devices, although a tracheal intubation access is preferred, under certain considerations. Patients with PCAS have a higher risk of developing acute respiratory distress syndrome, as well as a high rate of pulmonary infections and injuries. Over 30 % of the patients present with a full stomach, which involves a higher risk of aspiration during airway management. Moreover, chest compressions may result in lung contusion. Finally, increased infection rates have been described, secondary to the ischemia-reperfusion condition, with subsequent disruptions in the inflammatory response, the coagulation cascade and pulmonary permeability. 43,44
According to the literature, a conservative oxygen therapy results in fewer episodes of fever, shock, liver failure and bacteremia in therapies with higher oxygen concentrations, Therefore, the recommendation is to frequently monitor oxygen levels and deliver an oxygen concentration to achieve an oxygen saturation (SO2) between 92 %-97 % which is close to a PaO2: 90-100 mm Hg. 43
There is a complex relationship between PaCO2 and neurologic outcomes. PaCO2, is also associated with the hemodynamic effects of a positive ventilatory pressure, since it leads to increased intrathoracic pressure that hinders venous return and reduces cardiac output. 43 Additionally, PaCO2 directly affects the brain tissue, since per every mm Hg drop, there is a reduction of around 3 % in cerebral flow. Consequently, although its clinical value is only now being recognized, moderate hypercapnia - a PaCO2 between 50-55 mm Hg - may be a beneficial intervention in the medium and long term. 45
In order to reduce the changes in CO2, although no strategy has been established to determine the optimum values for artificial ventilation, the management of ventilation with a low tidal volume (≤ 6 mL/ kg) has been shown to reduce the absolute mortality risk by up to 9%. Therefore, an active search for pneumonitis and respiratory infections must be conducted, particularly in patients with bronchoaspiration events or seizures witnessed by the healthcare staff, or patients with prolonged mechanical ventilation, in order to deliver timely management. 43
Interventions to optimize neurologic recovery
Targeted temperature management
Notwithstanding the low level of evidence, the Advanced Life Support Task Force of the International Liaison Committee on Resuscitation makes a strong recommendation for patients experiencing an in-hospital or out-of-hospital cardiac arrest, with defibrillable rhythms and who remain unconscious following ROSC (except those with severe systemic infection and pre-existing coagulopathies), to undergo a targeted temperature control between 32 °C-36 °C for 24 to 72 hours. 46 Such strategy could result in a 35 % improved estimated relative survival, and improved short and medium term neurologic outcomes. 47
Studies conducted in humans and animal models show that the induction of mild hypothermia could have a neuroprotective effect, with improved primary outcomes in patients who have experienced a period of cerebral ischemia-hypoxia as a result of a cardiac arrest. From the pathophysiological perspective, these results are explained by a decreased metabolic oxygen rate (approximately 6 % per degree of reduced body temperature), in addition to the reduction in the release of excitatory amino acids and free radicals, hence preventing intracellular calcium elevation, which translates into fewer activations of the pathways of apoptosis. 11
The effects of controlled hypothermia are not limited to the neurologic realm. At the cardiovascular level, it has been said for example, that lower temperatures result in increased peripheral vascular resistance, which would translate into increased blood pressure. Furthermore, in the conduction system - as will be discussed later - the major manifestation of hypothermia is sinus bradycardia which could involve a longer diastolic time, with a potential improvement in the relationship between myocardial oxygen delivery and demand; this finding has been associated in several studies with improved neurologic outcomes. 10,48,49 In contrast, this temperature approach involves other risks, such as hydroelectrolytic disorders -hypophosphatemia, hypokalemia, hypomagnesemia and hypocalcemia -, altered hemostasis with a mild increase in the risk of bleeding, though there is yet no significant clinical evidence 50, and finally, a decreased immune response that some studies have associated with higher rates of lower respiratory tract infection, but with no changes in survival. 51
The targeted temperature management approach comprises three phases: induction, maintenance and rewarming. Induction should be early and some authors recommend intra-arrest induction. 46,48 The patient's temperature may be lowered using either central or peripheral methods; the former include cold saline infusion through a central venous line or a peripheral line - not recommended for patients with congestive signs because of the risk of pulmonary edema -, transanal evaporative cooling and ECMO. Surface methods include placement of gel cushions, ice packs or wet towels in the patient's inguinal, neck and axillary regions. This method is inexpensive but it is time-consuming for the nursing staff, may result in more temperature fluctuations and does not allow for controlled rewarming. 52 The maintenance phase shall include sedation and neuromuscular block as mechanisms to prevent tremors. (46) Special emphasis should be placed on reducing temperature fluctuations, preferably with indwelling devices such as bladder or esophageal thermistors. Finally, at the end of the period of hypothermia, the rewarming phase begins; this should be gradual to avoid marked variations in electrolyte concentrations or in the effective intravascular volume; the ideal rates are between 0.25 °C to 0.5 °C per hour. 48
Seizures control
Seizures are frequent following a cardiac arrest and present in one third of the patients who remain in comma after ROSC. This may result in a status epilepticus identified on an EEG in about 23 %-31 % of the cases, with subsequent increases in the cerebral metabolic rate and flaring of the injury, which may be associated with a poor neurologic prognosis. 11 The most common presentations are myoclonus, focal seizures, and tonic-clonic seizures. 53,54 The use of intermittent EEG is recommended for identification when patients are suspicious of seizures, since the motor manifestations are not seizure-specific and systematic monitoring has not proven to be effective in early detection. 11,53 Seizures can be managed with sodium valproate, levetiracetam, phenytoin - which is not the case in myoclonus due to poor effectiveness -, benzodiazepines, propofol or barbiturates. After the first event, maintenance therapy should be initiated, once the potential precipitating causes have been ruled out, such as intracranial hemorrhage or electrolyte imbalances. The use of prophylactic anticonvulsants after cardiac arrest is not recommended because of their potential adverse effects and because there is not enough supporting evidence. 11,53
Glucose control
There is a strong association between high blood glucose levels after ROSC and a poor neurologic prognosis. 52 Two approaches have been suggested to control this occurrence: intensive glucose monitoring with a target level between 81 and 108 mg/ dL 11 and a more permissive management where only glucose levels above 180 mg/ dL are intervened. It has been shown that overtreating high glucose levels, even in non-diabetic patients, may increase the 90-day mortality, particularly in comatose patients who may experience unidentified hypoglycemia. Keep in mind that mild hypothermia has been associated with episodes of hyperglycemia, with increased insulin requirements in diabetic patients. 55
Peri-cardiac arrest arrythmias
Cardiac arrhythmias are relatively frequent in the peri-arrest period and may precede any of the arrest rhythms, or develop after a successful resuscitation. Nielsen et al. observed that the most common side effects following resuscitation are bradycardic episodes in 41 % of the cases and tachycardia in 33 %. 56 The most important risk factors for the development of arrhythmias are persistent coronary artery lesions with unstable plaque or fresh thrombus in patients with acute coronary syndrome, cardiomyopathies, massive pulmonary embolism, acid-base and electrolyte imbalances, and poisoning. 11,57-59
As already mentioned, ROSC initiates an injury cascade through myocardial perfusion which is in part ROS-mediated and high levels of circulating cytokines. 21 These processes develop electrical instability during the period between 24 to 72 hours after ROSC, which may give rise to a broad spectrum of arrhythmias ranging from premature ventricular complexes to ventricular tachycardia (VT), ventricular fibrillation (VF) or accelerated idioventricular rhythm, classically described after reperfusion in acute coronary syndromes. 58
There are several contributing factors to cardiac electrical instability after ROSC. These include elevated levels of endogenous catecholamines, persistent effects of vasoactive agents such as vasopressin and epinephrine, administered during the arrest, the subsequent use of ionotropic agents and vasopressors. These factors increase the left ventricular aftertload, may induce coronary vasospasm and increased myocardial oxygen demand, which predisposes to arrythmias. 10 Moreover, the left ventricular dysfunction, both acute systolic and diastolic due to myocardial stunning, is frequent following cardiac arrest and also predisposes to recurring arrhythmias. Finally, patients under targeted temperature management as a strategy to minimize the hypoxic brain injury, may develop bradycardia and prolonged QTc (corrected QT interval) which predisposes to VT and polymorphic VF. 58 An interesting ECG finding in patients with hypothermia, even those with controlled hypothermia, is the Osborn wave, a vertical deviation from point J in the ECG, considered a risk predictor for ventricular arrhythmia. However, recent evidence suggests that this finding may be considered a benign physiological phenomenon, associated with lower mortality in univariate analysis. 59
With few exceptions, the treatment of arrhythmias following cardiac arrest should not adjourn the acute treatment of the initial arrhythmias that led to the arrest. Every effort must be made to correct and treat the precipitating factors and the reversible causes, with particular emphasis on coronary revascularization for those patients who developed ventricular fibrillation. 10,58 With the exception of patients with VF/VT over the 48 hours following an acute myocardial infarction with ST segment elevation, the current guidelines of the European Society of Cardiology and the American Heart Association recommend placing an implantable cardioverter defibrillator as secondary prevention in patients undergoing cardiac arrest. The long term use of adjuvant antiarrhythmic drugs should be reserved for patients with recurrent ventricular arrhythmias. 58
Identification and management of the cardiac arrest trigger
If possible any potentially reversible causes of a cardiac arrest, for which there is a specific treatment, should be identified and ruled out throughout the resuscitation process. Typically, these causes have been grouped into two categories to facilitate a systematic approach by the practitioners in charge. These are the H and the T categories, as shown in Table 2. The identification of any of these entities demands immediate intervention, since as already mentioned, patients with a persistent trigger pathology for cardiac arrest in the early stages of the post-cardiac arrest syndrome, exhibit worse outcomes than those patient treated for the underlying cause. 60
TABLE 2 Major cardiac arrest etiologies.
| Abnormality | Risk factors | Management | Survival |
|---|---|---|---|
| Category H | |||
| Hypoxia | Patients with SARS-CoV-2 infection | Secure the airway and administer supplementary oxygen | Low survival rates may present with secondary neurologic injuries |
| Foreign body airway obstruction (extreme age groups) | Maneuvers to clear the airway obstruction | ||
| Hypokalemia | Severe burns, ostomies, prolonged use of diuretics, renal tubular disorder | Potassium and magnesium supplementation | |
| Hyperkalemia | Renal failure, diabetes mellitus, potassium sparing drugs, rhabdomyolysis | Diagnose any ECG alterations, avoid myocardial depolarization, favor potassium cell inflow, potassium clearance (dialysis) | Varies according to the level of hyperkalemia and concomitant cardiac alterations |
| Acidosis (H+) | Chronic renal disease, sepsis, obstructive lung disease, toxic agents | Varies according to the etiology | Varies according to the etiology |
| Hypovolemia | Blood losses due to trauma, sharp injuries, puerperium. Diarrhea or emesis in patients with low tolerance to volume losses or high output (cholera) | IV fluid replacement or blood products based on the suspicious cause | Non-corrected hypovolemia is the primary cause of arrest rhythm called pulseless electrical activity (PEA),death |
| Hypothermia | Ice-water drowning | Prevention of arrhythmias, hospital re-warming (IV fluids, electric blankets) | High survival rates overall and good neurologic prognosis |
| Hyperthermia | Use of o de sympathomimetic substances, heat shock (elderly, dehydration, obesity, alcoholism), malignant hyperthermia | Cooling down form the tine of detection | Mortality between 10 and 50 % in heatshock |
| Category T | |||
| Cardiac tamponade | Stab wound in the precordial region, pericardial effusion from neoplasm, infectious or inflammatory causes | Pericardiocentesis, pericardial window | Varies according to the cause |
| Coronary thrombosis | High blood pressure, high cholesterol levels, DM, obesity, smoking, family history, atherosclerotic disease: previous MI, peripheral artery disease | Reperfusion therapy, anti-ischemic management and plaque stabilization | Coronary thrombosis is the main cause of cardiac arrest in adults |
| Tension Pneumothorax | Chest trauma | Needle decompression, tube thoracostomy | Survival depends on diagnosis and early management |
| Pulmonary thromboembolism | Deep venous thrombosis, cancer, recent major surgery, immobility, fractures | Fibrinolysis, anticoagulation, thrombectomy, ECMO (extracorporeal membrane oxygenation) | Low survival rates in case of massive pulmonary thromboembolism |
| Poisoning | Industrial accidents, occupational hazards, psychiatric disorders, sexual assault or sexual abuse victims | Decontamination, limit absorption, favor clearance, use of specific antidotes | The survival rate depends on diagnosis and early management |
SOURCE: Authors, based on Soar et al. 60.
NEUROLOGIC PROGNOSIS
According to the Advisory Statement on Neurological Prognostication in Comatose Survivors of Cardiac Arrest, up to 80 % of the patients admitted to the ICU exhibit hypoxic ischemic injury; of these, two thirds die mostly from discontinuation of vital support therapy based on a falsely pessimistic prognosis of a poor neurologic outcome. 11,61 Therefore, in patients with severe anoxic injury, the risk of any falsely pessimistic prognosis should be minimized 62, since such prognosis may lead to a hasty decision to discontinue treatment.
In view of the large number of predictors to assess the neurologic prognosis, a practical, systematized approach is needed, based on the most sensitive and specific predictors, with the largest possible evidence. For the sake of simplicity, the protocol suggested by ERC-ESICM is an excellent tool which begins with early robust sensitivity prognostic tests, not prone to misleading interpretations from factors such as sedation, neuromuscular block, hypothermia, severe hypotension and metabolic or respiratory disorders. Some of the predictors include the absence of pupillary reflex after 72 hours or more of ROSC, and the absence of a bilateral N2O SSEP wave following rewarming; then, if these findings are not present, other predictors are used which, despite their limitations, exhibit adequate sensitivity and specificity. These include:
Myoclonic status before 48 h.
High neuron-specific enolase (NSE) levels at 48 to 72 h after the return of spontaneous circulation (>71 mcg/L).
Malignant, non-reactive EEG pattern (burst-suppression, status epilepticus) after re-warming.
Diffuse ischemic injury identified with CT-scan 24 hours after ROSC or after 2 to 5 days with MRI, combining at least two of these predictors.
Similarly, there is a strong need to continue with prolonged observation of the indeterminate results, since this allows for the identification of "late awakening"; i.e., awakening time between 48 hours and 10 to 12 days from initial sedation interruption.
Finally, it is important to highlight the availability of many other tools for an adequate assessment of the neurologic prognosis, such as CPC, mRS, GOS rating scales, and GCS motor score, electrophysiology tests, EEG and short-latency somatosensory evoked potentials 61, neuroimaging (CT and MRI) and automated analysis; some of these diagnostic modalities 11,61 supported by the physical examination as the foundation for prognosis 54, allow for the best prognostic approach. However, there are still many challenges because of the need for an adequate assessment methodology and for a definition ofthe values associated with poor neurologic outcomes, supported with clinical trials that provide a satisfactory level of evidence for widespread use (Figure 2).
CONCLUSIONS
The SPPC concept has experienced some changes over time, based on the understanding of its pathophysiological mechanisms and in response to a scientific research interest derived from the high incidence rates of cardio-respiratory arrest and its impact on the short and long-term morbidity and mortality. Consequently, numerous interventions have been suggested, which according to the recent evidence, increase the survival rates and probably impact the neurologic prognosis of these patients. First and foremost, there is a need to train healthcare practitioners to be able to identify and approach early the disruptions that represent a higher risk for the development of cardiac arrest, since preventing the arrest is the cornerstone for a favorable prognosis. Timely management of the underlying cause of the cardiac arrest is another intervention with a major impact on mortality, as is protective ventilation. Other strategies, such as specific temperature control, management of peri-arrest arrythmias, blood sugar control and management of seizures, while all are physiologically relevant, their impact is more closely associated with the neurologic prognosis and a lower morbidity, rather than with improved survival. Another requirement is the development of local clinical practice guidelines designed for the biological, social and economic characteristics of each population, such as the Latin American people.











 text in
text in