Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biomédica
Print version ISSN 0120-4157On-line version ISSN 2590-7379
Biomédica vol.24 no.2 Bogotá June 2004
Degeneration of primary afferent terminals following
brachial plexus extensive avulsion injury in rats
Vilma Muñetón-Gómez 1,4 Julian Scott Taylor 2, Sharon Averill 3,
John V. Priestley 3 , Manuel Nieto-Sampedro 2,4
1 Laboratorio de Neurociencias, Instituto Nacional de Salud, Bogotá, D.C., Colombia.
2
Unidad de Neurología Experimental, Hospital Nacional de Parapléjicos, Toledo, España.3
Department of Neuroscience, Queen Mary University of London, London, United Kingdom.4
Instituto de Neurobiología Ramón y Cajal, CSIC, Madrid, España.Important breakthroughs in the understanding regeneration failure in an injured CNS have been made by studies of primary afferent neurons. Dorsal rhizotomy has provided an experimental model of brachial plexus (BP) avulsion. This is an injury in which the central branches of primary afferents are disrupted at their point of entry into the spinal cord, bringing motor and sensory dysfunction to the upper limbs. In the present work, the central axonal organization of primary afferents was examined in control (without lesion) adult Wistar rats and in rats subjected to a C3-T3 rhizotomy. Specific sensory axon subtypes were recognized by application of antibodies to the calcitonin gene-related peptide (CGRP), the P2X3 purinoreceptor, the low-affinity p75-neurotrophin receptor and the retrograde tracer cholera toxin subunit b (TCb ). Other subtypes weres labeled with the lectin Griffonia simplicifolia IB4. Using immunohistochemistry and high resolution light microscopy, brachial plexus rhizotomy in adult rats has proven a reliable model for several neural deficits in humans. This lesion produced different degrees of terminal degeneration in the several types of primary afferents which define sub-populations of sensitive neurons. Between the C6 and C8 levels of the spinal cord,,deafferentation was partial for peptidergic GCRP-positive fibers, in contrast with elimination of non peptidergic and myelinated fibers. Dorsal rhizotomy has provided an adequate experimental model to study sensory alterations such as acute pain and allodynia as well as factors that affect regeneration into the CNS., Therefore, the differential deafferentation response must be considered inr the evaluation of therapies for nociception (pain) and regeneration for brachial plexus avulsion. The anatomical diffierences among the primary afferent subtypes also affect their roles in normal and damaged conditions.
Key words: primary afferents, cervical spinal cord, dorsal root avulsion, lectin Griffonia simplicifolia (IB4), cholera toxin subunit b (CT
b ), calcitonin gene-related peptide (CGRP), purinoreceptor (P2X3).Degeneración de los terminales aferentes primarios de rata luego de lesión extensa por avulsión del plexo braquial
El uso de las neuronas sensoriales primarias ha aportado avances en el entendimiento de las razones por las cuales falla la regeneración cuando el sistema nervioso central (SNC) es dañado. La rizotomía dorsal se puede usar como un modelo experimental de las lesiones por avulsión del plexo braquial, una lesión en la cual son desprendidas, en su punto de entrada en la médula espinal, las ramas centrales de los aferentes primarios causando una disfunción motora y sensorial grave e irreversible del miembro superior. En el presente trabajo, se examinó la organización central de los aferentes primarios en ratas Wistar adultas. Éstas fueron divididas en controles normales no lesionados y en animales rizotomizados entre los niveles cervical 3 y torácico 3 (C3-T3). Se estudió la deaferentación de los subtipos de axones sensoriales utilizando anticuerpos específicos contra el péptido relacionado con el gen de la calcitonina (CGRP), el receptor purinérgico (P2X3), el receptor de baja afinidad p75 para el factor de crecimiento nervioso (NGF) y contra la subunidad â de la toxina de cólera (TC
b ). Otro subtipo fue marcado con la lectina Griffonia simplicifolia IB4. La inmunohistoquímica y la microscopía óptica de alta resolución demostraron que el modelo animal de rizotomía completa del plexo braquial reproduce diversos déficit observados en las lesiones humanas. Esta lesión produce diferentes grados de degeneración terminal entre los diversos tipos de aferentes primarios que definen subpoblaciones de neuronas sensoriales. En los niveles de la médula espinal estudiados (entre C6 y C8), la deaferentación fue parcial para las fibras peptidérgicas GCRPpositivas, en contraste con la eliminación de las fibras no peptidérgicas y las mielinizadas. La rizotomía dorsal es un modelo experimental apropiado para estudiar las alteraciones sensoriales como el dolor agudo y la alodinia, así como los factores que podrían afectar la regeneración en el SNC. Por tanto, la respuesta de deaferentacion diferencial debe ser tenida en cuenta para la evaluación de terapias antinociceptivas y regenerativas tras la avulsión del plexo braquial. Se discute la anatomía de los subtipos de aferentes primarios y su papel en condiciones normales y después de la lesión.Palabras clave: aferentes primarias, médula espinal cervical, avulsión de la raíz dorsal, lectina Griffonia simplicifolia (IB4), subunidad b de la toxina de cólera (CTb), péptido relacionado con el gene de la calcitonina (CGRP), purinorreceptor (P2X3)
The cervico-thoracic zone of the spinal cord receives the projections of the nerves corresponding to the upper limbs and forms the brachial plexus, between C3 and T3 (1-3). Lesions produced by brachial plexus avulsion are frequently caused in adult humans during motorcycle, sports and industrial accidents, and in children by hyperstretching of the angle between the head and neck. The experimental lesion of dorsal rhizotomy and avulsion models in rats reproduce the damage produced by human avulsion (4-6). Depending on the extent of the lesion, consequences include severe motor and sensory deficit and upper limb paralysis, reflex loss, hypersensitivity, acute pain and allodynia and, in the most dramatic cases, self-mutilation (7).
Primary afferent neurons transmit sensory information from tissues and organs. Their cell bodies lie within the dorsal root ganglia (DRG) and are conveniently located for the study of the mechanisms controlling regenerative failure because they have axonal branches both in the central nervous system (CNS) and in the peripheral nervous system (PNS) (8). Following a lesion, the central and peripheral projections show different regenerative capacities. While the peripheral axons show a strong regenerative ability that is supported by their associated Schwann cells (9), the damaged central afferents do not regenerate into the spinal cord (10,11). Dorsal root section (rhizotomy) results in a fast and complete degeneration of the central terminal roots of the axotomized afferents (12). Thus, the primary afferent system has provided a useful model to study the mechanisms preventing regeneration in the central nervous system.
Primary afferent neurons feature a high-leve organization according to their chemical phenotype, conduction velocity, axonal diameter, peripheral receptors, presence or absence of myelin, and axonal arborization pattern within the dorsal horn (13-15). The afferents are classified into two main groups of fibers according to their axon diameter: large (A fibers) which have thick myelinated axons, and small which have nonmyelinated axons (C fibers) or thin-caliber myelinated axons (A delta fibers). Large and medium-sized DRG neurons may be identified by their content of phosphorylated heavy chain (200 kd) neurofilament (16,17). These neurons also express the GM1 ganglioside and give rise to myelinated axons. The beta subunit of cholera toxin (CT
b ), which binds to GM1 and is then internalized by endocytosis, can be injected into peripheral nerves and used to transganglionically label A beta terminals in laminae III and IV of the spinal cord and A delta terminals in lamina I (18,19). The small C fiber DRG cells have been classified on the basis of their function and phenotype (20) into two groups: peptidergic and non-peptidergic. The C fibers that express neuromodulatory peptides (e.g., substance P; SP, calcitonin gene related peptide, CGRP) project to lamina I and lamina II outer of the dorsal horn (21-23). Many of these peptidergic fibers also express the p75 neurotrophin receptor (24). The C fibre nonpeptidergic DRG cells project to lamina II inner of the dorsal horn. These fibers express a glycoprotein that binds the lectin Griffonia simplicifolia IB4 and may also be identified by their fluoride-resistant acid phosphatase activity (25-27). Most of them express the ionotropic purinergic receptor for ATP, P2X3 (28,29). Finally, there is a small population of DRG neurons that bind IB4 and also contain CGRP (24,30).Unilateral dorsal rhizotomy leads to neuroanatomical (31), propioceptive (5), sensory and motor alterations (2, 31), in the forepaw of adult rats. The objectives of present work are: 1) using rhizotomies, to establish an animal model with dorsal horn afferent elimination which allows the evaluation of regenerative and reparative therapies for brachial plexus avulsion; 2) by inmunohistochemistry, to evaluate the effect of complete rhizotomies of brachial plexus over several types of primary afferents. The present work shows how rhizotomy in adult rats reproduces the injuries reported in humans following brachial plexus avulsion. The anatomical results, and the role of the different types of primary afferents in normal and injured conditions, will be discussed further.
Methods
Surgery
Adult male Wistar rats (250-300 g) were obtained from the Instituto Cajal and maintained in standard conditions with access to food and water adlibitum. The experimental protocols adhered to the recommendations of the European Council and Spanish Department of Health for Laboratory Animals and were approved by the Animal Care Committees of our institutions. A total of 24 rats were divided into controls (n=8) and rhizotomy (n=16) animals. Rhizotomized animals were anaesthetized with sodium pentobarbital (Nembutal©, Abbott Laboratories; 50 mg/kg i.p.) and Rompún© (2-5,6-dihidro-4H-1,3-thiazine hydrochloride, Bayer; 3 mg/kg i.p.). The back of each animal was shaved and disinfected with polividone iodine (Asta Medica, Madrid). After opening the muscular layers and fascia with a No. 11 scalpel (Aesculap AG & Co. Kg), an extensive cervico-thoracic hemilaminectomy was performed under aseptic conditions, opening the dura mater with fine iridectomy scissors (Fine Science Tools). After topical application of tetracaine (0.5%, Alcon Cusi SA, El Masnou, Barcelona), the roots between C3 and T3 were identified and cut more than 1mm away from the DREZ using fine iridectomy scissors. The spinal cord was then covered with Gelfoam (Upjohn Co., Kalamazoo, MI), soaked in sterile saline, and the muscle and skin layers were closed together with chromic gut suture and surgical clips, respectively. The animals were maintained in a warm environment until full recovery from anesthesia. In order to evaluate the effect of the rhizotomy on the A beta and A delta sensory afferents, cholera toxin ß (CTß) (33) was used as a transganglionic tracer in three rats of both groups, the control and the rhizotomized animals. In these cases the animals were reanaesthetized 1-3 days prior to perfusion (see below) and 4 µl of 1% CTß was injected using a Hamilton syringe, after which the muscle and skin layers were closed and animals were allowed to recover.
Histology
After survival periods of 3 weeks, all the animals were deeply anaesthetized with sodium pentobarbital (Nembutal©, Abbott Laboratories; 60 mg/kg) and perfused through the ascending aorta with 200 ml of 0.01 M phosphate buffered saline (PBS) followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.4. The spinal cord was dissected out, postfixed in 4% paraformaldehyde for 1 to 2 hours, and cryoprotected in 25% sucrose in PB for 1-2 days. Tissue was included in Tissue-tek O.C.T. (Sakura Finetek), frozen in solid CO2 and transverse 30 µm cryostat sections were recovered throughout C6-C8 on electrostatically-charged glass slides (Merck).
Inmunohistochemistry
Sections were processed for immunofluorescence using a variety of antisera in order to detect different primary afferent subpopulations. These comprised goat antiserum to the cholera toxin T bb (CTß) subunit (1:2000, List Biological Laboratories), rabbit antiserum to CGRP (1:2000, Affiniti), rabbit antibody to the low-affinity p75-neurotrophin receptor (1:5000, Chemicon) and guinea pig antiserum to P2X3 (1:25,000, Neuromics). In addition, the lectin Griffonia simplicifolia IB4 (biotinylated IB4, 10 µg/ml, Sigma-Aldrich) was used as an alternative marker for nonpeptidergic primary afferents. Primary and secondary antibodies (except p75 antibody which did not require permeabilization) were diluted in 0.01 M PBS containing 0.1% sodium azide and 0.2% Triton X-100. Primary antibodies were incubated for 48 hours, followed by three 10 minute washes in PBS, and a 2 hour incubation in secondary antibody (dilution 1:1000) conjugated to tetramethyl rhodamine isothiocyanate (TRITC) or fluorescein isothiocyanate (FITC) (both from Jackson Immunoresearch Laboratories Inc). Detection of biotinylated IB4 was also carried out using ExtrAvidin-FITC (1:500, Sigma-Aldrich, Dorset, UK). Tyramide signal amplification (TSA) labelling was carried out for P2X3 according to Michael & Priestley 1999 (28). Briefly, following a 2 hr incubation period in biotinylated donkey antiguinea pig IgG (1:400, Vector Laboratories, Peterborough, UK), the sections were incubated for 30 min in avidin-biotin Vectastain® Elite peroxidase reagent (Vector Laboratories, Peterborough, UK) followed by biotinyl tyramide (1:75 NEN Life Science Products, Hounslow, UK; TSA-indirect kit) for 8 min, and ExtrAvidin-FITC (1:500, Sigma-Aldrich, Dorset, UK) for 1.5 h. After staining, sections were washed briefly in PBS and then mounted in PBS/glycerol (1:3) containing 2.5% (w/v) 1,4 diazobicyclo (2,2,2) octane (DABCO, antifading agent). Sections were examined on a Leica DMR epifluorescence microscope equipped with a Hamamatsu C4742-95 digital camera. Confocal analysis was carried out on a Leica TCS 4D microscope.
Results
The projections of the forepaw nerves were evaluated both by using the retrograde tracer CTß, and by immunohistochemistry for specific markers of DRG subpopulations following induction of terminal degeneration by rhizotomy of the brachial plexus. A variable elimination of the various types of afferents in the dorsal horn was demonstrated immunohistochemically. The rostrocaudal extension of the lesions (between C3 and T3), the variation in the dorsal horn anatomy at the levels of the spinal cord studied (between C6 and C8), and the variation between animals showed the necessity of a thorough comparison between control and experimental animals. The initial observations and the comparison between normal animals and the horns contralateral to the lesions showed a unilateral projection of the dorsal root fibers at the cervical C6-C8 levels and thus, the immunoreactivity of the horn contralateral to the lesion was used as an intrinsic control for the ipsilateral rhizotomy-induced deafferentation. In our work, findings were very similar among the 16 rhizotomized studied animals.
Retrograde tracing with cholera toxin ß (CTß)
After a 3 day survival period and sacrifice by perfusion, CTß-immunoreactive fibers were observed medially in laminae I, III and IV of the dorsal horn (
figure 1 A-B). In addition, CTß-positive motoneurons in the ventral horn were also demonstrated (figure 1A). The typical varicosities in this type of fiber and their trajectory in the dorsal horn as observed using confocal microscopy are illustrated in figure 1C. No CTß-positive fibers were observed in the horn ipsilateral to the rhizotomy or in the deafferented dorsal root, though some TCß-positive motoneurons were occasionally observed (data not shown).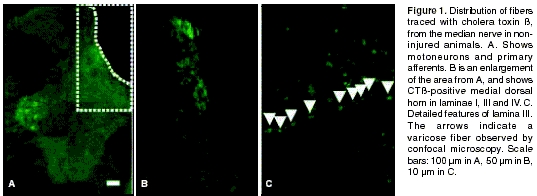
CGRP
Fibers which utilize the CGRP neuropeptide as neuromodulator were found in the dorsal roots and in laminae I and II of the dorsal horn of control animals. In rhizotomized animals, CGRP immunoreactivity was mainly present in axons contralateral to the injury and in some motoneurons in the ventral horn (figure 2). A significant decrease in immunoreactivity in the dorsal horn ipsilateral to the rhizotomy was observed. However, and in spite of the extent of the lesion that involved the whole 9-root section of the brachial plexus (between levels C3 and T3), complete elimination of CGRP-positive fibers in the dorsal horn did not occur (
figure 2). The residual fibers were found mainly in the dorsal horn's lateral surface. No immunoreactive fibers were observed in any areas of the dorsal horn that were different to the typical innervation of peptidergic fibers. Some ectopic fibers in the dura mater and arachnoid were observed in the injured root. Also, regenerating axons were observed and immunoreactivity in the injured root increased significantly when compared to the intact root.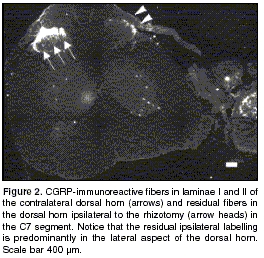
p75 neurotrophin receptor
Contralaterally to the lesion, axons were present in the dorsal root and dorsal horn laminae I and II (
figure 3A). Ipsilateral to the rhizotomy, a high p75-immunoreactivity was observed in both the dorsal (figure 3B) and ventral (data not shown) roots due to invasion by p75 immunoreactive Schwann cells. The primary afferent labeling in the dorsal horn ipsilateral to the lesion was virtually abolished by rhizotomy (figure 3B).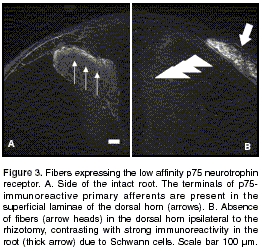
IB4 lectin
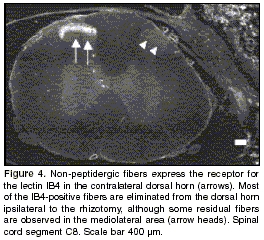
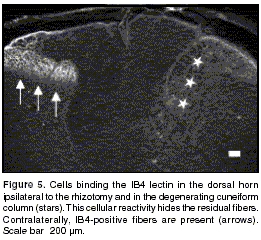
Fibers expressing the IB4-binding glycoprotein were present throughout the mediolateral extent of lamina II of the dorsal horn contralateral to the lesion. Most of the IB4-positive fibers were eliminated from the ipsilateral horn of rhizotomized animals, and only a few weakly labelled axons remained in lamina II (figure 4). This contrasts with the strong immunolabelling of the residual CGRP immunoreactive fibers in the lateral dorsal horn described above. The lectin IB4 also binds to microglia in normal and injured animals. Labelling of these cells was higher in injured animals (figure 5).
P2X3 purinoreceptor
A thin band of P2X3-immunoreactive fibers was demonstrated throughout the mediolateral extent of lamina II of the non-injured dorsal horn (figure 6).
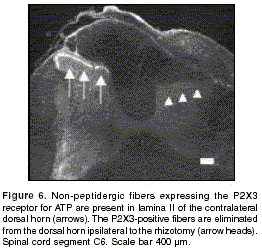
This marker is present in the same non-peptidergic fiber population that binds the IB4 lectin, but has the advantage that it is expressed exclusively by primary afferent axons and not by non-neuronal cells such as microglia. Following rhizotomy, a complete elimination of P2X3 immunoreactivity in the dorsal horn was observed, and some P2X3-immunoreactive fibers were identified in the dorsal root (
figure 6).Discussion
The axons studied in the present work define important subpopulations of primary afferents. This study evaluated the extent of the deafferentation and spontaneous regeneration in the central nervous system. The non-peptidergic fibers with binding sites for the lectin IB4 (many of which express the P2X3 receptor) (28,29) represent a population of non-peptidergic C fibers complementary to that of peptidergic afferents detected with antiserum to CGRP (20). Together with the population traced by CTß, they represent the whole population of DRG neurons. Previous studies have shown that there are very few bilateral dorsal root projections at the levels that we studied. Immunolabelling of the dorsal horn contralateral to the lesion was therefore used in the present study as an intrinsic control, in order to evaluate the same anatomical levels and the density of injured and non-injured primary afferents and to eliminate inter-animal variations (34). However, detailed studies of the response to the rhizotomy are necessary, including the determination of the amount of degenerating synaptic disks and terminals in the corresponding lamina, similar to those carried out for the sacral spinal cord (35).
Transganglionic labeling of cholera toxin ß was detected in the cervical spinal cord 4 days after injection into peripheral nerve, as has previously been demonstrated at the lumbar level (19). Median nerve fibers projecting towards the dorsal horn were observed medially in the dorsal horn between C6 and C8 levels, together with motor neurons dorsolaterally within the ventral horn, demonstrating the contribution of skin and muscle fibers. Similar findings were previously described using the CTß tracer (6) and using the WGA-HRP tracer injected into peripheral nerve (5). The A delta terminals in lamina I and the A beta terminals in laminae III and IV showed similar topographies to what has previously been reported (1,18). The medial distribution of the fibers traced in noninjured animals by tracer injection in the medial nerve, contrasts with the broader mediolateral distribution that is observed following the injection of CTß directly into the dorsal root ganglia (31). The complete elimination of fibers in the terminal field of the horn ipsilateral to the rhizotomy demonstrates its contribution from the primary afferents of the forepaw.
The neuropeptide CGRP is present in 50% of DRG neuron cell bodies and in their primary afferent terminals in the spinal cord (36). CGRP has been postulated as a useful marker for primary afferent terminals (37) due to its fast disappearance after rhizotomy and its absence from neuronal bodies or axon terminals of intrinsic dorsal horn neurons (37-39). CGRP-positive afferents are present in laminae I and II of the dorsal horn (22,26,36,37,40). Unilateral dorsal rhizotomy of nine consecutive segments of the cervico-thoracic spinal cord caused a significant, though incomplete loss of CGRP-immunoreactive fibers in the spinal cord ipsilateral to the rhizotomy. Residual axons were observed in the lateral dorsal horn at all the spinal cord levels studied. These results are compatible with those obtained by Traub RJ et al. (39), who observed the presence of CGRP-positive residual fibers in the lateral section of laminae I and IV after rhizotomy of five roots of the lumbar spinal cord in cats. They concluded that the smalldiameter primary afferents which are CGRPimmunoreactive are capable of projecting at least five segments from their entry segment and supply the superficial laminae of the dorsal horn with collaterals (39). The nature of these residual fibers is still unknown. At the cervical levels studied (C6 to C8), complete sectioning of their roots together with the three rostral roots (C3 to C5) and the three caudal roots (T1 to T3) to these segments studied was carried out in order to prevent axonal growth from non-injured adjacent intact roots into the denerved area. The residual immunoreactive fibers that we observed in the dorsal horn were not in areas that were different to those supplied by the normal, intact innervation. Although axonal collateral sprouts have been reported in the dorsal horn (15), this seems therefore unlikely to be the origin of the residual CGRP staining. However, in order to evaluate the presence of collaterals derived from distant intact roots, additional studies are required such as the use of multiple retrograde tracing (41) together with quantification of axons ipsilateral and contralateral to the rhizotomy and intra-axonal tracing before and after the lesion (42). Somatotopic maps of primary afferent fiber distribution derived from FRAP studies have shown that the lateral section of lamina II does not receive axons from the radial nerve but receives the projections of the dorsal cutaneous branch of the cervical spinal nerves IV-VI. The medial dorsal horn receives cutaneous terminals from the thoracic anterior nerve in the segments C8-T1 (5). Although FRAP is expressed in the non-peptidergic small fibers (26,43), it is thus possible that the residual CGRP-positive fibers may belong to these other nerve branches. In addition, the presence of residual CGRP is involved in the onset of the edema and the pain related with inflammation (44). It is possible that residual CGRP could contribute to and mediate the hyperalgesia response.
The study of p75 immunoreactivity showed axonal projections to laminae I and II of the dorsal horn contralateral to the rhizotomy (45), and a complete elimination of dorsal horn staining ipsilateral to the rhizotomy (31). The process of Wallerian degeneration was demonstrated by the increased p75 immunoreactivity in the lesioned roots. This process includes the proliferation and dedifferentiation of Schwann cells and invasion of the nerve by macrophages that remove myelin and axonal debris, leaving the basal lamina in an intact state. This basal lamina acts as a support for peripheral regeneration (46,47) and central regeneration as far as the dorsal root entry zone (DREZ) but dorsal horn p75 immunoreactive fibers were not observed, indicating a failure to reinnervate the CNS.
The IB4-positive fibers showed a normal projection pattern throughout the mediolateral extent of the dorsal horn lamina II (25-27) and were almost completely eliminated in the dorsal horn ipsilateral to the rhizotomy (27). As in cell culture, the lectin IB4 also binds to the microglial cells in the normal and injured spinal cord which express the glycoprotein (48). According to data by Bradbury et al., nearly 95% of neurons that express the P2X3 receptor belong to the C fiber group which binds the IB4 lectin (28). The P2X3-positive fibers normally project to lamina II of the dorsal horn (4,28,49). Immunoreactivity is exclusively observed in the primary afferents and no intrinsic spinal cord cells with immunoreactivity to the purinergic receptor have been identified (28). Consistent with previous studies, we found that P2X3 fibers were eliminated from the dorsal horn ipsilateral to the rhizotomy (4). The reason for the selective elimination of the P2X3 fibers and the presence of IB4-positive residual fibers following the rhizotomy is still unknown. It has been suggested that the absence of intrinsic P2X3, the short rostrocaudal extension of these fibers, or the difficulty for detecting low levels could contribute to the lack of residual P2X3 staining (31). However this complete elimination of residual afferents in injured animals may be a useful tool to enable detection of regenerating axons in future studies of strategies to promote neural repair.
Axonal degeneration in the DREZ area following stretch and transaction or rhizotomy in the cervical spinal cord have been demostrated by Nomura et al, using NF immunoreactivity. After 14 or 28 days NF IR was not longer present (50). Our data of extensive rhizotomy between C3-T3, complement interestingly this study in four aspects. In the first one, related with localization of primary afferents, our work complements Nomura´s work because we describe change of fibres not only in DREZ but also at the dorsal horn. The second one is related with types of primary afferents. As Nomura did with neurofilament inmunolabelling, we evaluated thick myelinated axons using CTß tracing. We found that CTß positive axons were eliminated by rhizotomy lesion after three weeks post lesion. Additionally other type of primary afferents: peptidergic (evaluated by CGRP or p75 immunoreactivity) and non-peptidergic (evaluated by P2X3 immunoreactivity or labelling with lectin IB4) were studied as the same post lesion time. These afferents were almost similarly affected by extensive rhizotomy. However, residual CGRP fibers were present at all cervical levels studied. One advantage of our model is that it allowed us to show differential CGRP afferent denervation between levels of cervical spinal cord. After extensive rhizotomy the presence of these residual fibres was high in the caudal levels of cervical cord, including thoracic (T1-T3) segments (31). In the work of Guenot M et al., the spinal level C7 studied for electrophysiological recordings has been chosen because it is exactly in the middle of the posterior cervical rhizotomy between C5- T1 (51), without taking into account the content of residual fibres and its difference between segments. Our discovery will help to design controlled studies and to make a more adequate choice of the level of spinal cord studied in order to measure the effect of deafferentation. The third one is related with levels of spinal cord injured. Despite the big rhizotomy between C3-T3, only thick and non peptidergic fibers were completely eliminated. This finding suggests that this model could be used especially to study re-afferentation by these types of afferents in future therapeutic assays. Finally, the complete section of the root is experimentally reproducible while the stretch lesion can be difficult to reproduce. The adequate force to cause the lesion is not established and is therefore a subjective parameter. In conclusion, reproducibility of our extensive rhizotomy lesion, the study of different type of primary afferents in both the root and the dorsal horn, and the differences between spinal segments guarantee our experimental model of brachial plexus avulsion.
Tactile allodynia is induced by activation of various types of fibers after damage to a peripheral nerve (52,53). The allodynia is evident as a marked hypersensitivity to stimuli which under normal conditions would induce an innocuous sensation. Roles for both CGRP and ATP have been demonstrated in nociception. Increased release of CGRP takes place in hyperalgesia and inflammation processes (44). In addition, tissuederived ATP promotes release of glutamate by P2X3-expressing primary afferents (54) and subsequent activation of NMDA receptors and calcium influx can result in increased neuronal excitability and plasticity of the postsynaptic dorsal horn cells (55,56).
In conclusion, dorsal rhizotomy of the brachial plexus represents a good experimental model for studying the changes which take place in the central and peripheral nervous system after damage, together with the mechanisms that inhibit regeneration, and therapeutic strategies that can be used to guide regenerating axons through inhibitory environments towards synaptic fields appropriate for recovery of lost function.
Acknowledgments
Financial support for this work was provided by the European Community (Biomed II contract BMH4-97-2586), the Spanish Health Department (FIS98/0830), The British Council Programme (#8375) of Acciones Integradas and the International Spinal Research Trust. The authors thank Dr. J. Collazos-Castro and Dr. A. Verdú for their helpful advice and critical corrections on the manuscript. Vilma C Muñetón-Gómez holds a Colciencias and a Fundación Carolina fellowships from the Colombian and the Spanish government respectively. We thank Carmen Hernández Capitán for the confocal photography of Ctßimmunoreactive fibers.
Correspondencia:
Vilma Muñetón, Laboratorio de Neurociencias, Instituto Nacional de Salud, Calle 26 No.51-60, oficina B-202, Bogotá D.C., Colombia
Recibido: 27/01/04; aceptado: 04/06/04
References
1. Castro-Lopes JM, Coimbra A. Spinal cord projections of the rat main forelimb nerves, studied by transganglionic transport of WGA-HRP and by the disappearance of acid phosphatase. Brain Res 1991; 542:187-2. [ Links ]
2. LaMotte CC, Kapadia SE, Shapiro CM. Central projections of the sciatic, saphenous, median, and ulnar nerves of the rat demonstrated by transganglionic transport of choleragenoid-HRP (B-HRP) and wheat germ agglutinin-HRP (WGA-HRP). J Comp Neurol 1991; 311:546-62. [ Links ]
3. Yoshida N, Nishiyama K, Tonosaki Y, Kikuchi S, Sugiura Y. Sympathetic and sensory innervation of the rat shoulder joint: a WGA-HRP tracing and CGRP immunohistochemical study. Anat Embryol (Berl) 1995; 191:465-9. [ Links ]
4. Ramer MS, Duraisingam I, Priestley JV, McMahon SB. Two-tiered inhibition of axon regeneration at the dorsal root entry zone. J Neurosci 2001;21:2651-60. [ Links ]
5. Taylor JS, Muneton-Gomez VC, Eguia-Recuero R, Nieto-Sampedro M. Transplants of olfactory bulb ensheathing cells promote fuctional repair of multiple dorsal rhizotomy. Prog Brain Res 2001;132:641-64. [ Links ]
6. He JW, Hirata K, Kuraoka A, Kawabuchi M. An improved method for avulsion of lumbar nerve roots as an experimental model of nitric oxide-mediated neurona degeneration. Brain Res Brain Res Protoc 2000;5:223-30. [ Links ]
7. Rossitch EJ, Oakes WJ, Ovelmen-Levitt J, Nashold BSJ. Self-mutilation following brachial plexus injury sustained at birth. Pain 1992;50:209-11. [ Links ]
8. Bradbury EJ, McMahon SB, Ramer MS. Keeping in touch: sensory neurone regeneration in the CNS. Trends Pharmacol Sci 2000;21:389-94. [ Links ]
9. Csillik B, Knyihar-Csillik E. Transganglionic regulation of primary sensory neurons. Acta Morphol Hung 1988; 36:35-46. [ Links ]
10. Liuzzi FJ, Lasek RJ. Astrocytes block axonal regeneration in mammals by activating the physiological stop pathway. Science 1987;237:642-5. [ Links ]
11. Tessler A, Himes BT, Houle J, Reier PJ. Regeneration of adult dorsal root axons into transplants of embryonic spinal cord. J Comp Neurol 1988;270:537-48. [ Links ]
12. Coimbra A, Ribeiro-da-Silva A, Pignatelli D. Effects of dorsal rhizotomy on the several types of primary afferent terminals in laminae I-III of the rat spinal cord. An electron microscope study. Anat Embryol (Berl) 1984;170:279-87. [ Links ]
13. Martínez M, Quiroga N, Castellanos JE, Hurtado H. Subpoblaciones neuronales presentes en el ganglio de la raíz dorsal. Biomédica 2000;20:248-60. [ Links ]
14. Wilson P, Kitchener PD. Plasticity of cutaneous primary afferent projections to the spinal dorsal horn. Prog Neurobiol 1996;48:105-29. [ Links ]
15. Woolf CJ. Physiological, inflammatory and neuropathic pain. Adv Tech Stand Neurosurg 1987;15:39-62. [ Links ]
16. Lawson SN and Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J Physiol 1991;435:41-63. [ Links ]
17. O'Brien C, Woolf CJ, Fitzgerald M, Lindsay RM, Molander C. Differences in the chemical expression of rat primary afferent neurons which innervate skin, muscle or joint. Neuroscience 1989;32:493-502. [ Links ]
18. Dodd J, Solter D, Jessell TM. Monoclonal antibodies against carbohydrate differentiation antigens identify subsets of primary sensory neurones. Nature 1984;311 : 469-72. [ Links ]
19. Robertson B, Grant G. A comparison between wheat germ agglutinin and choleragenoid-horseradish peroxidase as anterogradely transported markers in central branches of primary sensory neurones in the rat with some observations in the cat. Neuroscience 1985;14:895-905. [ Links ]
20. Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron 1998;20:629-32. [ Links ]
21. McCarthy PW, Lawson SN. Differing action potential shapes in rat dorsal root ganglion neurones related to their substance P and calcitonin gene-related peptide immunoreactivity. J Comp Neurol 1997;388:541-9. [ Links ]
22. Naim M, Spike RC, Watt C, Shehab SA, Todd AJ. Cells in laminae III and IV of the rat spinal cord that possess the neurokinin-1 receptor and have dorsally directed dendrites receive a major synaptic input from tachykinin-containing primary afferents. J Neurosci 1997;17:5536-48. [ Links ]
23. Todd AJ, Spike RC. The localization of classical transmitters and neuropeptides within neurons in laminae I-III of the mammalian spinal dorsal horn. Prog Neurobiol 1993;41:609-45. [ Links ]
24. Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci 1995;7:1484-94. [ Links ]
25. Silverman JD, Kruger L. Acid phosphatase as a selective marker for a class of small sensory ganglion cells in several mammals: spinal cord distribution, histochemical properties, and relation to fluorideresistant acid phosphatase (FRAP) of rodents. Somatosens Res 1988;5:219-46. [ Links ]
26. Silverman JD, Kruger L. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J Neurocytol 1990;19:789-801. [ Links ]
27. Bennett DL, Michael GJ, Ramachandran N, Munson JB, Averill S, Yan Q, et al. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci 1998;18:3059-72. [ Links ]
28. Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci 1998;12:256-68. [ Links ]
29. Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci 1999;19:1844-54. [ Links ]
30. Zwick M, Davis BM, Woodbury CJ, Burkett JN, Koerber HR, Simpson JF, et al. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci 2002;22:4057-65. [ Links ]
31. Muñetón-Gómez V, Averill S, King V, Yan Q, Doncel Pérez E, Caballero-Chacón S, et al. Transplantation of olfactory bulb ensheathing cells fails to promote significant axonal regeneration from dorsal roots into the rat cervical cord. J Neurocytol 2003;32:53-70. [ Links ]
32. Ramer MS, Bradbury EJ, McMahon SB. Nerve growth factor induces P2X(3) expression in sensory neurons. J Neurochem 2001;77:864-75. [ Links ]
33. Bradbury EJ, Khemani S, King VR, Priestley JV, McMahon SB. NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur J Neurosci 1999;11:3873-83. [ Links ]
34. Polistina DC, Murray M, Goldberger MF. Plasticity of dorsal root and descending serotoninergic projection after partial deafferentation of the adult rat spinal cord. J Comp Neurol 1990;299:349-63. [ Links ]
35. Chung K, McNeill DL, Hulsebosch CE, Coggeshall RE. Changes in dorsal horn synaptic disc numbers following unilateral dorsal rhizotomy. J Comp Neurol 1989;283:568-77. [ Links ]
36. Ju G, Hokfelt T, Brodin E, Fahrenkrug J, Fischer JA, Frey P, et al. Primary sensory neurons of the rat showing calcitonin gene-related peptide immunoreactivity and their relation to substance P-,somatostatin-, galanin-, vasoactive intestinal polypeptide- and cholecystokinin-immunoreactive ganglion cells. Cell Tissue Res 1987;247:417-31. [ Links ]
37. Navarro X, Valero A, Gudino G, Fores J, Rodriguez FJ, Verdu E, et al. Ensheathing glia transplants promote dorsal root regeneration and spinal reflex restitution after multiple lumbar rhizotomy. Ann Neurol 1999;45: 207-15. [ Links ]
38. Chung K, Lee WT, Carlton S.M. The effects of dorsal rhizotomy and spinal cord isolation on calcitonin generelated peptide-labeled terminals in the rat lumbar dorsal horn. Neurosci Lett 1988;90:27-32. [ Links ]
39. Traub RJ, Solodkin A, Ruda MA. Calcitonin generelated peptide immunoreactivity in the cat lumbosacral spinal cord and the effects of multiple dorsal rhizotomies. J Comp Neurol 1989;287:225-37. [ Links ]
40. Spike RC, Puskar Z, Sakamoto H, Stewart W, Watt C, Todd AJ. MOR-1-immunoreactive neurons in the dorsal horn of the rat spinal cord: evidence for nonsynaptic innervation by substance P-containing primary afferents and for selective activation by noxious thermal stimuli. Eur J Neurosci 2002;15:1306-16. [ Links ]
41. Guntinas-Lichius O, Angelov DN, Tomov TL, Dramiga J, Streppel M, Neiss WF, et al. Transplantation of olfactory ensheathing cells stimulates the colateral sprouting from axotomized adult rat facial motoneurons. Exp Neurol 2001;172:70-80. [ Links ]
42. Guntinas-Lichius O, Wewetzer K, Tomov TL, Azzolin N, Kazemi S, Streppel M, et al. Transplantation of olfactory mucosa minimizes axonal branching and promotes the recovery of vibrissae motor performance after facial nerve repair in rats. J Neurosci 2002;22: 7121-31. [ Links ]
43. Nagy JI, Hunt SP. Fluoride-resistant acid phosphatasecontaining neurones in dorsal root ganglia are separate from those containing substance P or somatostatin. Neuroscience 1982;7:89-97. [ Links ]
44. Traub RJ. The spinal contribution of substance P to the generation and maintenance of inflammatory hyperalgesia in the rat. Pain 1996;67:151-61. [ Links ]
45. Verge VM, Merlio JP, Grondin J, Ernfors P, Persson H, Riopelle RJ, et al. Colocalization of NGF binding sites, trk mRNA, and low-affinity NGF receptor mRNA in primary sensory neurons: responses to injury and infusion of NGF. J Neurosci 1992;12:4011-22. [ Links ]
46. Knyihar E, Csillik B. Effect of peripheral anatomy on the fine structure and histochemistry of the Rolando substance: degenerative atrophy of central processes of pseudounipolar cells. Exp Brain Res 1976;26:73-87. [ Links ]
47. Csillik B, Knyihar-Csillik. Transganglionic regulation of primary sensory neurons. Acta Morphol Hung 1988; 36:35-46. [ Links ]
48. Gudino-Cabrera G, Nieto-Sampedro M. Ensheathing cells: large purification from adult olfactory bulb, freezepreservation and migration of transplanted cells in adult brain. Rest Neuro Neurosc 1996;10:25-34. [ Links ]
49. Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, et al. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur J Neurosci 1998;10:3470-8. [ Links ]
50. Nomura H, Furuta A, Iwaki T. Dorsal root rupture injury induces extension of astrocytic processes into the peripheral nervous system and expression of GDNF in astrocytes. Brain Research 2002;950:21-30. [ Links ]
51. Guenot M, Bullier J, Sindou M. Clinical and electrophysiological expression of deafferentation pain alleviated by dorsal root entry zone lesions in rats. J Neurosurg 2002;97:1402-9. [ Links ]
52. Woolf CJ, Shortland P, Reynolds M, Ridings J, Doubell T, Coggeshall RE. Reorganization of central terminals of myelinated primary afferents in the rat dorsal horn following peripheral axotomy. J Comp Neurol 1995;360:121-34. [ Links ]
53. Woolf CJ, Fitzgerald M. Somatotopic organization of cutaneous afferent terminals and dorsal horn neuronal receptive fields in the superficial and deep laminae of the rat lumbar spinal cord. J Comp Neurol 1986;251: 517-31. [ Links ]
54. Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature 1997;389:749-53. [ Links ]
55. Benham CD, Bouvier MM, Evans ML. Changes in cytoplasmic calcium induced by purinergic P2X receptor activation in vascular smooth muscle cells and sensory neurons. Adv Exp Med Biol 1991;304:229-39. [ Links ]
56. Bouvier MM, Evans ML, Benham CD. Calcium influx induced by stimulation of ATP receptors on neurons cultured from rat dorsal root ganglia. Eur J Neurosci 1991;3:285-91. [ Links ]














