INTRODUCTION
Clinical personnel needs evidence-based information that is reproducible, accurate and free of potential risk to help with their decision-making regarding healthcare interventions. This requires studies that assess the efficacy and safety of the interventions implemented in daily practice, and randomized clinical trials have become essential tools for this purpose. However, the exponential growth of clinical trials calls for processes to synthesize all the available evidence to help facilitate reading and results interpretation. Systematic reviews and their quantitative synthesis, or meta-analyses, serve this purpose and also allow to verify the reproducibility of the information, increasing the accuracy of the effects of the interventions documented in individual studies 1.
Traditionally, systematic reviews and meta-analyses focus on comparing an intervention versus placebo or a control, be it a standard treatment or no intervention at all. However, there are clinical situations in which there are more than two therapeutic alternatives as well as multiple randomized clinical trials (RCTs) doing head-to-head comparisons. In view of such heterogeneity, methodologies such as network meta-analyses have emerged as a form of secondary study that allows to identify effects with greater accuracy as well as to identify gaps in the available evidence and estimate differences between interventions lacking head-to-head comparisons 1.
This special article is a summary with comments of a network meta-analysis published by the Cochrane Collaboration 2 on the topic of prophylactic interventions for postoperative nausea and vomiting (PONV) in adult patients undergoing general anesthesia. The aim is to discuss the article from the anesthesiology perspective in order to provide insights for critical reading and to gain a better understanding of the methods used in network meta-analyses.
BACKGROUND
The incidence of PONV is as high as 31% during the perioperative period in our setting 3, increasing length of stay in the postanesthetic recovery unit (PACU) and reducing postoperative satisfaction levels. A wide range of medications of varying efficacy are available for preventing nausea and vomiting in adults undergoing general anesthesia, creating uncertainty regarding which medications should be considered as the first option for PONV prevention.
What was the aim of the Cochrane review?
The aim of the review conducted by Weibel et al. 2 was to compare the efficacy and safety of various pharmacological therapies, used alone or in combination, versus no treatment, placebo or other therapies for PONV prevention in adult patients undergoing any type of surgery under general anesthesia, and to build a classification of the best therapies and the most adequate doses.
What did the Cochrane review study?
The review included adults undergoing any type of surgical procedure under general anesthesia. The eligible studies were RCTs, which included the use of any antiemetic of the following families as intervention: 5-HT3 receptor antagonists, D2 receptor antagonists, NKi receptor antagonists, steroids, antihistamines and anticholinergics for the endpoints of vomiting within the first 24 hours, serious adverse events (SAEs) and any adverse event (AE).
Literature search and identification of the Cochrane review
The authors of the Cochrane review conducted a search of original articles published between 1946 and April 20, 2020, with no language restrictions, excluding articles published only in abstract form, in the Embase, Medline, CENTRAL, CINAHL databases, the World Health Organization (WHO) protocols registry and the clinicaltrials.gov platform. An additional search of the references of six previous systematic reviews was also conducted.
What are the main results of the Cochrane review?
Eleven collaboration reviewers collected 585 studies including a total of 97,516 patients; mean age was 42 years, 83% were women, 72% ASA I and II, and 88% received intraoperative opioids. The studies assessed 44 individual antiemetics and 51 combinations, with ondansetron, dexamethasone and droperidol being the most studied in 246, 120 and 97 studies, respectively. Overall, 27% and 17% of the studies were found to have a low and a high risk of bias, respectively. The network of comparisons available among all the studied antiemetics is shown in Figure 1.
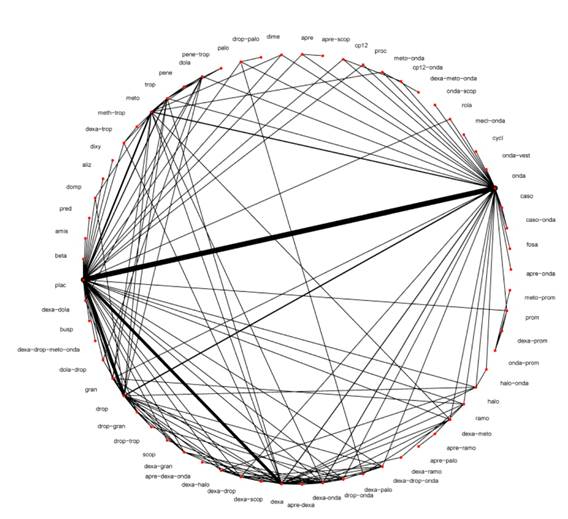
Source: Taken with authorization from Weibel et al. 2
Figure 1 Cochrane collaboration comparison network.
The authors of the review found that drug combinations are, in general, more effective than monotherapy. Moreover, with high certainty evidence, five drugs used alone have clinically relevant efficacy in reducing PONV in the first 24 hours: aprepitant RR 0.26 (95% CI [0.18; 0.38]), ramosetron RR 0.44 (95% CI 0.32; 0.59), granisetron RR 0.45 (95% CI 0.38; 0.54), dexamethasone RR 0.51 (95% CI 0.44; 0.57) and ondansetron RR 0.55 (CI 0.51; 0.60).
As for SAEs and AEs, the authors found, with low to very low evidence certainty, that single-dose medications had very little or no effect on the incidence of SAEs, while, with low to moderate evidence, they did not find apparently significant effects in relation to any AE, except an increase in AEs associated with dimenhydrate RR 5.70 (95% CI 1.36; 23.93) and scopolamine RR 2.12 (1.71; 2.64). Additionally, a reduced sedation level was found for ondansetron RR 0.87 (95% CI 0.79; 0.96), meaning that for every 1,000 patients there were 17 events of reduced sedation levels.
What do the authors conclude?
The authors of the review conclude, with high evidence certainty, that five single drugs (aprepitant, ramosetron, granisetron, dexamethasone and ondansetron) reduce PONV and, with moderate evidence certainty, that an additional two single drugs (fosaprepitant and droperidol) may reduce PONV when compared to placebo. These results can be extrapolated mainly to patients at a higher risk of PONV, such as anesthesia for otolaryngological and laparoscopic surgery, and pregnant women. No additional efficacy studies versus placebo are needed in view of the moderate to high evidence certainty for seven drugs of relevant benefit for vomiting prevention. However, further studies are needed to look into the potential long-term side effects of these medications and to examine patient populations at a lower risk, including general or pediatric populations.
Moreover, the authors of the review also conclude that high and recommended doses of granisetron (> 3 mg), dexamethasone (> 5 mg), ondansetron (> 4 mg IV) and droperidol (> 1.25 mg) were more effective in preventing vomiting than low doses. Dose related side effects were rarely found given the limited number of studies, except for the lower sedative effects of ondansetron when used at recommended and high doses.
In general terms, the Cochrane review found low or very low certainty evidence regarding adverse effects. However, there is moderate certainty evidence that granisetron and amisulpride probably have no effect on any adverse event when compared to placebo, and low certainty evidence regarding potential increase of adverse events with dimenhydrinate and scopolamine. Low evidence certainty was found regarding adverse effects for each drug family, except for moderate certainty in relation to the increase in headache and decreased sedation level with ondansentron.
What is the impact of this Cochrane review on the practice of anesthesia?
Synthesis initiatives, such as the Cochrane collaboration, are of the greatest importance for the practice of medicine. To begin with, they allow clinicians and researchers to make informed decisions and act based on evidence, considering the rigorous, transparent and thorough search of the available evidence. This evidence, in turn, helps resolve uncertainties and conflicting results, thus improving the accuracy of the estimated effects.
A Network meta-analysis, also known as multiple comparison meta-analysis, is an advanced statistical technique that allows to synthesize all the evidence regarding one question which includes three or more interventions for the same disease. The network meta-analysis uses two innovations to compare all the interventions 4. First, it allows to estimate effect differences between two interventions not previously compared, i.e., if A and B are different one from the other, using information from studies that have compared A and B against a common comparator, C (Figure 2). For example, suppose that ondansetron and amisulpride have not been compared against each other, but they have each been compared against placebo, then, with the information of those studies -ondansetron-placebo and amisulpride-placebo - it is possible to estimate differences between ondansetron and amisulpride, arriving at what is called indirect evidence. Second, the network meta-analysis allows to combine direct and indirect evidence and estimate mixed evidence to allow for higher statistical precision regarding the results 4,5.
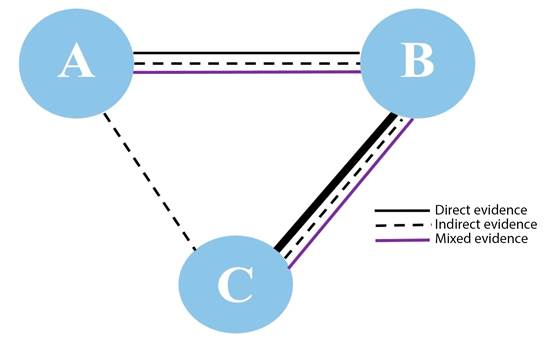
*Line thickness reflects the number of studies Source: Authors.
Figure 2 Schematic representation of the network meta-analysis*.
Network meta-analyses are considered reliable as long as they meet key assumptions of transitivity and consistency. Transitivity means that the evidence derived from head-to-head comparisons pertaining to population characteristics, interventions and endpoints in primary studies are sufficiently similar so as to allow to arrive at the relative effect of C based on the effects of A and B. Consistency refers to the fact that direct and indirect evidence must produce similar results 4. Provided they meet these assumptions, network meta-analyses are powerful tools to help resolve uncertainty regarding effect differences in several interventions for the same indication.
The authors of this special article analyzed a systematic review of the literature carried out by Weibel et al., which included 585 RCTs. The Cochrane review, assessed using the AMSTAR 2 tool 6, was found to be of very high quality and, therefore, offers reliable results to be used in practice. The AMSTAR-2 (A Measurement Tool to Assess Systematic Reviews 2) is a checklist that allows to discriminate the quality of a systematic review in accordance with four confidence levels: high, moderate, low and critically low. The tool is applied in seven domains: protocol registered before the review, adequate literature search, rationale for excluding studies, risk of bias of the individual studies included, appropriate meta-analytic methods, consideration of the risk of bias in interpreting the results of the review, and evaluation of the presence and probable impact of a publication bias.
Additionally, the Cochrane review presents the results with summary GRADE evidence tables 7 published in the full review version, to facilitate the synthesis and interpretation of the results. The GRADE evidence synthesis tables are tools that provide a rigorous format to include detailed information about an intervention. They list the most important clinical outcomes, the number of studies with their design, factor information regarding the quality of the evidence found - e.g., risk of bias, inconsistency, inaccuracy - or indirect evidence, baseline risk information - e.g., estimated incidence of nausea and vomiting without mitigating treatments - attributable risk, absolute and relative risk of the intervention and, finally, footnotes explaining judgements of the body of evidence.
PONV is a real problem in anesthesia, and different scientific and professional societies have developed consensus for its treatment 8,9. The primary impact of the Cochrane review is that the high degree of evidence certainty regarding the efficacy of aprepitant, ramosetron, granisetron, dexamethasone and ondansetron ought to be a key input for the development of recommendations. Added to efficacy, adverse events, cost-benefit ratio, patient preferences and other factors - such as those used in the GRADE ETD srategy 10- , these recommendations could suggest high quality standards of perioperative care that could also be implemented at a population level, for example in the form of clinical practice guidelines.
Although drug combinations could have a slightly greater effect, the Cochrane review suggests that monotherapy might be sufficient in a substantial percentage of patients, even with risk factors.
The results could help target therapies and improve the selection of treatment regimens associated with less drug-related adverse events and lower costs. In high risk patients in whom the use of combinations has been suggested, it is possible to consider, from the physiological perspective, combinations of different mechanisms of action with the aim of increasing efficacy or reducing adverse events. In the systematic review discussed, the most frequent combinations were: dexamethasone-ondansetron, dexamethasone-granisetron and droperidol-ondansetron. Metochlopramide, a very popular antiemetic used in daily practice, which was 92 in the list of the Cochrane review, deserves special mention given the lack of certainty of the results due to methodological limitations, inaccuracy and inconsistency of the original studies. Finally, it is worth highlighting that the Cochrane review only included pharmacological therapies, precluding extrapolation to other types of interventions.
The review by Weibel et al. has the limitations inherent to network meta-analyses. For example, having included a large number of clinical trials, it may be that some differences between the study populations, i.e., transitivity limitations, might not be correctly considered and analyzed. Furthermore, sample sizes vary among studies and, for some comparisons, evidence comes from studies with a small sample size, creating uncertainty regarding differences between interventions. For this reason, classifications assessed on their own can be misleading. Recently, the GRADE group proposed an approach for deriving conclusions from network meta-analyses using clusters, in order to better capture uncertainties regarding differences between individual drugs 11. This approach could be used when deriving conclusions from reviews in the future, bearing in mind the uncertainty described. Finally, the treatment classifications do not include clinical or context variables such as availability or cost, limiting them only to efficacy.
As far as impacts are concerned, the effect on the clinical equipoise and the direction of original research efforts are worth highlighting. Clinical equipoise refers to the genuine uncertainty regarding the superiority of one treatment over another 12,13. In this case, according to the Cochrane review authors, uncertainty regarding the efficacy of five medications is resolved and, therefore, there is no longer reasonable doubt that would justify future research on the efficacy of those drugs versus placebo. If needed, new RCT's could be conducted to compare new drugs versus those already established as standard of care to determine if they are more efficacious (superiority) or have a better safety profile (non-inferiority); or, as the synthesis suggests, direct research towards superiority studies of adverse events related to drugs used for PONV prophylaxis. Finally, large-scale pragmatic studies and their systematic reviews are required in order to assess differences in terms of drug-related adverse events in clinical practice.
CONCLUSION
This article assessed and discussed the results of a Cochrane high-quality systematic review which may have a constructive impact on the practice of anestheisa in terms of PONV prevention and the introduction of evidence synthesis with new methodological tools. This form of dissemination is an integral part of the Cochrane Corners strategy.











 text in
text in 



