What is the new contribution of this study?
The only strategy with evidence resulting from systematic reviews with meta-analyses -with high quality reports - is dexamethasone.
In adult patients undergoing surgical procedures under general anesthesia, dexamethasone at doses between 1.5 mg to 18 mg has proven to be effective for the prophylaxis of PONV.
Despite the low quality of the reports of published studies, other strategies show effectiveness for PONV prophylaxis.
INTRODUCTION
Postoperative nausea and vomiting (PONV) is one of the most frequent perioperative complications in the post-anesthesia care unit described in the literature. 1-3 It may present as postoperative nausea (PON), postoperative vomiting (POV), or both (PONV), at different stages of post-anesthesia recovery. 1-3 This complication, together with pain, is perceived by patients as one of the less tolerable symptoms during the postoperative period and greatly impacts patient satisfaction and quality of care. It is associated with increased care costs and longer postoperative recovery in the post-anesthesia care unit (PACU). 2,4 Similarly, Smith et al. 5 described this phenomenon as the acute postoperative complication with the highest relative economic burden according to the patients.
The large number of systematic reviews on the topic hinders both the management of the information and reaching rapid and practical conclusions regarding the clinical prevention of PONV, in addition to the identification of ethical-methodological concerns according to several publications by Dr. Fujii, which challenges the effectiveness of some pharmacological strategies in perioperative care. 6-8 In this context, there are different systematic reviews - with or without meta-analyses -on the use of multiple pharmacological and non-pharmacological treatment options for PONV prophylaxis, which have shown to be effective. 9
This document approaches and summarizes the available information from the evidence collected through systematic literature reviews and meta-analyses on the effectiveness of pharmacological and non-pharmacological strategies for the prevention of PONV assessed in adult patients undergoing surgical procedures under general anesthesia, regardless of age, gender or other sociodemographic factors.
METHODS
An overview of systematic reviews and meta-analysis or umbrella review was conducted, pursuant to the steps recommended by the PRISMA Guidelines 10 and the PRIOR checklist. The PICO model was established as follows: Population: immediate postsurgical patients undergoing general anesthesia; Intervention: administration of medicines and non-pharmacological measures for PONV prophylaxis; Comparator: antiemetic or PONV prophylactic medications, or placebo; outcome: effectiveness of the intervention in terms or reducing the number of PONV events.
The research protocol registered under Prospero CRD42021251999 was submitted for approval of the Curricular Committee of the School of Health and Sports Sciences of Fundación Universitaria del Área Andina. Based on its nature, it was classified as "risk-free", under Resolution 8430 of 1993 of the Ministry of Health of Colombia 11, hence no approval by the ethics in research committee was required.
Advanced and systematic searches were conducted in PubMed, EBSCO, Embase, Cochrane Database of Systematic Reviews, ScienceDirect and Scopus. The initial search was completed in March 2021 and the last search update was in September 2021. There were no restrictions as to the original date of publication of the article. All systematic reviews with meta-analyses of clinical trials assessing the effectiveness of the pharmacological and non-pharmacological strategies available for the prevention of PONV in adult patients - regardless of gender or clinical condition of the patient - were included. The search was limited to articles in Spanish, English and French. The search structures may be referenced in Complementary content 1.
One of the authors simultaneously searched in all databases, using the terms and strategies set forth in the protocol, and exported the results to Excel® tables. Then, independently and in duplicate, two of the authors screened the articles in accordance with the predefined eligibility criteria. The reasons for exclusion were listed. Then, the complete texts were collected and screened again pursuant to the eligibility criteria, and the reasons for exclusion were also specified. Any disagreements among reviewers were settled by consensus. Failure to reach consensus led to the third author to cast the deciding vote. A qualitative synthesis of the results was conducted and whenever feasible - based on the quality of the articles and the appropriate methodology - these were included in the quantitative summary of the evidence.
The data mining process was conducted independently and in duplicate in Excel® forms. The primary outcome assessed postoperative nausea and vomiting in terms of incidence of the event among the various groups of intervention, between 0 and 24 hours after surgery, as described by the authors of the previous reviews. The methodological quality assessment of the studies was implemented using the AMSTAR-2 tool. 12,13 All the summary measures described in the articles published were used in the review, trying to convert any effect measures into risk ratios (RR) for comparative purposes. 95% confidence intervals (CI) were included.
The results were organized in accordance with the type of intervention (pharmacological and non-pharmacological, incidence of PONV for phases 1 and 2 of the postoperative (POP) period according to the report of the previous reviews, and quality of the meta-analysis included. The PMID/DOI, primary authors, year of publication, number of studies included, specific population with the search strategy used in terms of doses, frequency and route of administration, and primary outcome assessed were all recorded for each review and meta-analysis. Any subgroup analyses reported data were also included.
The level of heterogeneity among trials was estimated using the I2 value, based on a cutoff figure for high heterogeneity of >50 %, very high heterogeneity >75 %, together with an assessment of uncertainty of the estimate based on the 95% CI. The statistical significance was estimated according to the Qtest based on X2 and a p<0.05 was considered significant.
Initially, the idea of the protocol was to assess publication biases in the trials using funnel plots to assess any asymmetries in the publications, as well as meta-regressions and sensitivity analyses by subgroups. Given that only three papers were selected for the quantitative synthesis, these types of results are not presented since that would imply a statistical error based on the tests design. The information was analyzed using The Cochrane Collaboration RevMan v5.0 statistical software which is freely available.
As an additional assessment to the meta-analysis, the Number Needed to Treat (NNT) was estimated as a clinical comparison measurement between strategies. 14 This approach has been discussed with regards to reliability, since it may be subject to biases in the presence of factors such as the heterogeneity of the participants in the various trials, the dissimilar follow-up observation times and the differences in randomization. 15,16 This review included the NNT estimated with two methodologies: the classical treat-as-one-trial of the pooled results and a second methodology which according to the Cochrane Manual is called corrected, for systematic reviews of interventions 17, described in the text as NNTc. Both methodologies were presented to allow for contrasting of the results.
The overlap analysis is a recommended strategy by various sources in the literature to mitigate any potential overestimates of effects for secondary literature studies. 18,19 It is also covered in chapter 15 of the Cochrane Handbook for Systematic reviews of interventions 20. The overlap analysis was conducted for this study, in order to appreciate any potential interpretation biases or overestimates of the studies included in the quantitative summary.
Any potential sources of plagiarism were identified at all times to avoid this problem. The authors declare they do not have any conflict of interest to disclose.
RESULTS
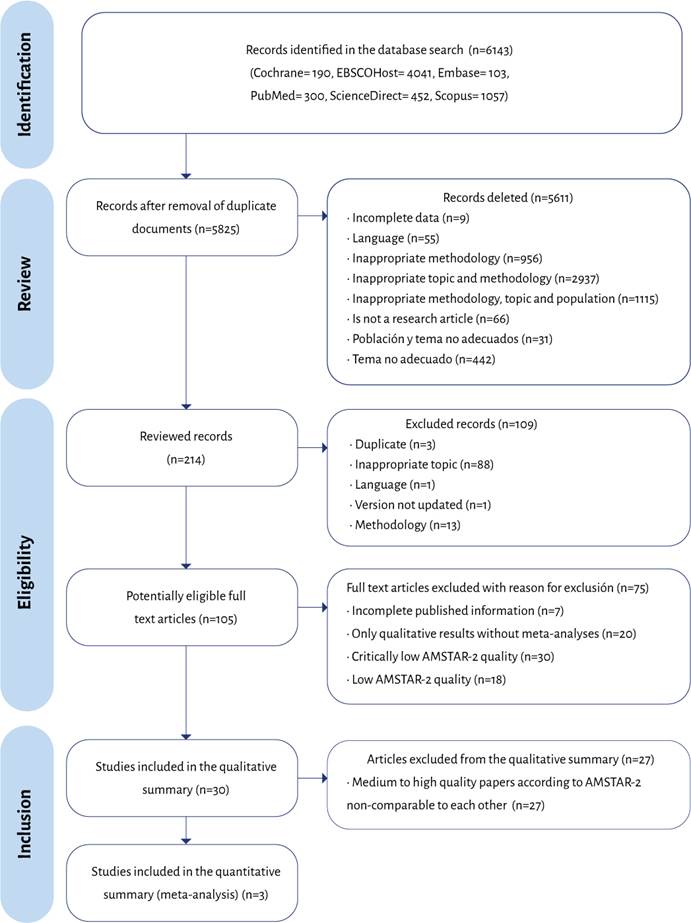
Figure 1 depicts the PRISMA flowchart for the process of identification, selection and inclusion of the studies. In order to enhance the review of the available literature, the authors decided to include in the assessment all articles with a moderate and high score.
The application of the AMSTAR-2 guidelines for the 80 studies included in the review showed that 3.8 % (n=3) were of high quality, 30.0 % (n=24), moderate quality, 23.8 % (n=19) low quality and 42.5 % (n=34) were of critically low quality. Eight of the 16 questions asked by the tool failed to reach a compliance score above 50 % in the set of research papers included. These were questions 2, 3, 4, 7, 8, 9, 10 and 15.
Henee, the weakest areas identified are:
1. Failure toreportthe sources offinancing of the studies included in the systematic reviews and meta-analyses.
2. Lack of a clear explanation about the reasons to choose specifìc methodological designs to be included in the systematic reviews and meta-analyses.
3. Lack of comprehensiveness when establishing search strategies; this involves searches in at least two databases (relevant to the research question), providing in the written paper the keywords or search strategies used, a clear description of the restrictions applied and justifìcations, search of the studies included in the reference lists, search in records of clinical trials or studies, experts advise in the area of interest, grey literature search when applicable, all within a period of less than 24 months upon completion of the review.
4. Failure to explicitly state the review methods previously selected with a justifìcation - when applicable -regarding any significant deviation from the protocol.
5. Weaknesses to satisfactorily assess the risk of bias of the studies included in the review, particularly because of the use of non-standardized scales or scales that fail to include the sources of bias described in the tool. These bias sources are usually included in the scale of risk-of-bias of the Cochrane Collaboration tool for randomized trials, ROBINS-I for non-randomized studies of interventions, Newcastle-Ottawa for observational trials, interalia.
Description of fìndings
The fìndings of each subgroup of strategies are available in Table 1. Twenty seven papers were rated as moderate to high quality according to AMSTAR-2, which describe the fìndings on acetaminophen, acupuncture and acupression, 5HT3 antagonists, NKi antagonists, dexamethasone and corticosteroids, dexmedetomidine, gabapentinoids, ginger, fluids, metoclopramide, midazolam, mirtazapine, naloxone, non-disaggregated non-pharmacological measures, total intravenous anesthesia (TIVA).
Table 1 Qualitative description of the subgroups of strategies.
| Strategy | Authors | Year | Relevant outcome | Number of studies included | Clinical context of surgery | Intervention | Comparator | Effects model used | Effect measurement report | Value of effect measure with CI | Heterogeneity I2 % (p value) | AMSTAR 2 | Sponsors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetaminophen | Doleman B, Read D, Lund JN, Williams JP. | 2015 | POV | 5 | Multiple | Preventive Acetaminophen | Post-incision Acetaminophen | Random | RR | 0,5 (0,31-0,83) | 0 % (0,96) | Moderate | No disclosures |
| Acupuncture and acupressure | Chen J, Tu Q, MiaoS, Zhou Z, Hu S. | 2020 | POV | 7 | Multiple | Electrical stimulation at the acupression site | Placebo | Fixed | RR | 0,59 (0,49-0,71) | 27 % (0,22) | Moderate | No disclosures. |
| PONV | 7 | 0,46(0,33-0,65) | 46 % (0,09) | ||||||||||
| PON | 7 | 0,54 (0,42-0,69) | 0 % (0,8) | ||||||||||
| Acupuncture and acupressure | Cheong K.B., Zhang J.P., Huang Y., Zhang Z.-J. | 2013 | POV | 3 | Multiple | PC6 Acupuncture | No intervention | Fixed | RR | 0,36 (0,19-0,71) | 0 % (0,63) | Moderate | Disclosed in the document |
| POV | 4 | PC6 Acupuncture | No intervention | 0,82 (0,48-1,38) | 0 % (0,79) | ||||||||
| POV | 6 | PC6 Acupression | Simulated | 0,62 (0,49-0,8) | 29 % (0,22) | ||||||||
| POV | 5 | PC6 Electrostimulation | Simulated | 0,5 (0,36-0,7) | 0 % (0,57) | ||||||||
| PONV | 6 | PC6 Acupression and others | No intervention, impostor | 0,29 (0,17-0,49) | 0 % (0,73) | ||||||||
| POV | 4 | Acupression at points other than PC6 | No intervention, impostor | 0,32 (0,17-0,61) | 0 % (0,83) | ||||||||
| PONV | 5 | Acupression at points other than PC6 | No intervention, impostor | 0,63 (0,49-0,81) | 44 % (0,13) | ||||||||
| PON | 3 | PC6 Acupuncture | No intervention | 0,64 (0,34-1,19) | 74 % (0,15) | ||||||||
| PON | 3 | PC6 Acupuncture | No intervention | 0,25 (0,10-0,61) | 0 % (0,41) | ||||||||
| PON | 6 | PC6 Acupression | Simulated | 0,71 (0,57-0,87) | 13 % (0,33) | ||||||||
| PON | 5 | PC6 Electrostimulation | Simulated | 0,49 (0,38-0,63) | 0 % (0,75) | ||||||||
| PON | 3 | Acupression at points other than PC6 | No intervention, impostor | 0,41 (0,24-0,69) | 0 % (0,59Moderate | ||||||||
| 5HT3Antagonists | Ahn E, Choi G, KangH, Baek C, Jung Y, Woo Y, Lee S,Chang Y. | 2016 | PON | 4 | Multiple | Palonosetron | Ramosetron | Fixed | RR | 0,66 (0,45-0,96) | 0 % (0,66) | Moderate | Disclosed in the document |
| POV | 8 | 0,66 (0,42-1,03) | 28 % (0,22) | ||||||||||
| PONV | 4 | 1,66 (1,27-2,18) | 0 % (0,93) | ||||||||||
| 5HT3Antagonists | Mihara T, Tojo K, Uchimoto K, Morita S,Goto T. | 2013 | POV | 7 | Multiple | Ramosetron | Placebo | Fixed | RR | 0,48(0,31-0,74) | 0 % (0,55) | Moderate | No disclosures |
| POV | 7 | Ramosetron | Placebo | Fixed | 0,5(0,35-0,73) | 0 % (0,5) | |||||||
| POV | 6 | Ramosetron | Ondansetron | Fixed | 0,5(0,28-0,9) | 7,5 % (0,37) | |||||||
| POV | 6 | Ramosetron | Ondansetron | Fixed | 0,53(0,34-0,81) | 0 % (0,93) | |||||||
| PON | 7 | Ramosetron | Placebo | Fixed | 0,59(0,47-0,73) | 0 % (0,51) | |||||||
| PON | 7 | Ramosetron | Placebo | Random | 0,65(0,49-0,85) | 32 % (0,18) | |||||||
| PON | 6 | Ramosetrón | Ondansetron | Random | 0,79(0,51-1,23) | 71,1 %(0,004) | |||||||
| PON | 6 | Ramosetron | Ondansetron | Random | 0,78(0,6-1,02) | 32,8 % (0,19) | |||||||
| NK1Antagonists | Liu M, Zhang H, Du BX, Xu FY, Zou Z,Sui B, Shi XY. | 2015 | POV | 3 | Multiple | Aprepitant 80 mg | Placebo | Fixed | RR | 0,13(0,04-0,37) | 0 % (0,79) | Moderate | Disclosed in the document |
| POV | 3 | Aprepitant 40 mg | Ondansetron 4 mg | Fixed | 0,47(0,37-0,6) | 0 % (0,38) | |||||||
| POV | 2 | Aprepitant 125 mg | Ondansetron 4 mg | Random | 0,32(0,13-0,78) | 86 % (0,008) | |||||||
| POV | 2 | Casopitant 50 mg | Placebo | Fixed | 0,38(0,26-0,53) | 0 % (0,53) | |||||||
| PON | 3 | Aprepitant 80 mg | Placebo | Fixed | 0,6(0,47-0,75) | 0 % (0,96) | |||||||
| NK1Antagonists | Singh, Preet Mohinder | 2015 | POV | 15 | Multiple | Aprepitant 40,80, 125 | Medication and placebo | Fixed | RR | 0,48(0,34-0,67) | 63,4 % (NR) | Moderate | Disclosed in the document |
| POV | 7 | 0,54(0,4-0,72) | 71,5 % (NR) | ||||||||||
| PONV | 7 | 1,37 (1,1-1,7) | 67,9 % (NR) | ||||||||||
| Dexamethasone & corticosteroids | Chen CC, Siddiqui FJ, Chen TL, Chan ES, Tam KW. | 2012 | PONV | 5 | Total thyroidectomy | Dexamethasone 5-8 mg | Placebo or droperidol | Fixed | RR | 0,38(0,30-0,49) | 30 % (0,22) | Moderate | Disclosed in the document . |
| Dexamethasone & corticosteroids | Chen P, Li X, Sang L, Huang J. | 2017 | PONV | 9 | Total jointarthroplasty | Dexamethasone or equivalent agents 4-40 mg | Placebo/saline | Fixed | NNT | 0,56(0,44-0,73) | 33,1 %(<0,001) | Moderate | No disclosures |
| Dexamethasone & corticosteroids | Fan Z, Ma J, Kuang M, Zhang L, Han B, Yang B, Wang Y,Ma X. | 2018 | PON | 6 | Knee arthroplasty | Dexamethasone IV | Placebo/control | Fixed | OR | 0,33(0,23-0,47) | 0 % (0,47) | Moderate | Disclosed in the documen |
| Dexamethasone & corticosteroids | Li B, WangH. | 2014 | PONV | 7 | Thyroid surgery | Dexamethasone 4-10 mg IV | Placebo | Random | OR | 0,23(0,13-0,41) | 66 % (0,002) | Moderate | No disclosures |
| Dexamethasone & corticosteroids | Yang Q, Zhang Z, Xin W, Li A. | 2017 | PONV | 6 | Hip Arthroplasty | Dexamethasone IV | Control | Fixed | RR | 0,41(0,30-0,57) | 0 % (0,550) | Moderate | No disclosures |
| Dexamethasone & corticosteroids | Zou Z, Jiang Y, Xiao M, Zhou R. | 2014 | PONV | 11 | hyroidectomy | Dexamethasone between 1.5 - 18 mg IV | Placebo | Random | RR | 0,52(0,43-0,63) | 56 % (0,003) | Moderate | No disclosures |
| Dexmedetomidine | Wang G, Zhang L, Lou S, Chen Y, Cao Y,Wang R, Zhang L, Tang P. | 2016 | POV | 5 | Laparoscopic surgeries | Dexmedetomidine infusion 0.2-1 µg.kg.min | Placebo | Fixed | RR | 0,36(0,18-0.72) | 0 % (0,62) | High | No disclosures |
| PON | 6 | 0,43(0,28-0.66) | 9 % (0,36) | ||||||||||
| Gabapentinoids | Alayed N, Alghanaim N, Tan X,Tulandi T. | 2014 | PON | 11 | Abdominal hysterectomy | Gabapentin (300to 1200 mg) | Placebo | Random | RR | 0,76(0,66-0,88) | NR | Moderate | Disclosed in the document . |
| POV | 11 | 0,67(0,52-0,87) | |||||||||||
| Ginger | Tóth B, Lan tos T, Hegyi P, Viola R, Vasas A, Benkő R, GyöngyiZ, Vincze Á, Csécsei P, Mikó A, Hegyi D, Szentesi A, Matuz M,Csupor D. | 2018 | POV | 11 | Multiple | Ginger >1 g | NR | Random | Log RR | -0,194(-0,4920,104) | NR | Moderate | Disclosed in the document |
| POV | Ginger >1 g | -0,360(-0,7440,023) | |||||||||||
| PONV | 11 | Ginger >0,3 g | -0,151 (-0,3510,048) | ||||||||||
| PONV | 6 | Ginger >0,3 g | -0,256(-0,5180,007) | ||||||||||
| Fluids | Jewer, JK; Wong, MJ; Bird, SJ; Habib, AS; Parker, R; George, RB | 2019 | PON | 18 | Multiple | 10-30 mL/kg crystalloids IV | No treatment | Random | RR | 0,62(0,51-0,75) | 56,76 % (0) | High | No disclosures |
| PON | 20 | 0,67(0,58-0,78) | 9,01 % (0,34) | ||||||||||
| PON | 17 | 0,47(0,32-0,69) | 38,27 %(0,05) | ||||||||||
| POV | 20 | 0,50(0,40-0,63) | 30,88 %(0,08) | ||||||||||
| POV | 19 | 0,56 (0,41-0,76) | 0 % (0,92) | ||||||||||
| POV | 15 | 0,48(0,29-0,79) | 0 % (0,9) | ||||||||||
| Fluids | Lee, Myeong Jong; Lee, Cheol; Kang, Hyun; Kim, Hyungtae | 2019 | PONV | 7 | Multiple | Crystalloids | Colloids | Random | RR | 0,802(0,561-1,145) | NR | Moderate | Disclosed in the document |
| Fluids | Yokoyama C, Mihara T, Kashiwagi S, Koga M, Goto T. | 2020 | PON | 10 | Multiple | Dextrose | Crystalloids | Random | RR | 0,76(0,59-0,99) | 28 % (NR) | High | Disclosed in the document |
| PON | 9 | 0,65(0,48-0,89) | 0 % (NR) | ||||||||||
| POV | 9 | 1,00(0,81-1,25) | 0 % (NR) | ||||||||||
| Metoclopramide | De Oliveira GS Jr, Castro-Alves LJ, Chang R, YaghmourE, McCarthy RJ. | 2012 | PON | 10 | Multiple | Metoclopramide 10 mg | Placebo or No treatment | Random | OR | 0,51 (0,38-0,68) | 8 % (0,03) | Moderate | Disclosed in the document . |
| PON | 13 | 0,49(0,35-0,68) | 7 % (0,001) | ||||||||||
| PONV | 13 | 0,58(0,43-0,78) | 0 % (0,38) | ||||||||||
| PONV | 11 | 0,52(0,36-0,75) | 24 % (0,08) | ||||||||||
| POV | 10 | 0,51(0,40-0,66) | 0 % (0,07) | ||||||||||
| POV | 12 | 0,44(0,29-0,65) | 5 % (0,03) | ||||||||||
| Midazolam | Ahn EJ, Kang H, Choi GJ, Baek CW, Jung YH, Woo YC. | 2016 | PON | 10 | Multiple | Midazolam (0,04-5 mg IV) | Placebo | Fixed | RR | 0,53(0,39-0,71) | 31 % (0,16) | Moderate | Disclosed in the document . |
| POV | 12 | 0,46(0,33-0,65) | 0 % (0,87) | ||||||||||
| PONV | 10 | 0,46 (0,36-0,57) | 31 % (0,16) | ||||||||||
| Midazolam | Grant M.C., Kim J., Page A.J., Hobson D.,Wick E., Wu C.L. | 2016 | PON | 5 | Multiple | Midazolam(0.035-0.075 mg/kg IV) | Saline solution, Haloperidol, Dexamethasone, Ondansetron or Ramosetron | Random | RR | 0,62(0,40-0,94) | 16 % (0,32) | Moderate | Own resources |
| PONV | 8 | 0,55(0,43-0,70) | 15 % (0,31) | ||||||||||
| POV | 8 | 0,61(0,45-0,82) | 1 % (0,42) | ||||||||||
| Mirtazapine | Bhatta charjee, Debamita; Doleman, Brett; Lund, Jonathan; Williams, John | 2018 | PONV | 3 | Multiple | Oral mirtazapine 30 mg tablet,1 hour before surgery | Placebo | Random | RR | 0,44 (0,32-0,62) | 0 % (0,933) | Moderate | Own resources . |
| Naloxona | Barrons RW, Woods JA. | 2017 | PON | 8 | Multiple | Naloxone (IV infusion or Infusión PCA) | Controls (unclear vs what) | Random | RR | 0,80(0,67-0,95) | 61 % (0,01) | Moderate | Own resources . |
| POV | 9 | 0,83(0,63-1,09) | 34 % (0,15) | ||||||||||
| Non- pharmacological | Lee A, Done ML. | 1999 | PON | 5 | Multiple | Different forms of acupression, acupuncture,electro-acupunctu re, transcutaneous peripheral nerve stimulation | Placebo or antiemetics (metoclopramide, droperidol, pro chlorperazine) | Random | RR | 0,47(0,34-0,64) | NR | Moderate | No disclosures . |
| POV | 13 | 1,13(0,95-1,35) | |||||||||||
| TIVA | Schaefer M.S., Kranke P., Weibel S., Kreysing R., Kienbaum P. | 2016 | PON | 9 | Multiple | Total IV anesthesia | Balancedanesthesia | Random | RR | 1,17(0,78-1,76) | 60 % (0,01) | Moderate | Declared in the document |
| PONV | 12 | 1,06(0,85-1,32) | 54 % (0,01) |
Source: Authors.
Acetaminophen
The use of IV acetaminophen as a prophylactic strategy for PONV was described by Doleman et al. 21 with moderate quality assessment result according to AMSTAR-2. Doleman et al. obtained RR=o.5 (95 % CI [0.31-0.83]; p=o.96) for the incidence of postoperative vomiting (POV), without mentioning the exact observation time. This research focused on pain dynamics, but the analysis of the incidence of POV was secondary.
Acustimulation, acupuncture and acupression
The research conducted by Chen et al. 22 included seven meta-analyses, the results of which pointed to transcutaneous electrostimulation as a prophylactic measure for PONV versus placebo. This research showed results for nausea with a RR=0.54 (95 % CI [0.42-0.69]; p<0.001), for the prevention of vomiting RR= 0.59 (95 % CI [0.49-0.71]; p=0<0.001) and for the prevention of PONV a RR=0.46 (95 % CI [0.33-0.65]; p<0.001).
Cheong et al. 23 assessed the effectiveness of acupuncture and acupression at different sites, with various techniques, as compared against medication, placebo or impostor operator. Different electrostimulation - acupuncture strategies were reviewed at point PC6 -, pooled in the 30 randomized clinical trials (RCT) included. The findings indicated that acupuncture at PC6 for prophylaxis of nausea during the first 6 hours is not effective (RR=0.64; 95 % CI [0.34-1.19]; p=0.150), neither for postoperative vomiting prophylaxis over the first 24 hours (RR=0.82, 95 % CI [0.48-1.38]; p=0.450). However, practicing acupuncture for early and late nausea did prove to be effective (RR=0.36; 95 % CI [0.19-0.71]; p=0.003 and RR=0.25; 95 % CI [0.10-0.61]; p=0.002, respectively).
This same paper by Cheong showed that acupression at PC6 reduces the occurrence of nausea and vomiting during the first 24 hours postop (RR=0.71; 95 % CI [0.57-0.87]; p=0.001 and RR=0.62, 95 % CI [0.49-0.80]; p<0.001, respectively). Electrostimulation at point PC6 acted as prophylaxis for nausea and vomiting in the same postoperative period (RR=0.49; 95 % CI [0.38-0.63]; p<0.001 for PON and RR=0.50, 95 % CI [0.36-0.70]; p<0.001 for POV). The simultaneous stimulation of PC6 and other acupuncture points had a prophylactic effect for PONV over 24 hours postop (RR=0.29; 95 % CI [0.17-0.49]; p<0.001). The stimulation of different acupuncture points other than PC6 was effective in the prophylaxis of PONV over the first 24 hours (RR=0.63; 95 % CI [0.49-0.81]; p<0.001). Notwithstanding the results submitted, the authors declare a low quality of the studies included with a high possibility of bias, so the recommendation is to be cautious in the interpretation of the results.
5HT3 antagonists
Ahn et al. 24 summarized the evidence with respect to the use of palonosetron versus ramosetron as a strategy for PONV prophylaxis; no statistically significant differences were identified (RR=1.07; 95 % CI [0.60-1.92]; p=0.03). They argued limitations in the data of the articles included in the analysis given the nature of the studies and the low reliability of the results, because the study populations were small.
Mihara et al. 25 conducted a systematic review with meta-analysis, and did not include the data reported by Fujii because of a potential bias. They assessed the impact of administering ramosetron versus ondansetron or other pharmacological strategies as comparators in the prevention of PONV, concluding superiority versus placebo (RR= 0.59; 95 % CI [0.47-0.73]; p<0.001); however, such superiority was no better than the findings in other meta-analyses. They concluded that ramosetron may be more effective than ondansetron in the prevention of POV, but its clinical significance is questionable based on the high NNT in the interventions.
NK1 receptor antagonists
Liu et al. 26 systematically described the reviewed evidence up to 2014 with regards to the use of aprepitant and other NK1 receptor antagonists in terms of their impact on PONV. They concluded that the use of this group of medicines, aprepitant in particular - with the most available evidence in the medical literature so far -, were effective in controlling PONV as compared to placebo (for nausea RR=0.6; 95 % CI [0.470.75]; p<0.001; and for vomiting RR=0.13; 95 % CI [0.04-0.37]; p<0.001).
Singh et al. 27, in the assessment of aprepitant for PONV prophylaxis, found that it reduces the incidence of vomiting during the first postoperative day (OR=0.48; 95 % CI [0.34-0.67]; in the absence of a p value report), though such finding involves a heterogeneity which is difficult to explain according the statement of the authors; this reduces the strength of the evidence. They also reported that its use reduces the need for rescue medication and that the oral administration of the drug is equivalent to the parenteral administration.
Dexamethasone and corticosteroids
Chen et al. 28 summarized the results of five trials assessing the use of dexamethasone for PONV prophylaxis in patients undergoing thyroidectomy. They found that the drug reduced the incidence of PONV (RR=0.38; 95 % CI [0.30-0.49]; with no p value report) and the need for postoperative analgesia.
Fan et al. 29 collected information about the postoperative outcomes of dexamethasone in patients undergoing total knee arthroplasty, assessing pain and PONV outcomes. They found a lower incidence of PONV (OR=0.33; 95 % CI [0.23-0.47]; p<0.001). The investigators recommend expanding the number of studies to improve the confidence of the results.
Li and Wang 30 conducted a meta-analysis on the available evidence in 2014 with regards to the use of dexamethasone for the prevention of PONV in thyroid surgery patients. They included the Fujii trials and they even found that one of them may have a confounding factor due to its poorer quality as compared to other trials. The conclusion was that the use of one dose of preoperative dexamethasone reduces the incidence and the severity of PONV in patients undergoing thyroidectomy (SMD=0.23; 95 % CI [0.130.41]; p<0.001).
Yang et al. 31 summarized the scientific evidence available on the use of corticoids -dexamethasone, methylprednisolone or hydrocortisone, taking equivalent doses with dexamethasone as the benchmark- versus controls for the prevention of PONV and postoperative pain. The conclusion was that the incidence of PONV and pain is significantly reduced during the postoperative period following total hip arthroplasty with the use of corticoids (RR=0.41; 95 % CI [0.30-0.57]; p<0.001); however, they recommend caution when considering these results because of dose variations, the methodological quality, and the strength of the trials in terms on the number of patients included.
Zou et al. 32 conducted a meta-analysis on the use of dexamethasone in the pre-operative period for patients undergoing thyroidectomy, in order to reduce the incidence of PONV. They found that administering a dose of between 8 to 10 mg before the induction of anesthesia is a safe and effective strategy for reducing the incidence of PONV (RR=0.52; 95 % CI [0.43-0.63]; p<0.001), reducing the use of rescue antiemetics, postoperative pain and the need to use rescue analgesia.
Dexmedetomidine
Fifteen prior studies were used by Wang et al. 33 to determine the effectiveness of the prophylactic use of dexmedetomidine for PONV (for nausea, RR=0.43; 95 % CI [0.280.66]; p<0.001; and for vomiting RR=0.36; 95 % CI [0.18-0.72]; p=0.004), as well as to reduce postoperative tremor and the need for rescue antiemetics in patients under-going laparoscopic surgery.
Gabapentinoids
Notwithstanding the availability of another primary outcome for the assessment of gabapentin in perioperative medicine, Alayed et al. 34 found that the administration of gabapentin versus placebo is effective as prophylaxis for nausea (RR= 0.76; 95 % CI [0.66-0.88]; with no p-value report).
Ginger
Chaiyakunapruk et al. 35 conducted a meta-analysis of five studies assessing the use of ginger for PONV prophylaxis. They argued that the administration of 1 gr of ginger proved to be effective as compared to placebo (for nausea RR=0.69; 95 % CI [0.54-0.89]; without a p-value report. For vomiting RR=0.61; 95 % CI [0.45-0.84]; without p-value report). In contrast, Tóth et al. 36 reviewed 10 research papers on the use of ginger as PONV prophylaxis versus placebo and failed to find any statistically significant differences, mainly because of the high heterogeneity of the original research projects.
Fluids
Jewer et al. 37 conducted a statistical analysis of the information from 41 original papers assessing the use of crystalloids and found that their use in elective surgeries reduced the incidence of PONV, but there was no clarity about the outcomes in emergency surgeries because of the variability of the measurements of the primary studies.
Lee et al. 38 reviewed the available evidence of nine clinical trials assessing the incidence of PONV on groups receiving colloids, versus crystalloids. They found that the perioperative administration of colloids does not reduce the incidence of PONV (RR=0.8; 95 % CI [0.56- 1.15]; p=0.224) as compared to crystalloids in surgeries, regardless of the surgical time; but there was a difference for surgical procedures exceeding 3 hours in which colloid infusion was used (RR=0.63; 95 % CI [0.44- 0.89]; p=0.009) as compared to the group receiving crystalloids infusion (RR=1.24; 95 % CI [0.4-2.09]; p=0.408). Despite this statistical finding, the authors state the need for improved methodological models with a larger number of patients to prove this association.
Yokohama et al. 39 analyzed the information from 11 prior research papers and found that the administration of dextrose decreases the incidence of nausea, as compared to placebo (for early nausea RR=0.76; 95 % CI [0.59-0.99]; with no p-value report. For late nausea RR=0.65; 95 % CI [0.48-0.89]; with no p-value report), but they found no differences in the incidence of vomiting.
Metoclopramide
De Oliveira et al. 40 reviewed 30 original articles on the use of metoclopramide in PONV prophylaxis. They found that the 10 mg IV dose is effective for the prevention of PONV as compared to placebo (RR=0.58; 95 % CI [0.43-0.78], with no p-value report). They also conducted sub-analyses of three articles published by Fujii, because of the risk of bias involved, but did not find any alterations in the final results.
Midazolam
Ahn et al. 41 summarized the available evidence for the use of IV midazolam as a prophylactic strategy for PONV. They found that a dose between 0.04 and 5 mg is effective in reducing postoperative events (RR= 0.45; 95 % CI [0.36-0.57]; p<0.001).
Grant et al. 42 assessed the efficacy of midazolam during the pre or intra-operative period to prevent the incidence of PONV and it proved to be effective (RR=0.55; 95 % CI [0.43-0.70]; with no p-value report) for up to 24 hours, as compared to the use of placebo.
Mirtazapine
Since mirtazapine is an antidepressant with various mechanisms of action including serotonin 5HT3 receptor antagonist, it has been described as an agent for PONV prophylaxis. The study by Bhattacharjee et al. 43 identified in three out of seven studies included in their review, that the oral administration of 30 mg of mirtazapine 1 to 2 hours before surgery, significantly reduced PONV, with a RR=0.44 95 % CI [0.32-0.62], I2 0 %), and these results were confirmed with a trial sequential analysis (TSA). In a single low quality trial, a comparison against the drug ondansetron was identified with findings of equi-effectiveness. Based on low-quality studies, the advantages of the drug include a reduction in preoperative anxiety; however, increased sedation during the postoperative period was also identified.
Naloxone
The only study on this drug conducted by Barrons et al. 44 and published in 2017 highlighted the importance of perioperative opioid administration as a source of PONV. They suggested the administration of low doses of naloxone which could have a dose-dependent effect with regards to specific outcomes, particularly PONV. The paper described a benefit in the perioperative administration of naloxone, in studies with minimum 24 hours of observation for PON with a RR=0.80 (95 % CI [0.67-0.95]; p=0.01). However, such benefit did not materialize in the case of postoperative vomiting, reporting a RR=0.83 (95 % CI [0.63-1.09]; p=0.18).
Non-pharmacological measures
Lee and Done 45, in 1999, documented a moderate quality meta-analysis and systematic review summarizing the scientific evidence on non-pharmacological approaches for PONV, including acupuncture, electroacupuncture, transcutaneous electrical nerve stimulation, acupoints stimulation and acupression. They concluded that these techniques are effective for the prevention of early nausea, but not of early or late vomiting. They failed to assess the compounded PONV outcome. This article is described separate from the previously mentioned acupuncture articles because there was no separation among the groups of intervention, as was the case with acustimulation, acupuncture and acupression.
Total intravenous anesthesia (TIVA)
According to the findings by Schaefer et al. 46 in 2016, total intravenous anesthesia as compared against balanced general anesthesia plus a single pharmacological intervention (anti 5HT3 or droperidol) are equally effective to prevent PONV episodes with a RR=1.06 (95 % CI [0.85-1.32]) for PONV and a RR=1.17 95 % CI [0.78-1.76] for nausea. The quality of the evidence in this trial was moderate as compared with the critically low evaluation of the study by Tramer 47, and so the results were not taken into account.
Qualitative analysis
Table 2 lists the NNT for the studies included as moderate to high quality using the treat-as-one-trial methodology and the corrected NNT (NNTc) according to the methodology described in the Cochrane Manual of systematic reviews of interventions version 6.3 of 2022. The results for PON, POV and PONV are described in accordance with the original report of the referred authors.
Table 2 Number Necessary to Treat and Corrected Number Necessary to Treat with the Cochrane method for the studies potentially included in the meta-analysis.
| Author, year | Strategy assessed | Vomiting | Nausea | PONV |
|---|---|---|---|---|
| Acupuncture and acupression | ||||
| Chen J, Tu Q, Miao S, 2020 22 | Electrical stimulation at acupression points (P6, L14, LU7, LI11, ST36, SP6, BL13, SP9, BL23). | NNT=7; 95 % CI [6.52-7.33] NNTc=8 | NNT=13; 95 % CI [11.87-13.46] NNTc=7 | NNT=6; 95 % CI [5.88-6.55] NNTc=6 |
| Cheong et al., 2013 23 | Acupuncture PC6. | (6h) NNT=7; 95% CI [6,53-7,89]. NNTc=5 (0-24h) NNT=35; 95% CI [31.86-37.62] NNTc=19 |
(6h) NNT=13; 95% CI [11.30-13.98]. NNTc=9 (0-24h) NNT=7; 95% CI [6.33-7.44]. NNTc=4 |
- |
| Antagonists 5HT3 | ||||
| Mihara et al., 2013 25 | Ramosetron | (6h) NNT=13; 95% CI [12.30-13.45]. NNTc=6 (24h) NNT=11; 95% CI [10.15-11.21] NNTc=7 |
(6h) NNT=6; 95% CI [5.67-6.47]. NNTc=8 (24h) NNT=7; 95% CI [6.65-7.61]. NNTc=10 |
- |
| NK1 Antagonists | ||||
| Liu et al., 2015 26 | Aprepitant 80 mg. | NNT=5; 95% CI [4.30-5.10] NNTc=4 | NNT=3; IC95 % [2,94-3,75] NNTc=8 | - |
| Casopitant 50 mg. | NNT=6; 95% CI [5.76-6.41] NNTc=5 | - | - | |
| Dexamethasone and corticosteroids | ||||
| Fan Z et al., 2018 29 | Dexamethasone 8-10 mg IV | - | NNT=5; 95% CI [4.68-5.31] NNTc=7 | - |
| Li Wang, 2014 30 | Dexamethasone 4-10 mg IV | - | - | NNT=3; 95% CI [2.65-3.04] NNTc=5 |
| Zou et al., 2014 32 | IV Dexamethasone 1.5-18 mg | - | - | NNT=5; 95% CI [4,65-5,06] NNTc=7 |
| Dexmedetomidine | ||||
| Wang G. et al., 2016 33 | Dexmedetomidine infusion de 0.2-1 µg/kg/min | NNT=10; 95% CI [9.24-10.53] NNTc=5 | NNT=7; 95% CI [6.65-7.68] NNTc=6 | - |
| Gabapentinoids | ||||
| Alayed et al., 2014 34 | Gabapentine 300 to 1200 mg. | NNT=11; 95% CI [9.78-11.65] NNTc=10 | NNT=8; 95% CI [7.78-8.95] NNTc=14 | - |
| Ginger | ||||
| Chaiyakunapruk N et al., 2006 35 | Ginger >0,3 g. | NNT=13; 95% CI [11.60-13.70] NNTc=13 | - | NNT=7; 95% CI [6.21-7.57] NNTc=13 |
| Fluids | ||||
| Jewer JK et al., 2019 37 | 10-30 mL/kg crystalloids IV. | (6h) NNT=20; 95% CI [19.40-20.34]. NNTc=8 (24h) NNT=7; 95% CI [6.49-6.97] NNTc=7 |
(6h) NNT=10; 95% CI [9.31-9.99]. NNTc=10 (24h) NNT=6; 95% CI [5.43-5.94] NNTc=9 |
|
| Metoclopramide | ||||
| De Oliveira et al., 2012 40 | Metoclopramide 10 mg. | (6h) NNT=10; 95% CI [9.33-10.13] NNTc=15 | (6h) NNT=7; 95% CI 7.15-7.89] NNTc=29 | (6h) NNT=7; 95% CI [6.76-7.67]. NNTc=76 (24h) NNT=8; 95% CI [7.39-8.45] NNTc=-32 |
| Midazolam | ||||
| Ahn et al., 2016(2) 41 | Midazolam 0.04-5 mg IV. | NNT=8; 95% CI [7.74-8.55] NNTc=6 | NNT=7; 95% CI [6.82-7.79] NNTc=7 | NNT=3; 95% CI [2.96-3.49] NNTc=6 |
NNT= Number needed to treat. 95% CI= 95 % Confidence Interval. NNTc= Corrected Number Needed to Treat with Cochrane method. ND= Not determined in the study.
Source: Authors.
Quantitative analysis
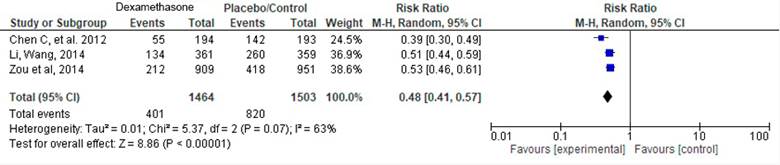
Keeping in mind that the populations were comparable, as well as the interventions assessed in the studies, a meta-analysis of three scientific articles was then conducted:
Chen (2012), Li (2014) and Zou (2014). Table 3 depicts the information of the articles included.
Table 3 Studies included in the meta-analysis.
| Authors | Chen et al. (2012) | Li, Wang (2014) | Zou et al. (2014) |
|---|---|---|---|
| Studies included | 5 | 7 | 11 |
| Intervention | Dexamethasone 5-8 mg IV | Dexamethasone 4-10 mg IV | Dexamethasone 1,5-18 mg IV |
| Comparator | Placebo or droperidol | Placebo | Placebo |
| Analysis based on weight of the samples | Yes | Yes | Yes |
| Sample size | 387 | 720 | 1860 |
| Effects model used | Fixed | Random | Random |
| I2 % (p) | 30 % (0.22) | 66 % (0.002) | 56 % (0.003) |
| Effect measure reported | RR | OR | RR |
| Value of the effect measure with CI | 0.38 (0.30-0.49) | 0.23 (0.13-0.41) | 0.52(0.43-0.63) |
| AMSTAR 2 | Moderate | Moderate | Moderate |
| Sponsors | Authors' own resources | No disclosures | No disclosures |
Source: Authors.
Postoperative nausea and vomiting prophylactically managed with dexamethasone
The tree chart on Figure 2 shows a RR=0.48 (95% CI [0.41-0.57]; p<0.001), with 63 % heterogeneity (p=0.07). The overlap analysis of the three trials assessing dexamethasone are shown on Table 4, the estimate for the corrected covered area (CCA) was 50 %, which according to Pieper et al. 48 corresponds to a very high overlap, indicating that the source for the analysis of the studies had several clinical trials in common, a situation that could result in overestimating the RR. The study with the largest number of original papers was Zou et al. (2014). Table 4 depicts the overlap matrix for the visual assessment thereof. Using this matrix, the corrected covered area (CCA) was then estimated. The CCA for the dexamethasone studies was 50 %. This number, according to the cutoff points suggested by Pieper et al. 48, is interpreted as a very high overlap.
Source: Authors.
Figure 2 Evaluation by items of the articles included in the study using the AMSTAR-2 tool.
Table 4 Overlap analysis of original studies included in the reviews.
| Secondary study | Feroci (2011) | Fujii (2007) | Lee (2001) | Wang (1999) | Worni (2008) | Barros (2013) | Doks-rod (2012) | Song (2013) | Bononi (2010) | Zhou (2012) | Fujii (2000) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chen et al. (2012) | X | X | X | X | X | ||||||
| Li, Wang (2014) | X | X | X | X | X | X | X | ||||
| Zou et al. (2014) | X | X | X | X | X | X | X | X | X | X |
Source: Authors.
In view of the risk of bias based on the heterogeneity data and the nature of the studies, the sensitivity-based adjustment was implemented disaggregating one by one the reviews included in this study, with no differences identified in the results.
No meta-regression was conducted based on the findings in one single type of results, without subgroup analysis or covariables. 49,50
DISCUSSION
The available evidence from systematic literature reviews and meta-analyses on PONV prophylaxis with pharmacological and non-pharmacological strategies is clinically heterogeneous, of variable quality and with limitations in terms of the results and their comparisons. However, the studies by Li and Wang 30, Zou et al. 32 and Fan et al. 29, included in the meta-analysis, suggest a reduction in the number of PONV events (RR=0.48; 95% CI [0.41-0.57]; p<0.001; I2=63 % - p=0.07) with NNTc 5 and 7 for 0-6 POP hours and 6-24 POP hours.
Reference was made to the results of the evaluation according to AMSTAR-2 and whether the quality standards were met. This condition of the evaluation of the trials and the impact on quality ratings could be explained in several ways. One is that different publishing groups have limitations with regards to the length of research reports - a determining factor when describing in an article the details considered to be most relevant by the group of researchers. This may result in overlooking details that could underestimate the final appreciation using the AMSTAR-2 tool, depending on the domain affected.
Additionally, any changes in the recommendations of the report for systematic review-type papers with meta-analyses and their demands, result in the older publications receiving a lower rating. AMSTAR-2 is a strict tool, developed after the publication of several papers, so in many cases the resulting quality rating was low or critically low.
In an effort to increase the number of available studies to conduct a qualitative summary of this paper, moderate quality studies were also included, assessing together with the high quality studies, the possibility to conduct meta-analyses. In view of the clinical heterogeneity exhibited by the various studies, it was impossible to conduct meta-analyses in most scenarios, with the exception of the prophylactic use of dexamethasone.
One frequent finding in the studies included was the need expressed by the authors to enhance the quality and comprehensiveness of the studies assessing this outcome.
This methodology which presumably should contribute to compile the available evidence on various therapeutic options into one single outcome, is limited because the primary studies - in this case meta-analyses - fail to explore the interactions among the various strategies. Despite the numerous records resulting from the initial search, there is a significant variability with regards to the definitions of nausea, vomiting and nausea and vomiting over time.
Another limitation is the lack of studies assessing the impact of different strategies administered simultaneously to prevent the occurrence of the events - PON, POV, PONV -. This limitation makes it impossible to assess the cumulative effect of the strategies since it disregards the potential synergy or antagonism of the drugs or of the non-pharmacological strategies from the clinical point of view.
With regards to the biological plausibility of the results identified, in terms of the mechanism of action of the drugs and the well-known pathophysiology of PONV, no mismatches in the results were found. Overall, the medications with direct or indirect antiemetic effects, although they are of poor quality in most cases, show evidence supporting their use during the perioperative period.
Among the strategies used, the administration of naloxone to reduce postoperative nausea should be highlighted, probably as a result of its antagonistic effect on the opioids administered during the intraoperative period. However, the effect that this agent may have on the outcomes driving the administration of opioids during the intraoperative period is unclear; for instance, controlling acute postsurgical pain within the framework of a multimodal strategy.
One consideration to keep in mind is that despite the statistical possibility of a quantitative synthesis on the intervention with dexamethasone, there is clinical heterogeneity that could impact the conclusions derived from statistical testing. This is due to the differences in the doses administered to the participants in the trials included in the original reviews.
As a conclusion, with regards to the prevention of postoperative nausea and vomiting using pharmacological strategies, the clinician has available a broad range of tools which may help to reduce the risk of developing this post-anesthesia complications Among these strategies, the use of intraoperative dexamethasone at doses between 4 to 18 milligrams is the option with the best quality studies and the strongest support. However, in the group of low-to-moderate quality studies, other drugs such as NK1 antagonists, 5HT3 receptor antagonists, metoclopramide, midazolam, dexmedetomidine and gabapentinoids, also delivered promising results in their respective meta-analyses.
For the prevention of the compound event of postoperative nausea and vomiting, the strategy used and which allowed for the smallest NNT was IV dexamethasone at a dose of between 4 and 10 mg (corrected NNT =5) and IV midazolam at doses between 0.04-5 mg (corrected NNT=6). In the case of 5HT3 antagonists, one of the most widely used group of medications for the prevention of PONV, only comparative trials between members of the same group were identified, and in case comparisons against placebo, the only drug included was ramosetron. In this case, the corrected NNT for vomiting was 6-7 and for nausea was 8-10.
Among the non-pharmacological strategies, the most widely studied was acupuncture at point P6, with a corrected NNT of 6, similar to the above-mentioned strategies (dexamethasone, ramosetron and midazolam).
Although the quality of the overall evidence is low, the results of these studies suggest effectiveness in the prevention of individual outcomes and of the compound event, with medications as different as: acetaminophen, amisulpride, ondansetron, aprepitant, dexamethasone, dexmedetomidine, scopolamine, gabapentin, pregabalin, metoclopramide, midazolam, mirtazapine, naloxone and perphenazine.
Drugs such as haloperidol, droperidol, dimenhydrinate, with biologically feasible mechanisms of action, low cost and good safety profile, deserve further consideration in future studies.
Notwithstanding the fact that the studies conducted after the dissemination of the AMSTAR-2 strategy show a compliance percentage slightly higher than their counterparts before the implementation of the instrument (34.3 % vs 38.8 %), in general the lack of compliance with the quality criteria is high. It would be ideal to further disseminate this strategy to drive scientific studies that meet higher quality standards.
ETHICAL RESPONSIBILITIES
Endorsement of the ethics committee
The research protocol registered under Prospero CRD42021251999 was submitted for approval by the Curricular Committee of the School of Health and Sports Sciences of Fundación Universitaria del Área Andina. Based on the nature ofstudy, it was classified as "no risk", pursuant to Resolution 8430 of 1993 of the Ministry of Health of Colombia (11). Therefore the approval of the ethics in research committee was not required.
Protection of humans and animals
The authors declare that no experiments in humans or in animals were conducted for this research. The authors declare that the procedures followed were consistent with the ethical standards of the committee for responsible human experimentation and pursuant to the World Medical Association and the Declaration of Helsinki.
ACKNOWLEDGEMENTS
Contribution by the authors
All of the authors significantly contributed to the conception or design of the study, and to the collection, analysis or interpretation of the data. They produced the design or the critical review of the document for a significant intellectual content and have approved the final version for publication.











 texto en
texto en