INTRODUCTION
In 2021, the theme selected by the World Breastfeeding Week, celebrated every year in August, was "protect breastfeeding," reinforcing the notion of a shared responsibility. This celebration came into being as a result of the Innocenti Declaration signed in 1990, which declared the protection, promotion and support of breastfeeding in the different countries of the world 1 .
The World Health organization (WHO) and the United Nations Fund for Children (UNICEF recommend initiating breastfeeding ideally within the first hour of life, maintaining it exclusively until 6 months of age and then supplementing it with food until 2 years of age 2,3. However, while close to 96% of mothers initiate breastfeeding, only 39% and 15% continue exclusive breastfeeding (EBF) at 3 and 5 months, respectively. Most of them discontinue within the first 6 weeks, mainly due to reduced milk production or "unhappy infant" (27.2%), nipple or suction issues (14.7%) and the mother's return to work/study activities (10.4%) 4,5.
Globally, the prevalence of EBF at six months is close to 40% 3. In Latin America, it ranges between 24 and 68%, with Peru and Bolivia at the top with figures above 60%, followed by Chile (43%), Colombia (36%), Brazil (35 %) and Paraguay (24%) 5. The WHO set a global target of increasing exclusive breastfeeding for infants under 6 months to at least 50% by the year 2025 3.
Breast milk meets all the nutritional and immune requirements of the neonate, protects against infection, favors bonding with the mother and reduces the risk of sudden death, obesity and diabetes. For the mother, breastfeeding plays a preventive role in breast cancer and, probably too, in ovarian cancer 6. From a cost perspective, it is the most cost-effective and complete food source which helps prevent potential causes of child morbidity and mortality in all socioeconomic brackets 7,8. The risk of mortality is 14 times higher in infants who did not receive EBF 9. According to the meta-analysis by Victora et al. scaling breastfeeding to a higher level could prevent close to 823,000 deaths per year in children under 5 years of age, and 20,000 deaths from breast cancer every year 6.
These statistics point to the importance of giving adequate counseling regarding the safety of many drugs, in order to promote successful, uninterrupted breastfeeding. Contact with medical care can be a risk factor for early weaning, considering that the advice given to mothers at the present time is nonuniform and inconsistent, leading to the delay in initiating breastfeeding by 2448 hours, pumping and dumping of breast milk due to the potential adverse effects of drug transfer 10. Consequently it is of the utmost importance that all health officials are aligned in their efforts to promote successful EBF.
The objective of this work is to conduct a non systematic review of breastfeeding and drugs used in anesthesia, both intra/ postoperatively as well as during childbirth/ postpartum, in order to prepare a series of recommendations and ensure that anesthetists have the necessary knowledge to help women who want to breastfeed and avoid interruptions.
REVIEW OF THE EVIDENCE
A search of reports was conducted in the PubMed (Medline), Coogle Scholar, SciELO, Embase and Cochrane Library databases using the terms: "breastfeeding," "anesthesia," "epidural anesthesia," "breastmilk," "preoperative assessment," and "surgery". The language of the search was restricted to English and Spanish. Specific breastfeeding-related sources were also included: UK Drugs in Lactation Advisory Service (UKDILAS) 11, Drugs and Lactation Database (LactMed) 12) , E-lactancia 13, references of selected articles, and grey literature. This structured search yielded 293 articles. After they were analyzed by the two authors, those that did not offer relevant information, duplicate articles, unavailable full texts and articles published more than 20 years ago were excluded. Finally, 51 articles with relevant information to carry out this review were identified.
DEVELOPMENT
Anesthetist role as promoter of breastfeeding
Anesthetists are active members of the surgical team. Their participation in the realm of lactation promotion includes:
Establishing communication with the mother in advance, answering preoperative questions regarding analgesia during labor or anesthesia for cesarean section, as well as explaining the advantage of breastfeeding. Just like many anesthetists use the preanesthetic assessment to counsel against smoking, mothers could be encouraged to initiate skin-to-skin contact, particularly after cesarean section, when it is often postponed, affecting maternal satisfaction and, eventually, breastfeeding 14.
Documenting the history of breastfeeding in the preanesthetic assessment record of all female patients with children under 2 years of age. This history is rarely considered or mentioned before surgical procedures.
Preparing an anesthesia plan for puerperal women to ensure adequate surgical conditions and allow for continuation of breastfeeding by selecting the least disruptive type of anesthesia. In this regard, neuraxial techniques are preferred over general anesthesia.
Providing adequate postoperative analgesia and preventing postoperative nausea and vomiting (PONV). Mothers with poorly managed postoperative pain and vomiting will have difficulty breastfeeding 15,16.
Identifying and providing early treatment for low blood pressure after spinal techniques and for postpuncture headache, as they can interfere with effective breastfeeding 17.
Promoting immediate skin-to-skin contact in cesarean delivery.
Discussing a plan reviewed by experts in breast milk storage in the event the surgery is prolonged or the infant cannot remain with the mother.
Mothers must be made aware of the risk of not breastfeeding, including obstruction of mammary ducts (mastitis), exposure of an allergic infant to artificial formula, inability of the infant to be fed by bottle and the risk of necrotizing enterocolitis in premature infants when breast milk is temporarily replaced by formula 18. All of these factors must be countered and any doubts regarding adverse effects and transfer of drugs in breast milk must be resolved.
Factors affecting drug concentrations in breast milk
Transfer of drugs to breast milk occurs mainly by passive diffusion, proportional to plasma concentration of the drug. Other drug characteristics that influence passage include protein binding, fat solubility, molecular weight and pKa (negative log of the acid-base dissociation constant or pH at which balance between the ionized and non-ionized form of the drug is attained). In general, drugs that are highly soluble in fat, have low molecular weight, low protein binding or high pKa, are associated with higher penetration in breast milk. However, drug excretion in breast milk does not necessarily imply toxicity for the neonate.
Mammary secretory epithelial cells form the blood-milk barrier consisting of tight intercellular junctions that prevent milk components from leaking. Loss of junction integrity has been associated with reduced mammary secretion and increased paracellular transport of plasma components into the milk and viceversa. This barrier is quite leaky before childbirth, allowing the passage of large molecules such as antibodies, immune factors and blood cells into the colostrum. A rise in prolactin and glucocorticoids at childbirth favors closure of those junctions, making them almost impervious to greater exchange between blood and milk components. Thus, formation and integrity of this barrier early in breastfeeding is critical to ensure the initiation of abundant milk production 19.
There are search platforms in the Internet, such as the US National Library of Medicine LactMed, where a search by the drug name in English yields more relevant data, including summary of use and bibliographic references 12. The Association for the Promotion and Scientific and Cultural Research into Breast Feeding (APILAM) hosts E-Lactancia, a rigorous website for queries on the compatibility between drugs and breastfeeding. The tool has received international endorsement from the Academy of Breastfeeding Medicine and the Maternal Lactation Committee of the Spanish Pediatrics Association. This didactic and comprehensive website in Spanish (with an English version as well), includes recommendations from LactMed and the United States Food and Drug Administration (FDA). Additionally, the platform is more intuitive and patient-friendly, classifying drugs according to risk during breastfeeding as very low, low, high and very high. This classification is shown in Table 1 13.
Table 1 Risk classification of medications according to E-lactancia.
| Risk | Definition | Examples |
|---|---|---|
| Very low risk | Safe and compatible medications. Minimum risk for breastfeeding and the infant. Ample scientific literature on absence of proven toxicity and frequent use in neonates and infants. | Propofol, thiopental, etomidate, cefazolin, local anesthetics, muscle blockers, atropine, fentanyl, methadone, tramadol, midazolam, halogenated agents, acetaminophen. Adrenaline, noradrenaline, labetalol. |
| Low risk | Quite safe medications, probably compatible. Mild or improbable risk. Their pharmacokinetic characteristics make adverse effects unlikely. | Pethidine, pregabalin, ephedrine, morphine, acetylsalicylic acid, ketamine, tapentadol, nitrous oxide, phenylephrine, ephedrine. |
| High risk | Unsafe medications. May trigger moderate or severe adverse effects in the infant. Use a safer alternative or discontinue breastfeeding until the drug is washed out from the mother (5 to 7 TJ4)1. Published data are few and pharmacokinetic characteristics make the occurrence of adverse effects likely. | Diazepam, oxycodone, codeine, metamizol, atenolol, clonidine, tobacco, alcohol. |
| Very high risk | Very unsafe medications, contraindicated. Use an alternative or discontinue breastfeeding. Published data show that they can be toxic for the infant. | Cyclophosphamide, retinoids, antineoplastic agents, phentermine, marihuana. |
1 T½: drug elimination half-life.
Source. Authors, based on 13.
However, there are instances in, which recommendations are inconsistent, as is the case with tramadol which metabolizes to 0-desmethyl-tramadol (M1), with both being excreted in breast milk. E-lactancia classifies it as compatible (very low risk), stating that no problems have been observed in mothers using this medication, although they use phrases such as "there are ultra-fast tramadol metabolizers who might accumulate a higher concentration of M1" or "it is wise to use the minimum sufficient dose and monitor the infant for signs or sedation or difficulty feeding" 13. On the other hand, LactMed is less permissive in this regard and is more in agreement with the FDA, not recommending tramadol administration when breastfeeding and, in case it is used in the mother, close monitoring of the infant for excess sleepiness, difficulty feeding and/or respiratory depression is required 12.
Factors influencing the risk of adverse effects in the neonate20
Timing of the dose: breastfeeding before taking the medication helps, with a lower concentration reaching the infant. Some exceptions to this principle are drugs with a longer half-life such as diazepam, where the timing of breastfeeding does not make a big difference.
Age of the infant: most adverse effects occur in neonates and infants under 2 months of age, and they are very rare beyond 6 months. Special care is required with infants under 6 weeks of corrected gestational age. In the case of opioids, the order of sensitivity to adverse effects due to immature liver and renal function is premature babies>neonates>infants. In a neonate, the metabolization and excretion capacity is only one-third that of an 8-9 month-old infant.
Pharmacokinetic factors: drug characteristics for compatibility with breastfeeding are summarized in Table 2.
Table 2 Summary of pharmacokinetic characteristics for compatibility with breastfeeding.
| Relative dose <10 % |
| Milk/plasma ratio <1 |
| Plasma protein binding >90% |
| Molecular weight >200 Da |
| Poor oral availability |
| Short half-life of the drug and its active metabolites |
| Drug certified for pediatric use |
Da: Dalton.
Source: Authors, taken from 20.
The main pharmacokinetic parameters are:
Oral bioavailability: Drug percentage absorbed after passing through the gut, the liver and the lungs. An example is gentamicin, which is administered intravenously to the mother. Since it is probably absorbed orally by the baby, the concentrations of the drug will not be reflected in the infant's plasma.
First pass metabolism: Reduces bioavailability. In breastfeeding, drugs inactivated due to this mechanism are preferred.
Plasma protein binding: Only the free fraction of the drug can cross biological membranes. The higher the protein binding, the lower the passage will be.
Milk/plasma ratio (M/P): Reflects protein free fractions in milk and plasma. Values >1 mean that the drug is not adequate for breastfeeding women.
Molecular weight: The higher the weight, the more difficult it will be for the drug to cross into the milk.
Half-life: Defined as the time elapsed until drug plasma concentration is reduced by one-half. It is determined by absorption, metabolism and excretion. The longer the half-life, the higher the risk of drug accumulation in the mother and the infant. Five half-lives must happen to reach a balanced state, hence the very low impact of timing the feeding to avoid maximum levels after that time. Likewise, after 5 half-lives and without receiving additional doses, almost 98% of the drug will have been cleared from the body. However, neonates do not metabolize as fast as adults.
Ionization percentage: Drugs cross membranes in non-ionized form. Milk is slightly more acidic (pH 7.2) than maternal plasma (pH 7,4) and, therefore, attracts weak bases such as oxycodone and codeine. These drugs are ionized and undergo ionic entrapment in milk.
Breast milk intake volume: The greater the volume, the higher the dose of the drug ingested by the infant. As such, drugs used during the first three postpartum days usually produce safe subclinical levels in the infant, given the limited colostrum volume.
Relative dose: It has been recognized as one of the most important parameters to determine the safety of a drug in a breastfeeding patient. Drugs with less than a 10% proportion are preferred. It is calculated by dividing the dose found in the infant by the dose received by the mother.
Maternal pharmacogenetic factors: Exemplified in codeine, with excess mother or infant sedation when they are fast or ultra-fast metabolizers, due to genetic excess if cytochrome P450 isoenzyme 2D6 (CYP2D6). This may occur in 3% of Afro-Americans, 10% of Caucasians or 30% of North Africans 12.
Perioperative medications and transfer to breast milk
Most medications are compatible with breastfeeding (Figure 1). Table 3 shows pharmacokinetic data and risks according to E-lactancia.
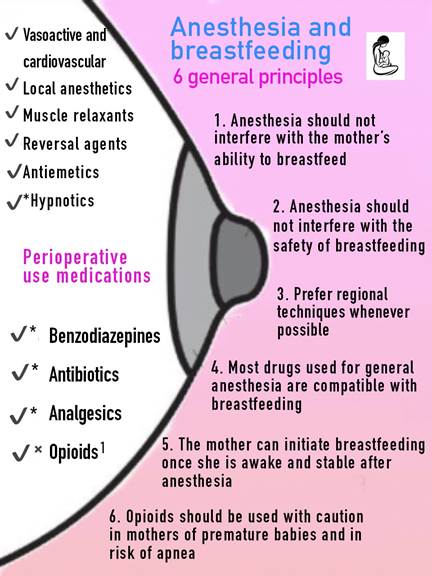
√ Compatible with breastfeeding. √*Most are compatible with breastfeeding. See details in the text. √× Non-compatible or compatible drugs, but with certain use restrictions. Infant monitoring is recommended. See details in the body of the text. 1Single-dose fentanyl (100 micrograms (μg) intravenous) is safe. Morphine and methadone are likely compatible but monitoring is recommended. Meperidine and oxycodone are high risk and have to be avoided. Source: Authors.
Figure 1 General principles of anesthesia/breastfeeding and drug compatibilities with breastfeeding.
Table 3 Pharmacokinetic parameters of the main perioperative anesthetics and risk classification according to E-lactancia.
| Family | Name | Plasma protein binding | Oral bioavailability | M/P ratio (ideal <1)* | Half-life (hrs) | Relative dose (ideal <10 %) | Risk E-lactancia** |
|---|---|---|---|---|---|---|---|
| Inducing agents/Sedatives | Propofol | 97 % | 3-12 | 0.007-0.5 | Very low | ||
| Midazolam | 98 % | 44 % | 0.15 | 2-3 | 0.1-1.4 | Very low | |
| Diazepam | 99 % | 100 % | 0.9 | 43-120 | 0.5-20 | High | |
| Etomidate | 77 % | 1.2 | 1.25 | 0.8-3.9 | Very low | ||
| Ketamine | 47 % | 16 % | 3 | Low** | |||
| Thiopental | 60 % | 0.4 | 12 | 2-6 | Very low | ||
| Opioid analgesics | Morphine | 35 % | 39 % | 1.6-5 | 1.5-2 | 7-15 | Low** |
| Methadone | 60-90 % | 90 % | 0.7-1.2 | 20-45 | 1.2-7 | Very low** | |
| Fentanyl | 80-85 % | 65 % | 2.1 | 2-3 | 1.2-4 | Very low** | |
| Remifentanil | 70% | 2-4% | 0.3 | Low | |||
| Codeine | 7-25 % | 65-100 % | 1.3-2.5 | 3-4 | 0.6-7 | High | |
| Tramadol | 20 % | 60-75 % | 2.4-3 | 6-7 | 2.6 | Very low** | |
| Pethidine | 60-80 % | 50-60 | 1.1-1.6 | 3-5 | 0.6-3.5 | Low** | |
| Oxicodone | 45 % | 60-87 % | 3.4 | 3.5-6 | 2.6-7.6 | High | |
| Non-opioid analgesics | Metamizole | 60 % | 85 % | 1.5 | 10-11 | 3.1-6.2 | High |
| Paracetamol | 10-25 % | 75-85 % | 0.9-1.4 | 1-3 | 2.3-5.2 | Very low | |
| Naproxen | 99.7 % | 95 % | 0.01 | 12-15 | 4.8 | Low | |
| Celecoxib | 97 % | 99 % | 0.3 | 11 | 0.3 | Very low | |
| Ketorolac | 99 % | 81 % | 0.02-0.04 | 4-6 | 0.18 | Very low | |
| Ketoprofen | 99 % | 90 % | 1.5-4 | 0.3 | Very low | ||
| Clonidine | 20-40 % | 65-99 | 2-4 | 6-24 | 13.3 | High | |
| Local anesthetics | Lidocaine | 66-70 % | 35 | 0.4 | 1.8 | 0.8-4 | Very low |
| Bupivacaine | 95 % | 0-1 | 2-4.6 | 0.2-6 | Very low | ||
| Antiemetics | Dexamethasone | 77 % | 70-78 % | 3.3-6 | High | ||
| Domperidone | 91-93 % | 15-20 | 0.25 | 7-9 | 0.01-0.1 | Very low | |
| Ondansetron | 75 % | 60 | 3 | Very low | |||
| Droperidol | 85-90 % | 2.2 | High | ||||
| Muscle blockers | Succinylcholine | 0-1 | 0.013 | Very low | |||
| Rocuronium | 30 % | 0-1 | 1.2-1.4 | Very low | |||
| Atracurium | 82 % | 0-1 | 0.3 | Very low | |||
| Reversal agents | Neostigmine | 15-25 % | 1-2 | 0 | 0.9-1.2 | Very low | |
| Sugammadex | 0 | 0 | 2 | Very low | |||
| Antibiotics | Cefazolin | 89 % | 0-1 | 0.02 | 1-2 | 1.6 | Very low |
| Ceftriaxone | 95 % | 0 | 0.04 | 6-9 | 0.15-0.6 | Very low | |
| Clindamycin | 94 % | 90 | 0.5 | 2.4 | 0.6-5 | Very low | |
| Cardiovascular agents | Atropine | 14 % | 90 % | 4.3 | Very low | ||
| Ephedrine | 85 | 3-5 | Low | ||||
| Phenylephrine | 95 % | 38 | 2-3.4 | Low | |||
| Noradrenaline | 0 | 1 | Very low | ||||
| Labetalol | 50 % | 25-40 | 1-2.6 | 6-8 | 0.05-0.45 | Very low | |
| Others | Pregabalin | 0 | 90 | 0.4-0.8 | 5-7 | 6 | Low |
| Tranexamic acid | 3 % | 30-50 | 0.01 | 2 | Very low | ||
| Omeprazol | 95-97 % | 40-60 | 1-2 | 0.9 | Very low | ||
| Magnesium sulfate | 25-40 % | 4-15 | 2 | 3-4 | 0.2 | Very low | |
| Unfractionated heparin | 95 % | 0 | 0 | 1-2 | Very low | ||
| Enoxaparin | 0 | 0.02-0.1 | 4-5 | 1.3 | Very low | ||
| Dalteparin | 0 | 0.02-0.2 | 3-5 | 1.3 | Very low |
*Some medications have incomplete data because no published information on their excretion in breast milk was found until the last update.
**This classification does is not consistent with some of the recommendations in this paper, based on the LactMed platform and other studies. For additional details, refer to the body of the text.
They are categorized according to their risk, as per E-lactancia, LactMed and they shall be discussed briefly, by family:
• lnducing agents/sedatives: Most inducing agents are compatible with breastfeeding because of low oral bioavailability and short half-life. Mothers can initiate breastfeeding as soon as they are awake, without the need for waiting time. However, sharing the bed at night with the infant is not recommended given the risk of asphyxia. A summary of inducing agents and sedatives is presented in Figure 2.
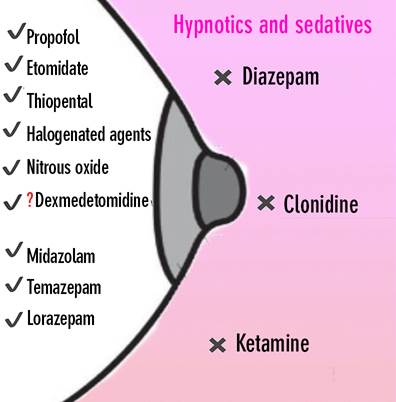
√ Compatible with breastfeeding. x Not compatible with breastfeeding. Must be avoided. √? No recommendations are available, but believed to be compatible. Source: Authors.
Figure 2 Summary of compatibility between breastfeeding and hypnotic and sedative drugs.
The main hypnotic and sedative agents are:
- Propofol: Small quantities (0.0025%) are transferred to breast milk.
- Thiopental: Because of its pharmacokinetic characteristics, quantities in milk tend to be very small.
- Etomidate: Quantities in milk are small and drop rapidly; not found in milk after three hours.
- Ketamine: It is the only inducing agent with scant data regarding transfer to human milk. Moreover, it can create hallucinations in the mother in the first 24 hours, thus failing to meet the principle of initiating breastfeeding as soon as the mother is awake and stable. However, because of its short half-life, rapid distribution from plasma to the tissues (4 minutes) and poor oral bioavailability, it is probably safe during breastfeeding. Hence its classification as low risk by E-lactancia 13. In contrast, the recommendation by LactMed appears to be more conservative in that it states that it should be avoided and, in the event it is used, close monitoring of the infant for signs of dizziness, sedation and lack of appetite is required 11,12.
- Inhaled agents (halogenated gases and nitrous oxide): Because of short half-life and high clearance, their use in anesthesia will not have major effects on subsequent breastfeeding.
- Benzodiazepines: Cenerally used for conscious sedation during procedures and sometimes as premedication. Lorazepam and midazolam are shorter acting than diazepam. Midazolam is the most widely used benzodiazepine. Civen its substantial first pass effect in the liver, bioavailability is low and, therefore, plasma levels in infants are low. A single dose is safe in breastfeeding, which can be initiated as soon as the mother is awake. Short-acting agents at a minimum effective dose are preferable, and repeated and chronic use should be avoided in mothers of neonates and premature babies, because of accumulation potential. In this regard, diazepam should be avoided because of its active metabolite desmethyldiazepam (high risk), which has a long half-life, transferring at significant levels into breast milk 12.
- Dexmedetomidine: There are just a few isolated reports that do not foresee a risk in infants and neonates. The drug is totally absent in milk within 24 hours of completing the infusion.
- Clonidine: It has multiple uses in hypertension, sedation, anxiolysis and analgesia. It can increase prolactin secretion, producing gynecomastia and galactorrhea. Because of this deleterious impact on breastfeeding, not to mention adverse effects on the neonate and high plasma concentrations, the suggestion is to avoid it. However, it has not been studied as a single neuraxial dose to reduce requirements.
• Analgesics: Analgesia must be individualized. If opioids are required, use of the minimum effective dose for the shortest time possible is recommended. Figure 3 illustrates non-opioid/opioid analgesics and their compatibility with breastfeeding.

√ Compatible with breastfeeding. x Not compatible with breastfeeding. Avoid. *Monitor before repeat doses. ** At analgesic doses. Low doses are safe (antiplatelet doses). Source: Authors.
Figure 3 Compatibility summary of opioid and non-opioid analgesics.
The main analgesic drugs are:
- Acetaminophen (paracetamol): Despite some unfavorable pharmacokinetic parameters (Table 3), it is excreted in insignificant amounts in milk, even much less than would be received in a normal oral pediatric dose. Because of liver immaturity in neonates and small infants (with EBF), low levels of cytochrome P450 enzymes hinder acetaminophen conversion into its more toxic metabolites, resulting in very rare instances of liver toxicity.
Non-steroidal anti-inflammatory agents:
- Ibuprofen, ketorolac, ketoprofen, celecoxib and diclofenac are compatible with breastfeeding, making them the first choice, after cesarean section, for example, especially intravenously.
- Naproxen: Because of its longer half-life and case reports of sedation in infants of mothers taking the drug, it should not be the first choice.
- Parecoxib and meloxicam: There is a paucity of data and reports, so the recommendation is to use safer options in lactation.
- Acetylsalicylic acid: Low risk when used in prophylactic anti-platelet doses (75325 mg). No levels of acetylsalicylic acid are detected in breast milk. In fact, it is used in high risk pregnancies to prevent preeclampsia 21. At higher doses for long periods of time (analgesic, anti-inflammatory) it is considered high risk due to the possibility of salicylic acid-related metabolic acidosis. Moreover, there are two old case reports of severe adverse events in infants. Reye's syndrome is associated with the use of this drug in children with viral infections, but risk of this syndrome related to the presence of salicylate in breast milk is unknown.
- Metamizole: Classified by E-lactancia as high risk for breastfeeding. The general recommendation is to use a safer alternative or initiate lactation 48 hours after the last dose 20). Metabolites are present in milk in significant amounts, and there is one published case of cyanosis and respiratory depression related to the use of this drug. A higher risk of acute lymphocytic leukemia has been reported in infants whose mothers received it during pregnancy and breastfeeding 13. Consequently, the FDA and the European Medications Agency (EMA) have limited the dose and use of this drug mainly due to adverse reactions and the risk of agranulocytosis in infants/children, and during breastfeeding 22. However, there are opponents, mainly in Israel and Latin America, where it is widely used, suggesting that the particular risk of agranulocytosis is associated with a specific HLA allele, and is of high risk among populations of celtic breton ancestry, while risk is nil or inexistent in other populations 23.
- Opioids: This group comprises drugs that pose the highest risk for breastfeeding, hence the importance of making clear distinctions among the various opioids. Accordingly, a single intravenous dose of fentanyl, commonly used in general anesthesia, is safe and compatible with lactation. On the other hand, neuraxial use of opioids does not pose a significant risk.
- Morphine: Excreted in milk in negligible amounts. Historically, it has been considered as one of the opioids of choice during the postpartum period when potent intravenous analgesia is required. However, there is also compatibility between other opioids and breastfeeding, including fentanyl, methadone and remifentanil. Moreover, when used at repeated intravenous doses, during the postoperative period for example, monitoring of the infant for signs of sedation, lack of appetite and respiratory depression is recommended.
- Methadone: Excreted in non-clinically significant amounts, with no problems reported in infants. In case of repeat doses, monitoring is recommended. Very low risk because of a lower milk/plasma ratio and relative dose as compared to other opioids.
- Fentanyl: Approximately 100 times more potent than morphine, highly lipophilic and potentially stored in mammary tissue. A study that assessed 5 women who received intravenous fentanyl 100 µg on anesthetic induction, found that less than 0.1% of the drug was present in breast milk 24. Because of its pharmacologic characteristic (low bioavailability and short action) it is a safe and very low risk in lactation, hence the recommendation to initiate breastfeeding in the immediate postoperative, provided the mother is alert and a reasonable (2 µg/kg) single dose of fentanyl has been used. The same applies to alfentanil.
- Remifentanil: Although there are not very many studies on remifentanil in breastfeeding, because of low oral bioavailability, context-sensitive half-life independent from infusion duration, metabolization by plasma stereases and high binding protein, it is an ideal opioid for breastfeeding mothers, especially if minimum postoperative pain is expected. 25. Concomitantly, this can be extrapolated to its intravenous use in analgesia during labor in cases of contraindicated neuraxial techniques, with evidence of significant advantages as compared to all other opioids, and minimum neonatal effects 18.
- Codeine: It is one of the drugs that should be avoided in breastfeeding mothers. Because of the considerable genetic polymorphism of the isoenzyme responsible for its metabolization, "ultra-fast" metabolizers produce high concentrations of morphine in breast milk which can result in neonatal depression in extreme cases 26. High fat solubility and low plasma protein binding allow secretion in breast milk, with the resulting effect on the infant. If the mother has already been taking codeine, she must be advised to discontinue or discard milk for a period of 15 hours. After this time, transfer is negligible 27.
- Tramadol: The FDA warning and some case reports of neonatal respiratory depression appeared to have placed a death sentence on tramadol in puerperal women 27). However, there are opponents who point to substantial differences with codeine: The M1 metabolite of tramadol has a weak opioid effect and, moreover, metabolization to M1 is diminished in small infants, with very small amounts excreted in milk 28. Notwithstanding, it is not an ideal drug due to its properties (Table 3) and the incidence of dizziness, nausea and vomiting in the mother. Therefore, when and if it is used, monitoring the infant for drowsiness, lack of appetite, respiratory depression and sedation is required.
- Oxycodone: Another opioid to be avoided because infants may receive >10% of the therapeutic dose. Its pharmacogenomic metabolism is similar to that of codeine and, moreover, there are case reports of sedation/respiratory depression in infants exposed to breast milk. The risk is higher if the mother receives doses higher than 30 mg/día 29.
- Hydromorphone: Almost 7 times more potent than morphine and has a 62% oral bioavailability 12. There are no major compatibility studies and it is classified as low risk by E-lactancia, albeit on the basis of scant information 13. However, it has been estimated that an infant on EBF would receive 0.15 µg/kg per day from a single maternal 2 mg dose of intranasal hydromorphone 12. Additionally, there is one report of respiratory depression in one 6-day old infant, requiring reversion with naloxone 30. Therefore, its use is not recommended, supporting the statement by LactMed 12.
- Pethidine or meperidine (demerol): Because of the long half-life of its metabolite (norpethidine), it is advisable to prefer other drugs, especially in cases of premature babies. Its use as continuous analgesic in labor can delay breastfeeding initiation 31.
• Local anesthetics: Their physical and chemical characteristics are ideal because they are long, polarized molecules that cannot easily cross maternal mammary ducts. Negligible concentrations of local anesthetics like bupivacaine and lidocaine have been demonstrated in breast milk. Concentrations of the pipecolylxylidide metabolite of bupivacaine were measured in breast milk and maternal blood, concluding that there was minimum transfer of the anesthetic and its metabolite and that it could be used safely. 16. Moreover, good pospartum pain control has been shown to promote earlier breastfeeding, so peridural local anesthetic injection after umbilical cord clamping, or adding levobupivacaine to wound infiltration in cesarean section enhances the success of breastfeeding.
• Neuromuscular blockers: The assumption is that these agents do not cross the blood-milk barrier, because of their properties: polarized quaternary ammonium complexes poorly soluble in fact, poor oral bioavailability, and high molecular weight. Because of its high ionization a physiologic pH, poor oral absorption and rapid clearing from maternal plasma, succinylcholine is also compatible with breast feeding, which can be initiated when the mother is awake after general anesthesia 18.
• Muscle relaxation reversal agents: Neostigmine and sugammadex are compatible with breastfeeding. Some cases of abdominal cramps in neonates, but of no great consequence, have been described with neostigmine (12. Civen that sugammadex could retain progestogens in its structure, it is recommended that puerperal women taking contraceptives should use a barrier method during the first 7 days after its administration 32.
• Antiemetics: Although there are no major studies on the transfer of setrons (granisetron and ondansetron), they are drugs of choice in the postpartum period because of their high distribution volume and short half-life that reduce the likelihood of excretion in significant amounts in breast milk. Dexamethasone, used widely in general anesthesia for nausea and vomiting prohylaxis, has shown to reduce the production of prolactin, but its short-term use is compatible with lactation. Moreover, patients with postoperative vomiting would have difficulty breastfeeding and, for this reason, prophylaxis offers more benefits than risks. Metoclopramide and domperidone, apart from being antiemetics are also used as galactogogues and could increase milk production. Droperidol, also used to treat postoperative nausea and vomiting, has favorable pharmacokinetic parameters (high protein binding percentage and short half-life), making it compatible with breastfeeding.
• Cardiovascular drugs
- Atropine: In isolated systemic doses like those used together with neostigmine, it is compatible with breastfeeding. In additional repeat doses, it may reduce milk production by altering prolactin and oxytocin secretion, and also induce muscarinic anticholinergic effects in the neonate. Therefore, monitoring is required.
- Ephedrine: Low risk. Civen its wide distribution volume, it is unlikely that it may cause effects in infants.
- Phenylephrine: It is one of the vasoactive agents of choice after spinal anesthesia in cesarean section. It is also compatible with breastfeeding given its poor oral bioavailability (38%) and high plasma protein binding.
- Ethylephrine: Though there is a paucity of data regarding its excretion in breast milk, it is probably compatible with lactation due to its short half-life and low oral bioavailability 13.
- Noradrenaline and adrenaline: Very low risk. Compatible with breastfeeding.
- Betablockers: Labetalol, metoprolol and propanolol (very low risk). Atenolol would be the exception (high risk) given clinically significant excretion in breast milk and the potential to affect the infant 13.
• Antibiotics: Only those that are used in antibiotic prophylaxis will be discussed.
- Cefazolin, ceftriaxone, ampicillin-sulbactam: Very low risk drugs, safe in lactation. It is normal to expect negative cultures in febrile infants and in infants with gastroenteritis due to altered gut flora 33.
- Clindamycin and metronidazole: One single dose is probably compatible with lactation, but it could alter the gut flora to a greater degree, causing diarrhea, candidiasis or, more rarely with clindamycin, pseudomembranous colitis.
• Other postoperative medications: Omeprazole, pregabalin, ranitidine, tranexamic acid, unfractionated heparin and low molecular weight heparins are compatible with lactation. For more details, refer to the sites mentioned previously.
Breastfeeding and social "drugs"
Though not anesthetic drugs, they are commonly used, and it behoves anesthetists to include their discussion as part of their duty to promote health. Tobacco, tetrahydrocannabinol (THC or marihuana), an alcohol must be avoided and considered as drugs that have the ability to cross the blood-milk barrier. Smoking, including passive smoking, is associated with sudden infant death. Motor development impairment was found after one year of follow-up of mothers who used marihuana 34.
Scenarios
With the aim of providing a more practical perspective of compatibility between anesthesia and breastfeeding, the four most common potential scenarios will be used as examples.
Pregnant woman in labor concerned about the effect of epidural anesthesia on breastfeeding
Neuraxial analgesia is the most effective technique to diminish pain in labor. The drugs most commonly used are a combination of local anesthetic and an opioid like fentanyl, because of their synergistic effects.
Because of their low oral bioavailability and physical and chemical properties, local anesthetics are safe and compatible with breastfeeding.
Adding fentanyl to the combined or the traditional epidural technique allows for lower concentration and a lower mass of the local anesthetic, helping to reduce maternal motor block and preventing negative effects from the epidural on the rate of instrumental delivery. However, epidural doses of more than 150 µg could influence breastfeeding, with reports of up of 19% difficulty with breastfeeding in the first 6 weeks, as compared to 2 and 6% in the group receiving lower or no doses of epidural fentanyl, respectively 35,36. Although it was statistically significant (p = 0.002), it may be marginal in clinical terms. Added to this, more recent reports like that by Lee et al., have stated that epidural solutions containing fentanyl concentrations as high as 2 µg/mL do not influence the rates of breastfeeding 6 weeks into the postpartum period 37. Finally, breastfeeding effectiveness was the same, with no difference in the neurobehavioral status of the infant between the mothers who received epidural analgesia and those who did not 38.
Answer to scenario 1
Neuraxial analgesia for labor is compatible with breastfeeding.
Patient scheduled for elective surgery who is concerned about the effect of spinal anesthesia on breastfeeding
There are cesarean section-related factors that can interfere with the initiation and duration of breastfeeding: increased pain, nausea and vomiting, the use of a larger number of drugs, potential for reoperation and complications such as fetal distress and postpartum bleeding 39,40. Intrathecal fentanyl is undetectable (<0.1 µg/L) in colostrum within 1 hour of its administration, not impacting breastfeeding rates and neonatal APCAR scores 41.
On the other hand, intrathecal morphine has an oral bioavailability of 30% and its metabolite -morphine-6-glucuronide- is pharmacologically active and more potent, resulting in lower bioavailability. Sedation in 50% of infants with morphine plasma concentrations of 125 ng/mL has been described in different reports. Feilberg et al. reported an 82 ng/mL morphine peak in breast milk 30 minutes after an epidural 4 mg morphine bolus 16,42.
Thus, intrathecal morphine in the usual doses of 50-100 µg can be used safely (in the absence of contraindications or risk factors for maternal respiratory depression) for intra and postoperative analgesia in patients taken to cesarean section who wish to initiate breastfeeding right away. In fact, according to the latest guidelines, it is the standard recommendation for pain treatment after cesarean section 43.
Postoperative pain is one of the factors leading mothers undergoing cesarean section to report difficulty carrying the infant and putting it to the breast 43. Anesthetists must identify a strategy to avoid postoperative pain, considering that many intravenous opioids (most widely used in immediate postoperative pain) can interfere with effective breastfeeding, particularly when given in repeated doses, and prioritize regional techniques (neuraxial morphine or abdominal wall block). Adequate pain management after cesarean section has been found to be associated with increased neonatal ponderal index gain 15.
Another factor that could impact early breastfeeding discontinuation is postoperative nausea and vomiting, which increase with neuraxial morphine. The use of dexamethasone and/or ondansetron could diminish those symptoms after cesarean section, if neuraxial morphine is administered 44,45.
Finally, keeping the mother-infant pair together is a safe and healthy practice. Evidence has shown that constant skin-to-skin contact during and after cesarean section promotes optimal maternal and fetal outcomes (46,47.
Answer to scenario 2
Spinal anesthesia for cesarean section is compatible with breastfeeding. Adequate postoperative analgesia should be advocated, ideally using intrathecal morphine to help mothers initiate early breastfeeding. Intraoperative skin-to-skin contact techniques result in higher maternal satisfaction and could influence a more successful EBF.
Patient who will receive general anesthesia for cesarean section
Close to 0.5-1% of cesarean sections are performed under general anesthesia. International guidelines recommend the use of neuraxial over general anesthesia in cesarean section, mainly due to the risk of difficult tracheal intubation, gastric content aspiration, better postoperative pain control and easier immediate skin-to-skin contact. Moreover, it also promotes mobility and faster return to everyday activities, with improved quality of life scores 48.
Mothers who receive general anesthesia for cesarean section do not initiate breastfeeding within the first hour of life, and this is associated with a lower frequency of EBF at 6 weeks and at 6 months when compared with neuraxial anesthesia 12,49.
If general anesthesia is selected, drugs compatible with breastfeeding, as described above, must be given priority, and the 6 principles must be followed (Figure 1), selecting a regional method (for example, transverse abdominis plane or TAP block) for postoperative pain control. If the mother is alert and stable, it is advisable to initiate breastfeeding as soon as possible, monitoring the neonate for eventual occurrence of sedation or respiratory depression. In hypotonic premature infants - and if the mother received higher and repeat opioid doses - it is advisable to discontinue lactation for a short period of time. If the mother is alert and stable, it is advisable to initiate breastfeeding (6-12 h). Finally, sharing the bed with the infant is not advisable when general anesthesia has been used.
Answer to scenario 3
Breastfeeding is less effective in cesarean sections under general anesthesia. Optimal pain and nausea/vomiting management is required so that the mother can initiate breastfeeding on awakening, monitoring the neonate for the onset of symptoms.
Puerperal patient with exclusive breastfeeding requiring elective or urgent surgery
Preanesthetic assessment is crucial to clear doubts and establish an intra and postoperative plan that is more compatible with breastfeeding. Mothers should be advised that pumping and dumping after anesthesia is not necessary. If prolonged surgery is anticipated and the child cannot remain with the mother, the need to pump and store milk beforehand has to be discussed. This must be carried out with the participation of an expert in breastfeeding.
The 6 principles illustrated in Figure 1 should be applied in this case. Regional intraoperative and postoperative techniques are the best choice to reduce the need for systemic analgesics. If general anesthesia is selected, opioid-sparing (single initial dose) or opiod-free techniques can be an effective option.
Antiemetic prophylaxis should be used to make breastfeeding easier during the postoperative period.
If general anesthesia is administered, the use of opioids is more likely. The effect may vary depending on pharmacokinetic (greater or lesser metabolism) and pharmacodynamic differences (different sensitivity to the same plasma level) (18.
It is advisable to assess for signs of excess opioid use in the mother as an indicator of potential effects on the infant, and avoid their use especially in mothers with infants less than 6 weeks of age, due to the higher risk of liver and renal immaturity. If opioids are required, single-dose fentanyl and morphine are the most appropriate.
Postoperative pain will be less if regional analgesic techniques are used together with general anesthesia (neuraxial or peripheral nerve block). Concomitant multimodal analgesia with non-opioid analgesics such as paracetamol and NSAID, both compatible with breastfeeding, should be indicated. If postoperative intravenous opioids are still required, the lowest effective dose should be used for the shortest time possible.
Outpatient surgery should be the first option in a breastfeeding woman. It is recommended that an adult caregiver remain with the patient during the first 24 hours after discharge to the home. Counseling and support should be offered in the event the mother needs to remain in the hospital for one or more days because of complications or any other reason 18.
Finally, given the current pandemic context, it is advisable to continue breastfeeding in case of maternal COVID-19 infection. The benefits of breastfeeding outweigh by far the potential risk of viral transmission, as long as hygiene and preventive measures are maintained 50.
Answer to scenario 4
Asking about breastfeeding should be part of the preanesthetic assessment. Most anesthetic drugs are compatible with lactation, and neuraxial techniques should be preferred in order to allow the mother to breastfeed as soon as she is awake and stable 51. Some intravenous opioids must be used with caution, in particular when repeat doses are given.
CONCLUSIONS
Breastfeeding is safe after anesthesia, considering that most drugs are compatible. The recommendations of this review are summarized in Table 4.
Table 4 Recommendations regarding breastfeeding and anesthesia.
| 1. | Puerperal women should be encouraged to initiate breastfeeding after surgery. |
| 2. | Anesthesia should not interfere with the safety of breastfeeding. |
| 3. | Neuraxial techniques are the most effective for pain control during labor and favor early initiation of breastfeeding. |
| 4. | Spinal anesthesia in cesarean section allows for immediate skin-to-skin contact and adequate pain management as compared to general anesthesia. |
| 5. | As a routine, all women with children under two years of age should be asked about breastfeeding during the preanesthetic assessment. |
| 6. | Milk pumping and dumping is not necessary in puerperal women after surgery. If a prolonged surgery and/or repeated postoperative use of intravenous opioids, or if the infant cannot stay with the mother, the need to pump and dump should be discussed in advance with an expert in breastfeeding. |
| 7. | Non-opioid anesthetic and analgesic drugs are compatible with breast milk given that they are transferred in very small amounts and cause no effects on the neonate. |
| 8. | Codeine should not be used in lactation due to some reports of deep sedation in infants related to differences in metabolism. |
| 9. | Drugs such as opioids and long-acting benzodiazepines must be used cautiously in mothers of children up to 6 weeks of age, especially after multiple doses. The infant must be monitored for signs of drowsiness and respiratory depression, even more so if the mother exhibits signs of sedation. |
| 10. | Opioid-sparing techniques or opioid-free general anesthesia are the preferred options in puerperal women. Regional anesthesia without sedation offers ample benefits in this regard because it interferes the least with the mother's ability to care for her child. |
| 11. | Outpatient surgery, if possible, should be the selection of choice to avoid disrupting normal routines. |
| 12. | Puerperal patients must have access to postoperative counseling and breastfeeding support. Likewise, updated information (brochures, informative social media) on compatibility between breast milk and the various anesthetics, as well as guidelines focused on promoting breastfeeding during the perioperative period must be made available. |
Anesthetists must act as agents for the promotion and maintenance of breastfeeding and must be willing to answer questions during the preoperative period and prepare an anesthesia plan that will not interfere with the safety of breastfeeding. Regional techniques, opioid-sparing techniques and outpatient surgery are preferred. Multidisciplinary support during the perioperative period as well as accurate counseling will ensure minimal disruption of this very important aspect of child care.











 texto en
texto en 


