What do we know about this problem?
Excessive fasting produces catabolism, dehydration, and irritability in children.
The elevation of ketone bodies in the blood reflects the catabolic state triggered by fasting in children.
Despite the recommendations of the preoperative fasting guidelines of different scientific societies to reduce fasting times, preoperative fasting times continue to be prolonged.
INTRODUCTION
The body has regulatory mechanisms designed to meeet the energy demands during prolonged fasting periods. Large amounts of fatty acids are transformed into ketone bodies via lipolysis in the liver and muscle glycogenolysis, which is subsequently used as a source of energy. 1 However, the small stores of liver glycogen and the high energy demands of infants and newborns leads to the development of earlier and more intense catabolic response than adults, even during short fasting periods, which are manifested through the increase of ketone bodies in the blood. 2
Additionally, the metabolism of carbohydrates, lipids, and proteins is affected by the release of counterregulatory hormones during the circadian cycle. During the day, food intake is accompanied by an increase in insulin levels creating an anabolic effect and leading to glycogenesis and lipogenesis. During the night, circulating insulin levels fall and counterregulatory hormone levels increase (mainly growth hormone, leptin, and glucagon), which, together with the absence of food intake, favor systemic catabolism: glycogenolysis, gluconeogenesis, and lipolysis. 3
In the perioperative setting, the time of surgery determines the type of fasting (day or night), that the patient shall undergo. However, the metabolic consequences and the degree of catabolism with regards to the circadian cycle of insulin release and counterregulatory hormones described are not fully known in the pediatric population. Therefore, the main objective of this study was to assesss the effect of the preoperative fasting time (day or night) on the preoperative concentration of ketone bodies in children younger than 48 months scheduled for outpatient surgery. The secondary objective was to determine the relationship of ketonemia with the type of food eaten, blood glucose levels, and the level of preoperative anxiety and dehydration.
MATERIALS AND METHODS
The study was approved by the ethics committee of the Rafael Henao Toro Children's Hospital in Manizales, Colombia (IRB number CBCS-091). The procedures were conducted in accordance with the Declaration of Helsinki -2013. An observational, prospective study was conducted between September 2020 to March 2021. Patients under 48 months of age, ASA (American Society of Anesthesiologists) I and II scheduled for outpatient surgery were included. Patients who received intravenous fluids during the fasting period, those with diabetics, on chronic or recent (within a week before surgery) steroid use, emergency cases, and hospitalized before the procedure were excluded.
Fasting recommendations were given to parents and patients according the ASA preoperative fasting guidelines (6 hours for solid foods, 4 hours for breast milk, and 2 hours for clear liquids). Patients with scheduled diurnal fasting with surgery in the afternoon (surgeries performed after 12:00 m) were assigned to group A and overnight fasting patients scheduled for morning surgery (performed between 7 am and 11:59 am) to group B.
In the preoperative admission area, the anesthesiologist interviewed the patient's parents and/or legal guardian and recorded the following patient data: age (months), sex (male, female), weight (kilograms), time of last food intake (hours and minutes) and type of food eaten (clear liquid, breast milk, formula milk, or solids) and assessed the degree of anxiety using a visual analog scale on a 100-mm line. A value > 30 mm was indicative of preoperative anxiety 4. The presence of one of the following signs: dry mucous, capillary filling > 2 seconds, or crying without tears was considered dehydration5. Additionally, a 5 uL capillary blood sample was obtained to measure the concentration of Beta-Hydroxybutyrate and glucose using the Freestyle Precision Neo H equipment (Abbott Laboratories, UK). A value greater than 0,5 mmol/L of Beta-Hydroxybutyrate was considered positive for ketonemia and less than 70 mg/ dL for hypoglycemia in all age ranges.
The patients were assigned to groups A or B according to the time of surfery, as discussed above. The sample size calculated for a significance level of 95% and a power of 80% was 74 patients based on the following outcome variables: preoperative anxiety, dehydration, and ketone bodies, with a Fleiss correction factor. Data were presented using median and interquartile ranges given the non-normal distribution estimated using the Kolmogorov Smirnov test. The weight was normally distributed, thus mean and standard deviation was used. Either the chi-squared (x2) test or the Fisher's exact test was used to compare categorical variables, as appropriate, according to the type of fasting and the presence of elevated ketonemia. For the univariate analysis of ketone bodies concerning age and weight, the Student's t-test was used; for fasting hours the Kruskal-Wallis test. Logistic regression analysis was performed to detect the variables that were associated with elevated ketone bodies (day or nighttime fasting, type of food, glucose). The level of significance was established with a P value of < 0.05. All analyses were performed in Stata 16.1 (StataCorp, Tx USA).
Sociodemographic information
A total of 92 patients were included, 40 in group A and 52 in group B (Figure 1). The median age was 24 months for group A (IQR 12.5 - 33.5) and 31 months for group B (IQR 21 - 38.5). No differences were found between the two groups in terms of sex, gender, and type of surgery. The characteristics of the patients included are summarized in Table 1.
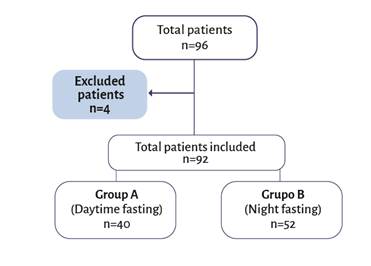
Four patients were excluded due to data loss. A total of 92 patients were included. Source.Authors.
Figure 1 Patient recruitment flow diagram.
Table 1 Clinical and demographic characteristics.
| Group A Daytime fast (n=40) | Group B Night fast (n=52) | P | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (Months) | 0.02 a | ||||
| Median | 24 | 31 | |||
| Interquartile Rage | 12.5 - 33.5 | 21 - 38.5 | |||
| Age group (Months) | 0.005 b | ||||
| <12 | 9 | 22.5 | 1 | 1.9 | |
| 12 - 23 | 11 | 27.5 | 14 | 26.9 | |
| ≥ 24 | 20 | 50.0 | 37 | 71.2 | |
| Gender | 0.38 c | ||||
| Female | 15 | 37.5 | 15 | 28.8 | |
| Male | 25 | 62.5 | 37 | 71.2 | |
| Weight (kg) | 0.01 d | ||||
| Mean/SD | 11.4 ± 0.46 | 12.9 ± 0.39 | |||
| Surgery | 0.47 b | ||||
| Pediatric Surgery | 19 | 47.5 | 36 | 69.2 | |
| Plastic Surgery | 7 | 17.5 | 4 | 7.7 | |
| Gastroenterology | 2 | 5.0 | 1 | 1.9 | |
| Neurosurgery | 1 | 2.5 | 1 | 1.9 | |
| Dentistry | 2 | 5.0 | 2 | 3.8 | |
| Orthopedics | 5 | 12.5 | 5 | 9.7 | |
| Otorhinolaryngology | 4 | 10.0 | 3 | 5.8 | |
| Type of food | 0.000 b | ||||
| Clear fluids | 27 | 67.5 | 4 | 7.7 | |
| Solid | 2 | 5.0 | 26 | 50.0 | |
| Breastmilk | 9 | 22.5 | 4 | 7.7 | |
| Milk Formula | 2 | 5.0 | 18 | 34.6 | |
| Fasting hours | |||||
| Median | 5.2 | 11.2 | 0.000 a | ||
| Interquartile range | 4.3 - 6 | 10 - 13 | |||
a Kruskal-Wallis test; b Fisher's exact test; cx2 test; d t Student test. SD: Standard desviation.
Source: Authors.
Fasting time and adherence to the preoperative fasting guidelines
There was a significant statistical difference between nocturnal fasting time versus diurnal fasting time, with a median of 11,2 hours (IQR: 10 - 13) and 5.2 hours (IQR: 4.3 - 6), respectively (P < 0.0000). Subgroups by type of food and their corresponding medians of the preoperative fasting times are shown in Table 1.
The analysus of the total number of patients showed that 9.6% (88 patients) had at least one hour of excess fasting according to preoperative fasting guidelines with no difference between the groups (Group A: 95%, 95% CI 89.2 - 99.1 and Group B96.l%, 95% CI 80.2-98.7, P = 0.58). A total of 48.9% presented to surgery with ketonemia (Figure 2y 3).
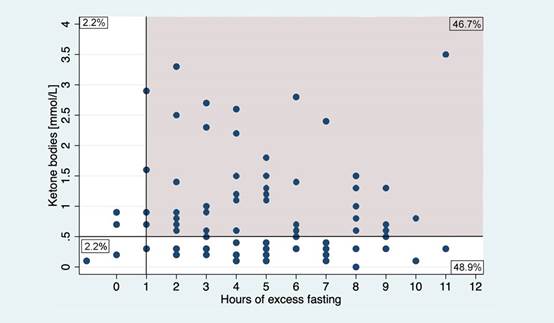
The gray área represents the patients who had an excess of at least 1 hour of fasting in relation to the ASA pre-surgical fasting guidelines. Source. Authors.
Figure 2 Ketone by hours of excess fasting among all patientas (n:92).
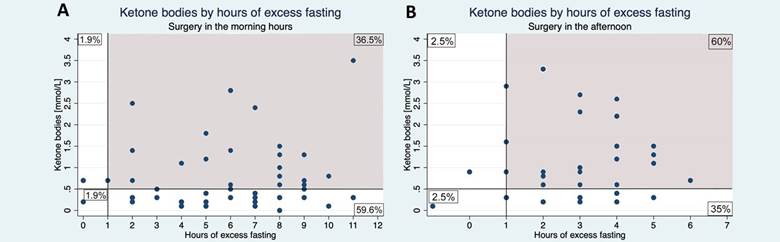
The gray area represents patients who had an excess of at least 1 hour of fasting to the ASA pre-surgical fasting guidelines. Source. Authors.
Figure 3 Ketone bodies levels in groups A and B according to excess fasting.
Ketone bodies and glucose blood levels
The median of ketone levels in group A was 0,85 mmol/L (IQR: 0.3 -1.5), while in group B was 0,35 mmol/L (IQR: 0,2 - 0,7), with no significant statistically differences (P: 0.07). When comparingthe incidence of ketonemia, there was a statistically significant difference in the concentration of ketone bodies with regerds to the time of preoperative fasting (day vs night fast), resulting in a higher incidence of ketonemia in group A (62.5%; 95% CI: 48.1 - 82) compared to group B (38.5%; 95% CI 26.5-52.5) P: 0.02. (Table 2).
Table 2 Comparison of ketonemia and secondary outcomes between groups A and B.
| Group A Daytime fast (n=40) | Group B Night fast (n=52) | P | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Ketone bodies (mmol/L) Median Interquartile range |
0.85 0.3 - 1.5 |
0.35 0.2 - 0.7 |
0.06 a | ||
| Ketonemia ≤ 0.5 > 0.5 |
15 25 |
37.5 62.5 |
32 20 |
61.5 38.5 |
0.02 b |
| Glucose concentration (mg/dL) | 0.08 c | ||||
| Mean (SD) | 78.6 (± 1.79) | 82.3 (± 1.28) | |||
| Hypoglycemia | 0.09 b | ||||
| Yes | 10 | 25.0 | 6 | 11.5 | |
| No | 30 | 75.0 | 46 | 88.5 | |
| Dehydration | 0.45 d | ||||
| Yes | 5 | 12.5 | 5 | 50.0 | |
| No | 35 | 87.5 | 47 | 48.0 | |
| Anxiety | 0.38 e | ||||
| Yes | 19 | 47.5 | 20 | 38.5 | |
| No | 21 | 52.5 | 32 | 61.5 | |
aKruskal-Wallis test; b X2 test; ct Student test ; d Fisher's exact test; e Kruskal-Wallis test. SD: Standard desviation.
Source: Authors.
There were significant differences in the distribution of type of food and its relationship with the level of ketone bodies, especially in terms of clear liquids versus other types of food (breast milk, formula milk, or regular diet), (OR: 3.23; 95% CI: 1.19 - 9-03, P=0.01). This was not related with the fasting time (day or nighttime), (Table 3).
Table 3 Comparison between elevated and normal ketone levels.
| Ketone bodies > 0.5 | Ketone bodies ≤ 0.5 | P | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (Months) | 0.49 a | ||||
| Mean (SD) | 26.5 ± 1.6 | 28.2 ± 1.8 | |||
| Age Group (Months) | 1.0 b | ||||
| <12 | 5 | 11.1 | 5 | 10.6 | |
| 12 - 23 | 12 | 26.7 | 13 | 27.7 | |
| ≥ 24 | 28 | 62.2 | 29 | 61.7 | |
| Gender | 0.45 c | ||||
| Female | 13 | 28.9 | 17 | 36.2 | |
| Male | 32 | 71.1 | 30 | 63.8 | |
| Weight (kg) | 0.99 a | ||||
| Mean/SD | 12.3 ± 0.42 | 12.3 ± 0.45 | |||
| Surgery | 0.64 b | ||||
| Pediatric Surgery | 28 | 50.9 | 26 | 49.1 | |
| Plastic Surgery | 5 | 36.7 | 7 | 63.6 | |
| Gastroenterology | 2 | 66.7 | 1 | 33.3 | |
| Neurosurgery | 2 | 100.0 | 0 | 0.0 | |
| Dentistry | 1 | 25.0 | 3 | 75.0 | |
| Orthopedics | 5 | 50.0 | 5 | 50.0 | |
| Otorhinolaryngology | 4 | 57.1 | 3 | 42.9 | |
| Type of food | 0.01 b | ||||
| Clear fluids | 21 | 46.7 | 10 | 21.3 | |
| Solids | 8 | 17.8 | 20 | 42.5 | |
| Breast Milk | 5 | 11.1 | 8 | 17.0 | |
| Milk formula | 11 | 24.4 | 9 | 19.2 | |
| Fasting hours | 0.18 d | ||||
| Median | 6.4 | 10 | |||
| Interquartile Range | 5.3 - 11.3 | 6 - 11.4 | |||
a t Student test; b Fisher's exact test; c x2 Test; d Kruskal-Wallis Test. SD: Standard desviation.
Source: Authors.
Blood glucose levels did not differ significantly between both groups (group A: 78,6 mg/dL; 95%CI 74,9 -82,2 - group B: 82,3 mg/dL; 95% CI 79.8 - 84.9; P: 0.09, Table 2). Only 16 patients presented to the preoperative area with blood glucose levels lower than 70 mg/dL (17,4%). There was no relationship between age and the presence of hypoglycemia. In the logistic regression model, a glucose level greater than 70 mg/dL was a protective factor for the development of ketosis, reducing the risk by 83% (OR 0.16 95% CI 0.04-0.69).
Preoperative anxiety and dehydration
Ten patients experienced significant levels of anxiety. (11%) There was a statistically significant difference in anxiety levels between the groups (P: 0,75). Significant levels of anxiety were found in 41-3% of patients with excess fasting of more than 1 hour. Ten patients (10.9%) experienced dehydration with no statistically significant difference between the groups (P = 0,48) (group A: 8.4%; 95% CI 2.8 - 18.6 - group B: 15.1%; 95% CI 5.1 - 31.8).
DISCUSSION
This study showed a higher incidence of ketonemia among patients under 48 months of age undergoing diurnal fasting versus patients undergoing nocturnal fasting. There was no difference in the incidence of dehydration, anxiety, or blood glucose between both groups. In a subgroup analysis, a glucose level greater than 70 mg/dL was a protective factor for the development of ketonemia, while clear liquids were found to be a risk factor for ketonemia.
Ketone bodies are produced in the mitochondria including B-hydroxybutyrate, acetoacetate, and acetone. During ketosis, the elevation of each type of ketone body is variable and the ketone body ratio changes (the proportion between concentrations of beta-hydroxybutyrate and acetoacetate). A normal value of 1:1 increases to a 6:1 ratio with fasting. 6 Therefore, beta-hydroxybutyrate is the predominant ketone body in most abnormal metabolic states. The reference value has been described as 0.3 mmol/L; however, levels above 0.5 mmol/L are more widely accepted in adult and children studies. 1,7
Previous studies have documented the elevation of ketone levels with prolonged preoperative fasting times. Dennhardt et al. in their study showed a correlation between elevated ketone bodies and preoperative fasting time in a pediatric population. 8 This effect could be explained by a lower concentration of liver glycogen which means that short periods of fasting are accompanied by significant concentrations of ketone bodies at levels similar to those found in the adult population after several days of fasting. 2,9
This study shows a significantly higher level of ketone bodies in the diurnal fasting group as compared to the nocturnal fasting group. Morimoto at al., observed a similar effect, identifying that diurnal timing for surgical procedures is a risk factor for the development of ketonemia (OR 4,16; 95% CI 1,29 - 12,76). 10 This could be caused by the type of food taken before surgery. In this work, the concentration of ketone bodies was higher among patients who took clear liquids before fasting as compared to other types of food. Thus, although patients who underwent surgery in the afternoon had fewer hours of fasting, the energy intake could be insufficient to stimulate the release of insulin, thus inhibiting the accelerated production of ketone bodies, as shown by Niiya et al. 11
It should be noted that the proportion of patients younger than 12 months was significantly lower than other age groups, which may be associated with a lack of statistically significant difference in the concentration of ketone bodies between daytime and overnight fasting in this population range.
This finding highlights the need to determine the number of calories included in clear liquids that should be recommended to patients. In addition, energy expenditure during the day is significantly higher due to higher levels of physical and mental activity than during the night 12, leading to a premature consumption of liver glycogen stores, which is scarce in young patients. 2 Insulin follows a circadian behavior, showing elevated levels during daylight hours. 3 However, greater insulin resistance has been identified in the afternoon. 13 All of these factors combined promote ketosis and catabolism during daytime fasting.
The use of preoperative oral carbohydrate therapy has been shown to limit catabolism and postoperative ketogenesis by decreasing insulin resistance by 50%, decreasing the levels of dehydration, and improving overall satisfaction in the adult population 14. Studies on pediatric patients are scarce. The administration of just 5 mL/kg of a 0.5 kcal/mL glucose solution 2 hours before surgery decreases the gastric volume and the incidence of postoperative nausea and vomiting when compared to the usual fasting recommendations. 15
Similar to what has been reported in other studies 8,16 blood glucose levels remained within normal ranges despite prolonged periods of fasting. There was no association between age, fasting hours and fasting time (day or night), and hypoglycemia. The impact of normal glycemic values to prevent ketonemia and the importance of an adequate caloric intake before surgery, is supported by our results.
Preoperative fasting is an emerging strategy to reduce the risk of bronchial aspiration. However, recent studies show a low incidence (9.3/10,000) 17 and lower mortality in the pediatric population. 18 Prolonged preoperative fasting does not result in additional benefits in terms of decreased gastric volume and pH levels. 19 A complete fasting time does not guarantee the absence of gastric content, since up to 6.2% of patients have findings of a full stomach by gastric ultrasound. 20 Therefore, prolonged preoperative fasting in the pediatric population is unjustified.
A similar prospective study 21 also reported prolonged fasting times in up to 88% of cases. In this research, excess fasting by one or more hours was accompanied by significant ketonemia in 48.9% of the patients, highlighting the need to maintain minimum fasting times to avoid preoperative catabolism.
Despite having informed the parents about preoperative fasting recommendations during the pre-anesthesia visit, the level of compliance in our study was very low. Lack of understanding and fear of complications derived from abbreviated fasting have been identified as relevant causal factors. 22 Interventional studies have focused on improving education and quality of information through written instructions and telephone calls the night before the surgical procedure resulting in a positive impact on compliance with the pre-surgical fasting recommendations. 23) Additionally, the findings reflect the need to adopt shorter fasting regimens in our setting. Anderson et al. describe in their study a mean fasting time for clear liquids of 4 hours with a 33% incidence of fasting greater than 6 hours when using the 6-4-2 regimen. 24 The introduction of the 6-41 fasting regimen recommended by the European Society of Pediatric Anesthesia 25, allows for the intake of clear liquids up to 3 mL/kg one hour before elective surgery and has not been correlated with an increase in the incidence of bronchial aspiration in pediatric patients. 26 Additionally, a reduction in fasting times for breast milk has recently been recommended. 27
The impact of prolonged fasting negatively impacts the patient's and family experience during the perioperative period. Increased hunger and thirst causes anxiety and sadness in patients and their families. 28 In this study, no relationship was found between the hours of fasting and the time of fasting (day or night) with these clinical outcomes. A contrasting result was observed in the clinical trial by Zamora et al. 16, describing increased irritability and dehydration in the group of patients with overnight fasting within an age range of 2 to 8 years.
The authors acknowledge some limitations of this work. Due to the dynamics of surgical schedules during the study period, we had a lower proportion of patients under 12 months of age in the diurnal fasting group, which may affect the analysis in this age group. Furthermore, the calorie intake before surgery was not measured, a factor that may have an impact on the resulting levels of ketone bodies and glucose. The lack of randomization and the type of study (observational) are limiting factors to reach conclusions and extrapolate the results. However, our results highlight the importance of shortening the fasting times, and of future recommendations about the calorie intake requirements before surgery to offset the negative effects of catabolism.
CONCLUSIONS
There is a higher risk of preoperative ketonemia in patients under 48 months of age who undergo diurnal fasting as compared with nocturnal fasting. The type of food eaten before surgery was the main associated factor. Further studies are required to determine the optimal calorie intake before surgery to decrease the risk of ketonemia. There is a need to enhance the strategies for reducing preoperative fasting times pursuant to the presurgical fasting guidelines.
ETHICAL DISCLOSURES
Ethics committee approval
The study was approved by the ethics committee of the Rafael Henao Toro Children's Hospital in Manizales, Colombia (IRB number CBCS-091). Parents and/or legal representatives gave their informed consent.
Protection of human and animal subjects
The authors declare that no experiments were performed on humans or animals for this study. The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
ACKNOWLEDGMENTS
Authors' contributions.
AT: Concept or original project, study planning, data collection, interpretation of results, and final drafting and approval of the manuscript.
AR: Study planning, data collection, interpretation of results, data analysis, and initial draft and approval of the manuscript.
FA: Study planning, interpretation of results, and final drafting and approval of the manuscript.











 texto en
texto en 



