INTRODUCTION
Medication errors are a cause of severe lesions and serious perioperative complications. These events are the result of poor standardization of the process of preparation, administration and labeling, particularly with regards to injectable medications.
During anesthesia, several drugs are simultaneously administered, which result in a wide range of effects, in an stressing environment with multiple distractions and a significant work load. On many occasions, there is poor visibility and in some drugs there is often a change in their presentation (depending on changes of pharmaceutical vendors). The process of preparation and administration of injectable drugs is not homogeneous and is sensitive to various causes of error. It is well recognized that medication errors during the perioperative period are a significant source of morbidity and rarely of fatalities. The incidence of error in anesthesia is estimated between 1:133 and 1:274 anesthesia procedures 1 and mortality as a result of anesthesia errors associated to medications is estimated to be 1:298 (0,3 %). 2 Considering that in average each anesthesiologist is responsible for four anesthesia procedures per day (1,252/ year), it is possible to have between four to nine errors of this nature per year. Over 30 years of practice, and if 1% have severe consequences, one to two patients may experience a severe of fatal lesion. 3
Some of the most frequently reported errors associated with the use of medications, include: 1) confusion with the route of administration (intravenous, arterial, epidural); 2) confusion with vials and syringes (Look-Alike Sound-Alike "LASA"), and 3) errors in the medical devices for administration of medications. 4
Historically, various strategies have been introduced to mitigate medication errors, including the use of color labels based on the pharmacological group and the introduction of capital letters to facilitate a rapid identification, concentration and route of administration. An analysis of critical incidents in anesthesia found that the introduction of labeling of syringes reduced the number of errors in the administration of mediations from 1:484 to 1:817 anesthesia procedures (59 %). 5
These are some of the recommendations used to reduce anesthesia medication errors: 1) careful reading of drug label before it is prepared and injected by the practitioner; 2) double check (nurse and anesthesiologist); 3) legible labeling of syringes and vials (color and letter type which facilitates an adequate identification of the manufacturer and the label placed on the syringe that will be used); 4) have the medications neatly organized in the work area used by the practitioner, and 5) proper storage. 6,7
The Safety and Quality Guidelines in Anesthesia of the European Union and the Declaration of Helsinki on patient safety, recommend implementing protocols ad guidelines for labeling of medications used in anesthesia, with a standardized international color code. 8,9
COLOR CODE EXCLUSIVELY FOR ANESTHESIA
A color-based classification code has been established to identify the various groups of pharmacological products which has been adopted and standardized by the Anesthesia Societies of various countries, including the United States, Canada, Spain, England and the United Kingdom, Australia and New Zealand, France, and Italy. 10-17
Following is a list of the groups of medicines frequently used in anesthesia (Table 1).
Table 1 Medications frequently used in anesthesia and their color code.
Source: Adapted from:
-Royal College of Anaesthetists, Association of Anaesthetists of Great Britain and Ireland, Faculty of Accident and Emergency Medicine, Intensive Care Society. Syringe labelling in critical care areas review 14.
- American Society of Anesthesiologist. Statement on labeling of pharmaceuticals used in the practice of Anesthesiology 10.
- Canadian standards association. Standard for use-applied drug labels in anesthesia and critical care 13).
- Sicurezza in Anestesia e Area Critica per la somministrazione dei farmaci 17.
- Recommendations for labeling of injectable drugs administered in anesthesia. Spanish reporting
system on Anesthesia and Resuscitation Safety - SENSAR 18.
φ ASTM: American Society for Testing and Materials
ᵮ RGB S3 color combination system: red, green and blue
* ISO: International Organization for Standardization
Ϫ Pantone System - a registered universal color coded scale (https://www.pantone.com/eu/es/)
RECOMMENDATIONS FOR THE PREPARATION OF MEDICATIONS IN ANESTHESIA
The following recommendations are derived from the recommendations of the Spanish Reporting System on Anesthesia and Resuscitation for labeling of injectable drugs administered in anesthesia. 18
Before preparing a medication it should be properly identified and the manufacturer's instructions for preparation and administration should be read thoroughly.
All syringes, bags or bottles containing medicines shall be labeled with the name of the medication inside.
Whenever possible, the same person should do preparation, labeling and administration of medications.
Any syringe or bag prepared should be labeled immediately, as soon as the medication is transferred.
Medicines should be prepared for each individual patient. Do not prepare the medications for several patients at the same time.
Each medication administered shall be recorded in the anesthesia record.
The lines or catheters of the systems used for the administration of medications shall be labeled (peripheral vein, central venous catheter epidural, intrathecal and intra-arterial).
Any of the drugs used in regional anesthesia should be stored in a separate area from the other medicines.
The use of more than one concentration of high risk medications, such as morphine, phenylephrine, etilefrine, heparin, etc., should be avoided.
LABEL
The following are the recommendations for labeling of injectable medications administered in anesthesia.
Generic name and concentration (units in mL) (the most common).
Date and time of preparation and initials of the person who prepared the medicine.
The name of the patient and route of administration may also be included.
Infusion bags: Place the total volume and the generic name and concentration (units per mL), date and time of preparation, initials of the person who prepared the medication and name of the patient.
Letter font and printing characteristics of the label for syringes
The text in the labeling should be designed for improved legibility of the medication and its concentration. These standards include recommendations on the size of the font, extra spacing for separating the name of the medication to facilitate reading and additional emphasis on the initial syllable or differentiating syllable for similar names.
Contrasting letters in the syringe labels
Except for the colors reserved for anesthesia, maximum contrast should be used between the text and the background with predetermined color combinations to avoid insufficient color contrast that makes reading difficult. Example: black letters over white background, blue letters over white or yellow background, white letters over blue background.
SYRINGE LABELING
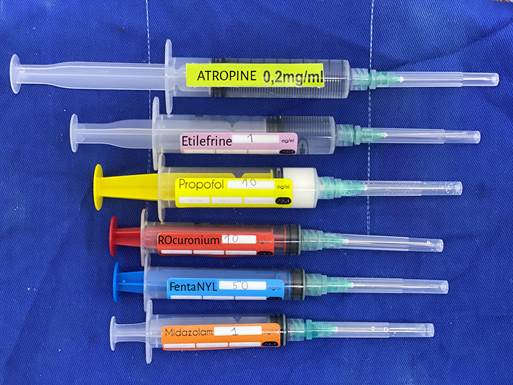
Each label should measure between 3.5 and 4.5 cm long and 1.5 to 2.5 cm de wide; made of adhesive material adequate to write with ballpen or any other indelible ink. The label is placed horizontally without covering the numbered markings on the syringe (Figure 1).
Other recommendations associated with labeling:
The color should be consistent with the Pantone® scale color code.
Antagonistic drugs are identified using diagonal 1 mm-long bars with the same color as the agonists tilted at 45° and alternating with white 1mm bars. The name of the drug is in the middle of the label with no bars around the name of the product.
The color of the letters should be black for improved contrast, except for adrenaline and succinylcholine; these
should be written on a black strip in the same color of the background .
For the text a legible font type should be used; Arial 10 at least.
The label shall show the generic name of the medication and the concentration in mL.
For drugs with similar names, - look alike, sound alike (LASA) - capital letters may be used in the name to avoid errors.
Micrograms should be written as mcg instead of μg (Table 2).
Table 2 Labeling of medications used in anesthesia and their color code.
Source: Adapted from:
- Royal College of Anaesthetists, Association of Anaesthetists of Great Britain and Ireland, Faculty of Accident and Emergency Medicine, Intensive Care Society. Syringe labelling in critical care areas review (14) .
- American Society of Anesthesiologist. Statement on labeling of pharmaceuticals used in the practice of Anesthesiology (10) .
- Canadian standards association. Standard for use-applied drug labels in anesthesia and critical care (13) .
- Sicurezza in Anestesia e Area Critica per la somministrazione dei farmaci (17) .
- Recommendations for labeling of injectable drugs administered in anesthesia. Spanish reporting system on
Anesthesia and Resuscitation Safety - SENSAR (18) .
LIST OF MEDICATIONS IN CAPITAL LETTERS
Since 2001 the FDA required the manufacturers of generic drugs with similar names to use a combination of Tall Man bold letters to minimize LASA errors and facilitate their identification, since the use of color based on the pharmacological group was not found to be effective enough 19-21.
These capital letters are not necessarily at the beginning of the name but may be used anywhere and it could 1 or more letters, to differentiate the molecules with a similar name (Table 3).
Table 3 List of medications frequently used in the OR, with a combination of capital letters.
| ceFAZoline | EPINEPHrine |
| cafaLOTiNE | fentaNYL |
| dexameTASONE | PHENobarbital |
| dexmedeTOMidine | HYDROcodone |
| diazePAM | HYDROmorphone |
| DOBUTamine | hydrOXYcine |
| DOPamine | oxyCODONE |
| ePHEDrine | prednisoLONE |
| propOFol | VECuronium |
| traMADol | CISATRacurium |
| tRAMadol | |
| REMIfentanil | LIDOcaine |
| ROcuronium | OXYtocin |
Source: Author.
It should be highlighted that, notwithstanding the implementation of these measures (such as color coding in anesthesia) implemented over two decades ago, its use is not yet widespread in the institutions in our country. Furthermore, compliance has been poor in the institutions where the label methodology is available.
There is a need to create awareness about the importance of using this type of measures in our country and in other countries in the region. Undoubtfully, training, implementation and management by the scientific societies and government agencies may translate into safer surgical care. It is essential to adopt guidelines for labeling of medications used in anesthesia at the national level. Finally, the implementation of a color code, and the inclusion of Tall Man Lettering are most helpful for the correct identification of anesthesia agents. Finally, the implementation of a color code, as well as the inclusion of Tall Man Lettering are extremely helpful for the correct identification of the drugs to be administered and represent an additional protective barrier for a safe anesthesia. Actively improving the implementation of these measures, with the involvement of the pharmaceutical industry and the national policies, with help in reducing these complications.











 text in
text in