INTRODUCTION
Wilms Tumor 1 (WT1) gene mutation is associated with the development of Nephroblastoma, the most common pediatric abdominal malignancy. WT1 has been associated with the development of glomerulopathy and chronic kidney disease (CKD) including a missense mutation causing focal segmental glomerulosclerosis (FSGS).1,2 In patients with end-stage renal disease (ESRD) associated with WT1 glomerulopathy, a prophylactic bilateral nephrectomy may be recommended prior to renal transplantation to reduce the risk for developing Wilms Tumor when the patient is immunosuppressed. 2 In patients with other WTi gene mutation-related syndromes, like Denys Drash, WAGR or Frasier syndromes, nephrectomy may be indicated independent of immunosuppression. In pediatric patients with ESRD, peritoneal dialysis (PD) is an attractive option for renal replacement therapy due to the flexibility of home dialysis and reduced risk of vascular complications from long-term hemodialysis (HD) line placement. 3 Pre-operative HD is also an independent risk factor for poor outcomes following renal transplant.
The prone, retroperitoneoscopic approach is well described in the pediatric surgical literature 4, and allows for a safe and effective nephrectomy without sacrificing the ability to maintain PD during the perioperative period. The unique anesthetic concerns around the perioperative management of such patients have not been reported. We highlight these concerns by reviewing a case of a 9-year-old patient with ESRD, secondary to FSGS, on daily PD, who underwent a staged prone, retroperitoneoscopic bilateral nephrectomy.
CASE DESCRIPTION
A 9-year-old female, 26 kg (representing i9th percentile for weight), developed ESRD secondary to FSGS; a renal biopsy identified an incidental WT1 mutation. She was initiated on PD approximately 5 months before and underwent nightly dialysis. Given the risk of malignancy following renal transplantation, the decision was made to proceed with a bilateral nephrectomy via a retroperitoneoscopic approach in order to maintain PD during the perioperative period. Her relevant medical history included hypertension and gastroesophageal reflux disease. Medications included Metoprolol, Amlodipine, Darbapoetin, Ferrum, Lansoprazole and Multivitamins. The patient had excellent exercise tolerance with no additional risk factors. The anesthetic plan included general anesthesia with endotracheal tube placement, large bore intravenous (IV) access, arterial line and erector spinae plane (ESP) block for postoperative pain control. The potential for intraoperative transfusion, post-operative complications related to the prolonged prone positioning and postoperative intensive care unit (ICU) was discussed with the family. The anesthetics for the two surgeries were performed by different pediatric anesthesiologists, so some differences were observed in the plan.
A left nephrectomy was done in the first stage and the right in the second stage, 8 days later. A description of the perioperative differences between the two surgeries is shown in Table 1. In both cases, the patient received oral acetaminophen preoperatively. Following induction, airway management and line placement, the patient was placed in the prone position. Subsequently, an ultrasound-guided single shot ESP block was performed. Intraoperative ketorolac and hydromorphone were administered as part of a multimodal analgesic plan.
Table 1 Perioperative differences between the left and right nephrectomies.
| Left Nephrectomy | Right Nephrectomy | |
|---|---|---|
| Preoperative medications | Metoprolol | Metoprolol |
| Medications held the day of surgery | Amlodipine | Amlodipine |
| Preoperative pain score | 8 (Leg pain)a | 7 (Leg pain)a |
| Preoperative blood pressure (mmHg) | 139/91 | 174/113 |
| Induction medications and dosage | Propofol (2 mg/kg) Fentanyl (1 mcg/kg) Rocuronium (0.5 mg/kg) |
Propofol (4 mg/kg) Fentanyl (3 mcg/kg) Rocuronium (0.7 mg/kg) |
| IV lines | 20 G, 14 G | 20 G, 18 G |
| Block performed | Bilateral ESP block | Right ESP block |
| Local anesthetic and volume | Bupivacaine 0.25%+Epinephrine 1:200.000 (20 mL) | Bupivacaine 0.25%+Epinephrine 1:200.000 (20 mL)- |
| Local anesthetic adjunct | None | Dexmedetomidine 0.5 mcg/kg |
| Maintenance medications | Sevoflurane Cisatracurium infusion (0.2 mg/kg/h) | Sevoflurane Remifentanil infusion (0.1 - 0.2 mcg/kg/min) Tranexamic Acid bolus (10 mg/kg) followed by infusion (10 mg/kg/h). |
| Hemodynamic response to incision or insufflation | Yes | No |
| Total maintenance fluids | Ringers Lactate (10 mL/kg) | Ringers Lactate (8 mL/kg) |
| Analgesics used and dosage | Preoperative acetaminophen (15 mg/kg) Hydromorphone (24 mcg/kg) Ketorolac (0.5 mg/kg) |
Preoperative acetaminophen (15 mg/kg) Hydromorphone (48 mcg/kg) Ketorolac (0.5 mg/kg) |
| Antihypertensives during surgery | Nitroglycerine infusion (0.01 - 0.05 mcg/kg/min) Esmolol (0.12 mcg/kg) |
Nitroglycerine infusion (0.5 - 2 mcg/kg/min) Labetalol 0.1 mg/kg (5 boluses) |
| Length of surgery | 3 h, 22 min | 3 h, 50 min |
| Surgical issues | Intraoperative surgical violation of the peritoneal cavity led to carbon dioxide tracking along the path of least resistance and out the pre-existing peritoneal dialysis catheter | |
| Anesthetic issues | Trend to hypertension, easily titrated | High hypertension spikes, requiring multiple antihypertensives at high doses. |
| PACU issues | None | None |
| Time to discharge from PACU | 1 h, 44 min | 1 h, 29 min |
| Pain score PODi | 4 [3 - 5] | 5 [4 - 8] |
| Opioids use PODi | Hydromorphone (4 doses) | Hydromorphone (4 doses) |
| Pain score POD2 | 3 [2 - 3] | 2 [0 - 5] |
| Opioids use POD2 | None | Hydromorphone (1 dose) |
| Pain score POD3 | 0 | 3 [2 - 4] |
| Opioids use POD3 | None | None |
a Chronic left leg pain. No preoperative abdominal pain reported. ESP: Erector Spinae Plane; PACU: Post-Anesthesia Care Unit; POD: Postoperative day.
Source: Authors.
Carefulattentionwasgiventofinalprone positioning ensuring adequate padding of pressure points, joints, eyes and other facial structures (Figure 1A). Figure 1B depicts the PD catheter attached to a Foley catheter drainage bag. During left nephrectomy, an hour after pneumoperitoneum, the anesthesiologist remarked rapid filling of the Foley catheter bag with insufflation gas (Figure 1C) and informed the surgical team. They determined that the peritoneal cavity had been breached and carbon dioxide was accumulating in the Foley bag. The defect was then visualized on the laparoscopic camera screen. Repeated emptying of the air from the Foley bag was needed until repair of the peritoneal cavity defect with titanium clips was complete. The second nephrectomy was uneventful.
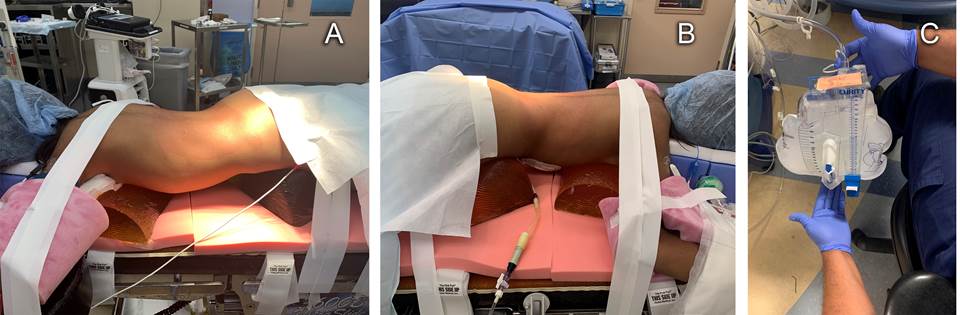
Source: Authors.
Figure 1 A) Positioning of patient for left retroperitoneal laparoscopic nephrectomy. B) Peritoneal dialysis catheter attached to closed system Foley catheter bag. C) CO2 insufflation of Foley catheter bag remarked by the anesthesiologist during intraoperative peritoneal access.
Preoperative blood pressure (BP) on the day of the left nephrectomy was 139/91 mmHg. Intraoperatively, an infusion of nitroglycerine (0.05 mcg/kg/min), repeated remifentanil boluses (1 mcg/kg) and an esmolol bolus of 0.5 mg/kg were needed to avoid significant BP increases.
Preoperative BP on the day of the right nephrectomy was 174/113 mmHg despite the patient taking her preoperative anti-hypertensive medications. Post-induction, the blood pressure dropped to 140/90, before experiencing another significant rise to 187/113 mmHg, immediately after placement of the ESP block medication, this time with 0.5 mcg/kg Dexmedetomidine mixed with the local anesthetic solution. This was managed by temporarilyincreasing the Nitroglycerine infusion to 1 mcg/kg/ min, which was subsequently titrated down to 0.5 mcg/kg/min. There was an additional BP elevation, unrelated to surgical stimuli, reaching 184/130 mmHg. This was treated by increasing the Sevoflurane (>1.5 MAC), a Remifentanil bolus (1 mcg/kg), increasing the Nitroglycerine infusion to 2 mcg/kg/ min and giving 5 sequential boluses of Labetalol of 0.1 mg/kg. The Nitroglycerine was titrated down to 0.5 mcg/kg/min and discontinued near the end of surgery. After extubation, blood pressure increased again to 196/118 mmHg which was treated with three boluses of Hydralazine 0.1 mg/kg and one 0.05 mg/kg bolus of Verapamil that finally controlled the hypertensive event. However, no end-organ damage was sustained, the patient vomited post-extubation, once BP was lowered.
The patient never required perioperative blood transfusions.
After the two surgeries, the patient was then transferred to the Post-Anesthesia Care Unit (PACU) for monitoring, before being discharged to the floor. After the second surgery, a multidisciplinary meeting involving Anesthesiology, Urology, Nephrology and ICU was held in the PACU to determine disposition. It was decided that Pediatric ICU monitoring would not be required, as the significant hypertension eventually resolved without the need for continuous infusions.
Post-operatively, there was no additional CO2 noted in the Foley bag, indicating that the peritoneal defect repair was air-tight, and therefore water-tight and likely safe for use for PD. PD was initiated uneventfully on postoperative day 2. She remained mildly hypertensive and required adjustment of the antihypertensive regimen as well as PD ultrafiltration. Pain was well controlled. Pathology from both kidneys revealed advanced global glomerulosclerosis without features suggestive of Wilms tumor. At the most recent follow up visit, the renal transplantation evaluation had been initiated.
DISCUSSION
Prone, retroperitoneoscopic nephrectomy allows patients to continue on PD, since the abdominal cavity remains intact, while waiting for the kidney transplant. This approach avoids hemodialysis, which is independently associated with increased morbidity and mortality (higher than PD), but requires especial anesthetic considerations. Firstly, these patients often have chronic hypertension requiring multiple antihypertensive medications that should be continued preoperatively. Intraoperatively, labile blood pressures are frequent; hence, being prepared for significant hypertension with the use of vasodilators is important to reduce the risk of end organ damage (myocardial ischemia, hemorrhagic stroke). Nitroglycerin infusions were administered for both surgeries, but noticed a significant difference in the doses needed between the two cases without an obvious cause. Moreover, shorter acting beta blockers IV boluses were required to maintain blood pressures within 20% of the preoperative BP readings. The intraoperative blood pressure management and the choice of antihypertensive drug, depends on the preferences of the managing anesthesiologist in these contexts 5. Although there is no strong evidence suggesting that Nitroglycerin should be considered as a first line agent, it was also used for the second procedure since it showed good effects during the first case. Post-operatively, these patients can be at risk of hypertension from missed doses of regular antihypertensive agents while under anesthesia. In the postoperative period of the second nephrectomy, IV doses of verapamil and hydralazine were given for significant hypertension despite no pain or agitation. Secondly, intraoperative surgical violation of the peritoneal cavity can lead to carbon dioxide tracking along the path of least resistance and out the pre-existing PD catheter. This situation calls for close communication between the anesthesia and surgical teams. This was the case during the left nephrectomy, with the Foley bag repeatedly filling with CO2 and requiring frequent emptying. Early identification of the defect and subsequent intraoperative repair in the peritoneal cavity using titanium clips finally resolved the leak. If the repair is too large or unmanageable, this may result in the child requiring temporary HD while the peritoneum heals. Thirdly, this type of surgery presents a risk for potential postoperative complications related to prone position, including, but not limited to, pressure sores and visual loss from posterior optic neuropathy; the latter is particularly concerning when the patient remains pronated for a long time and presents with labile intraoperative blood pressure. Though this complication was not seen in our case, it was one of the reasons for staging the surgeries.
This case also provided an opportunity to compare postoperative pain management with the use of an ESP block, with and without dexmedetomidine as an adjunct. In the first surgery an ESP block was performed with 0.8 mL/kg of Bupivacaine 0.25%. In the second surgery, an ESP block was performed with 0.8 mL/kg of Bupivacaine 0.25% and 0.5 mcg/kg of Dexmedetomidine. Both patients received preoperative Acetaminophen and intraoperative Ketorolac. Postoperative pain scores, total opioid use and patient satisfaction were similar; however, no additional benefit was noted with the use of dexmedetomidine as an adjunct to the single shot ESP block (Table 1). A rise in the patient's blood pressure was noted shortly after administering the dexmedetomidine- containing block. This may have been caused by the alpha 1 effect of systemically absorbed dexmedetomidine; a consideration to keep in mind with patients already managed for refractory hypertension. Though dexmedetomidine has been shown to be a beneficial adjunct in peripheral nerve blocks to prolong sensory block, motor block and duration of analgesia. 6
In summary, this case report highlights the unique anesthetic considerations for a prone, retroperitoneoscopic nephrectomy in a child on PD, facilitating the comparison of an ESP block with and without Dexmedetomidine, which in this case failed to improve the quality of the block.
ETHICAL DISCLOSURES
Ethics committee approval
According to the Canadian Scientific Publication Guidelines (TCPS2) and local regulation, there is no explicit consent. However, anonymity has been guaranteed in the reporting and monitoring of institutional guidelines.
Protection of human and animal subjects
The authors declare that no experiments were conducted on humans or animals for this study. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and pursuant to the Code of Ethics of the World Medical Association (Declaration of Helsinki).











 texto en
texto en 



