INTRODUCTION
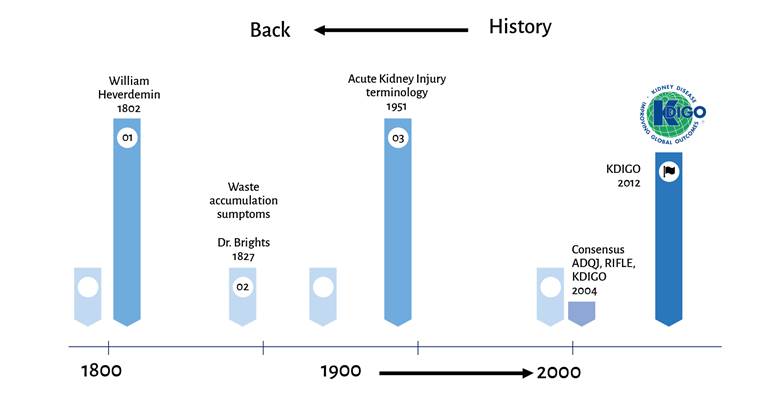
Acute kidney injury (AKI) is defined as a sudden reduction in the kidney's ability to clear toxic waste - such as nitrogen compounds- whether or not accompanied by a fluid and electrolyte homeostasis 1,2; henee, postoperative acute kidney injury (PAKI) is defined according to the same criteria for AKI but identified following a surgical exposure and up to the seventh postoperative day. 3 lt was first described in 1802 by William Heberden, under the term ischuria and in 2004 the Acute Disease Quality Initiative (ADQJ) adapted the modern terminology of acute kidney injury (Figure 1). 4,5 A broad range of classifications have been developed for its categorization and staging, but the most recognized and used are the Acute Dialysis Quality Initiative, RIFLE (risk, + injury + failure + loss + end stage) and the Kidney Disease: Improving Clobal Outcomes (KDICO). 6,7
Several predictive scales have been designed to estimate the incidence of PAKI, including key variables such as previous heart surgery, emergency surgery, and elevated intra or pre-operative creatinine levels. 8 Similarly, in patients with previously preserved kidney funetion undergoing major surgery, the condition was associated with age, peripheral arterial disease, bleeding, colloid infusion, and type of surgery, as the most relevant risk factors. 9 Various intraoperative strategies have been suggested, including hemodynamic management and goal-directed intravascular fluid management. 10,11 This article summarizes the current state-of-the-art relevant information.
With regards to the definition of major surgery, (excluding cardiovascular surgery) an expert consensus concluded that the definition shall be based on the duration and/or exposure to surgical stress >4 h and/ or procedures with estimated blood losses of >15 cm3/kg, and/or surgical procedures including the thoracoabdominal cavity; however, a clearly recognized definition is not yet available. 12
EPIDEMIOLOCY
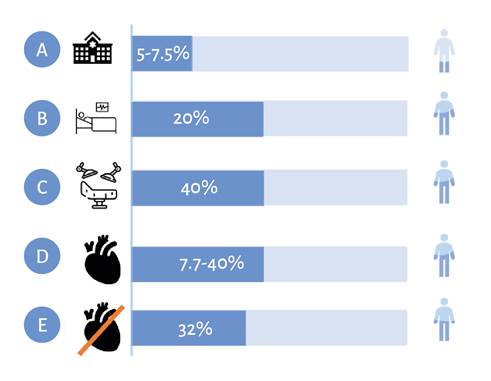
The incidence of PAKI varies according to the diagnostic criteria, the surgical stress exposure, and the coexisting pathologies. 13 Additionally, it has been associated with a higher incidence in particular population groups such as the elderly and obese undergoing major abdominal surgery 14; however, the epidemiological data have not been consistent and there is a broad variability in terms of the incidence of the disease. For instance, the incidence reported for PAKI in major surgery is 13 % 15, with a higher incidence among patients undergoing cardiovascular surgery of between 5 % and 42 %. 16 In fact, kidney damage secondary to cardiovascular surgery is the most frequent cause of renal pathology in the intensive care unit (ICU), following sepsis, with evidence of increased mortality directly proportional to the severity of the AKI (Figure 2). 16-20 However, the incidence of the condition has also been reported in major non-cardiac surgery and it is strongly associated with short and long term adverse outcomes, including a higher risk of chronic kidney disease, cardiovascular events and death. 3,21 Some studies for the identification of the incidence and risk factors associated with postoperative AKI have been conducted in Bogotá, Colombia, but only in the context of cardiovascular surgery. As of this date, there are no data available on the incidence of this pathology at a regional scale.
The mortality of patients with PAKI is one of the most widely studied outcomes. Both the early and late PAKI phenotypes (AKI <48 h and after 48 h, respectively) are associated with a higher mortality (10 fold risk) 23 at one year, as compared against patients with no kidney function impairment (19 % vs. 8 %, respectively). 13,23-25 In patients with severe PAKI progressing to require permanent or temporary renal replacement therapy, the mortality rises to 46 %. 26,27
With regards to PAKI associated healthcare costs, a retrospective study was published in 2015 including50.314 patients undergoing major surgery and concluded that the average cost al most doubles as compared to patients without AKI, and it is even higher when care involves extended mechanical ventilation, cardiovascular complications and sepsis. 28
Pathophysiology and etiology
The pathophysiology of PAKI is multifactorial and involves several aspects, including hypoperfusion secondary to the use of anesthetic agents that have a vasodilation effect, leading to compromised cardiac output (CO) and renal perfusion. Increased blood pressure also affects the glomerular filtration rate, basically as a consequence of increased intra-abdominal pressure, right ventricular dysfunction with edema secondary to fluid overload; hypoperfusion of distant organs with release of injury markers traveling to distant organs as a result of ischemia/hypoperfusion, or surgical trauma; the immune response activation and direct endothelial injury secondary to the inflammatory process, exposure to nephrotoxic agents and finally, the procoagulating activity that promotes the development of vascular microthrombi with the subsequent blood flow obstruction of the glomerular tuft. 3
The risk of developing PAKI will then depend on the exposure to nephrotoxic medications, surgical stress, underlying comorbidities and trans-operative hypoperfusion;consequently, it is important to preoperatively identify any risk factors that increase the incidence, including age >59 years, body mass index (BMI) >32 kg/m2, high-risk surgery, emergency surgery, vascular surgery, liver disease and chronic obstructive pulmonary disease (COPD), as the most representative risk factors. 29 With regards to surgery, cardiovascular interventions show the highest incidence of PAKI, followed by gastrointestinal (56,4 % and 26 %, respectively), due in part to the aging population and larger blood losses impacting kidney perfusion as compared to surgeries with a lower incidence in less morbid and younger population groups. 25,30
DIAGNOSIS
There are currently several classifications for diagnosis; however, the LDICO criteria (Table 1) have been used since 2012. Additionally, this same classification allows for staging of severity, taking two variables into consideration: serum creatinine level (sCr) and diuresis 31.
Table 1 KDIGO Criteria. Serum Creatinine.
| Stage | Serum Creatinine (sCr) in mg/dl | Urinary Output | Biomarker | |
|---|---|---|---|---|
| 1s | No changes or increased sCr <0.3 | No urinary output criteria | - | |
| 1a | Known increased sCr 1.5-1.9 | Diuresis <0.5 mL/kg/h | - | |
| 1b | or presumed to have occurred during the 7 days before, or increased sCr ≥0.3 within 48 hours | During 6-12 h | + | |
| 2a | Increased sCr 2-2.9 | Diuresis <0.5 during ≥12 h | mL/kg/h | - |
| 2b | + | |||
| 3a | Increased sCr 3 or sCr ≥4 or initiation of renal replacement therapy (RRT), or drop in the glomerular filtration rate (GFR) <35 mL/min | Diuresis <0.3 mL/kg/h during ≥24 h or anuria for ≥12 h | - | |
| 3b | + | |||
Source: Adapted from Kellum et al. 32.
Additionally, a tool was validated to estimate the risk of developing PAKI in 2009 33, which was then replaced by another PAKI predictive tool - Simple Postoperative AKI Risk(SPARK) (Table 2), which identifies the groups at higher risk of developing the disease, and includes results-based recommendations; unfortunately, these scales are not widely used during the perioperative period. 34
Table 2 Risk of simple postoperative AKI (SPAKI).
| Preoperative risk factors | Score | Class A |
|---|---|---|
|
|
|
Score <20 |
| AKI <2 % | ||
| Critical AKI <2 % | ||
|
|
|
Class B |
| Score 20-39 | ||
| AKI ≥2 % | ||
| Critical AKI <2 % | ||
|
|
|
Class C |
| Score 40-59 | ||
| AKI ≥10 % | ||
| Critical AKI ≥2 % | ||
|
|
|
Class D |
| Score ≥60 | ||
|
|
|
AKI ≥20 % |
| Critical AKI ≥10 % | ||
|
|
|
Source: Adapted from Park et al. 34
As a general rule, the creatinine levels in patients at high risk of PAKI should be measured at least once a day and even more often, following a risk exposure. 31 Any PAKI, whether it is mild or severe, regardless of its resolution, increases the postoperative morbidity and mortality. 13,35,36 Different biomarkers have been studied to assess the preoperative risk of PAKI; however, none of them have been validated as a presurgical assessment tool so far. 37
In terms of urinary output measurements, a retrospective trial conducted in patients undergoing lung resection under general anesthesia, identified that a urinary output of <0.8 mL/ kg/h is an independent risk factor for the development of PAKI. 38
MANAGEMENT
Avoiding renal hypoperfusion has become a real challenge, since even increasing the renal oxygen supply fails to ensure homogeneity of the perfusion in the renal cortex and medulla, and hence a genuine prevention of PAKI. 24 Low blood pressure and cardiac output, high central venous pressure and low levels of hemoglobin have shown to be good predictors of the development of PAKI. Consequently, some of the intraoperative measures for the prevention of PAKI include maintaining intraoperative hemodynamic stability, avoiding potential nephrotoxic agents, avoiding hypotension and goal-directed fluid management for maintaining euvolemia. 13
Goal-directed therapy includes invasive monitoring of the patient's hemodynamic stability to establish the amount of IV fluids and vasoactive agents to be administered. It is essential to define clear objectives in the new management. 39 Inotropes and vasopressors have the potential to rise the systemic blood pressure and hence renal perfusion, which turns them into a pillar to fight hemodilution, systemic inflammatory response and hypoperfusion evidenced during major surgical procedures. Phenylephrine, ephedrine, norepinephrine, epinephrine, dopamine and vasopressin are the most commonly used and recognized vasopressors in these categories. 40 However, to date, there are no consistent data regarding which vasopressor is more effective for the prevention of PAKI. 13
Now then, due to the significant numbers of kidney injury, fluid restriction therapy has been challenged, particularly in patients with high risk of complications during major abdominal surgery, as compared against more liberal fluid regimens 41; nonetheless, it continues to be a controversial topic.
Some of the available fluids are normal saline solution which has a high sodium and chlorine concentration, as compared with balanced solutions containing additional electrolytes such as potassium, magnesium and calcium and adds other components to achieve the acid-base balance, such as acetate or lactate. With regards to the incidence of postoperative RRT, no differences have yet been identified comparing the use of these types of fluids. 42 However, the use of high-chlorine solutions (normal saline solution), both during the perioperative period and in the intensive care units, is associated with higher rates of PAKI. 43
In terms of blood products transfusion and their association with PAKI, the evidence is limited. Different studies have described how the intraoperative transfusion of red blood cells independently increases the incidence of kidney injury and the morbidity and mortality during the immediate postoperative period, as compared to those who did not require an intraoperative transfusion. 44-46 Nonetheless, from the pathophysiology perspective, it is known that oxygen transport in the blood is essential to reduce kidney ischemia, even under deficient blood flow. The justification for early blood transfusion is based on the oxygen tissue availability. 45 The restriction of this therapy is based on the high risk of its associated adverse events such as lung injury, hemolysis, volume overcharge, immune reaction and infections. 46.
Anesthesia technique and PAKI
Considering the availability of multiple modalities for the delivery of anesthesia, and the fact that the choice of anesthesia depends on the individualized patient assessment, in accordance with the presurgical evaluation and the type of surgery involved, clinical trials have been conducted to assess the impact of certain anesthetic agents in terms of kidney function. However, the findings have been contradictory.
In a study developed in 2017, including 328,540 patients, comparing the postoperative complications between general and regional anesthesia, no difference was found with regards to renal complications. 47 It is well known that there is an important relationship between the choice of anesthetic agent and kidney function, recognizing that some of these agents, i.e.: propofol, dexmedetomidine and ketamine induce anti-inflammatory, anti-necrotizing and antiapoptotic effects; and both, sevoflurane and isoflurane provide preconditioning nephroprotective effects which are crucial in kidney injury from ischemia/reperfusion and become particularly relevant in kidney transplant surgery for organ preservation. 48,49
Inhaled anesthetic agents such as sevoflurane, desflurane, and enflurane have shown controversial results in terms of their role in protecting the kidney function; it has been reported that their use in patients with pre-existing kidney insufficiency did not worsen the condition. 48,50,51 However, recent studies compared general versus total intravenous anesthesia (TIVA) using propofol vs. halogenated agent (sevoflurane) in patients undergoing major abdominal surgery, and there was a higher incidence of PAKI in patients receiving sevoflurane (142 [11.2 %] versus 272 [8.9 %], p=0.02 according to the AKIN criteria, 94 [7.4 %] versus 157 [5.1 %], p=0.004 according to the RIFLE criteria) 52,53; apparently this is associated with deflourination and the production of compound A derived from the interaction with carbon dioxide absorbents, which forms a nephrotoxic haloalkane which is volatile and may be absorbed in the alveolar gas exchange. 48,49 One additional explanation could be the enhanced hemodynamic stability generally recognized with TIVA.
With regards to intravenous anesthetic agents, studies have been conducted with dexmedetomidine, ketamine and propofol, showing that they contribute to regulate the inflammatory pathways by inhibiting oxidative stress, hence granting them a protective effect against damages of various organs, including the kidney. 48,54 Some data support the protective effect of lipid emulsions against ischemia/reperfusion, and in the case of propofol presentations they seem to be the underlying mechanism for its nephroprotective effects 48,55,56, thus suggesting that TIVA with propofol may be an adequate choice with positive clinical results in terms of the incidence of PAKI. 48,52
In terms of the regional techniques, some studies have suggested a potential benefit with the use of epidural catheter-based analgesic techniques (use of local anesthetics such as lidocaine or bupivacaine) in combination with general anesthesia, with regards to the reduction in PAKI, particularly in patients undergoing cardiovascular surgery 48,57; however, the data regarding other types of interventions are not conclusive. 48
CONCLUSION
Following an examination of original and review articles with heterogeneous populations and different hospital settings, the authors of this narrative review conclude that the prevention of PAKI will depend on an individualized risk assessment, taking into account the preoperative risk scales (SPARK), risk factors control (comorbidities, hypovolemia, anemia, use of nephrotoxic agents, previous AKI, fluid electrolyte imbalances, etc.); the selection of an adequate anesthesia technique acknowledging the nephroprotective benefits of intravenous anesthetic agents, awareness of their role in the ischemic and reperfusion processes involved with the pathophysiology of AKI, and adequate vital signs control during the perioperative period. And of course, keeping in mind the variable incidence of this condition that in the absence of timely prevention, diagnosis and treatment, leads to early and late complications.
Readers are encouraged to consult the bibliography for further study of the concepts described in this narrative review, in order to make individualized decisions in the intraoperative management of patients with a potential risk of presenting POP AKI. Furthermore, research studies on this pathology are encouraged on a national scale in order to collect statistics that will enable the adoption of preventive actions for the benefit of the population at risk.











 text in
text in