Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biomédica
Print version ISSN 0120-4157On-line version ISSN 2590-7379
Biomédica (Bogotá) vol.24 no.4 Bogotá Dec. 2004
Protein malnutrition up-regulates growth hormone receptor
expression in rat splenic B lymphocytes
Wilson Mejía-Naranjo 1, 2, Myriam Sánchez-Gomez1
1 Laboratorio de Hormonas, Departmento de Química, Universidad Nacional de Colombia, Bogotá, Colombia.
2
Departmento de Nutrición y Bioquímica, Pontificia Universidad Javeriana, Bogotá, Colombia.The reciprocal interaction between the endocrine and immune systems has been the subject of active research during the last decade, and an important body of evidence has accumulated supporting the role of the GH/IGF axis in immune function. More recently, the GH/IGF axis has been postulated as playing an important role in the modulation of stress condtions, such as catabolic stages, aging-related disorders, immunodeficient aids patients and malnutrition. Whether these effects are exerted through endocrine, autocrine or paracrine mechanisms remains to be determined for different immune cell types and tissues. The aim of the current study was to define which specific subsets of lymphocytes are the primary targets for GH action. In addition, the regulatory role of stress induced by protein restriction was investigated with respect to the relative distribution of GH receptor positive lymphoid cells. Normal growing rats were fed isocaloric diets with variable protein content (0, 4, 8, 12 and 20%) for a period of 14 days. The lymphoid cells were then separated from spleen, lymph nodes and peripheral blood lymphocytes. Flow cytometry analysis measured the binding characteristics of Fluos-rrGH to lymphocytes together with specific PE-labelled mAbs defining CD4+ and CD8+ T cells and B lymphocytes. The pattern of expression of the GH receptor differed among the lymphoid tissues and cell subsets. Spleen was the most responsive organ to protein deprivation with highest GH receptor expression in B lymphocytes, followed by CD4+ T cells. As the protein intake was decreased from 20% to 0%, the percentage of GHR positive cells increased from 12% to 52% in splenic B lymphocytes and from 8% to 17% in CD4+ T cells. In contrast, only 10%-13% of lymphocytes in lymph nodes and 2%-4% in circulation, showed binding sites to GH associated with protein deprivation. In conclusion, the increase in GH receptors on lymphocytes under catabolic stress induced by protein malnutrition gives support to the hypothesis of a modulatory role of the GH/IGF axis in preserving the homeostasis of immune tissues.
Key words: growth hormone receptor, malnutrition, lymphocytes.
La desnutrición proteica estimula la expresión de receptores de la hormona del crecimiento en linfocitos B esplénicos de rata
La interacción recíproca entre los sistemas endocrino e immune ha sido objeto en la última década de numerosas investigaciones que han aportado evidencia sustancial sobre el papel del eje GH/IGF-I en el sistema inmune. Recientemente, se ha postulado que el eje GH/IGF-I ejerce una función importante en la modulación de condiciones de estrés, tales como estados catabólicos, trastornos asociados con el envejecimiento, síndrome de inmunodeficiencia adquirida y desnutrición. No se conoce aún si estos efectos son ejercidos a través de mecanismos endocrinos, autocrinos o paracrinos en los diferentes tipos de tejidos del sistema inmune. El objetivo de este estudio fue establecer los principales subtipos celulares linfoides que son blanco de la acción de la hormona de crecimiento. Además, se estudió el efecto regulador del estrés inducido por la desnutrición proteica sobre la distribución relativa de células linfoides positivas al receptor de GH (GHR). Ratas normales se sometieron a dietas isocalóricas de contenido variable de proteína (0, 4, 8, 12 y 20%) durante 14 días y se aislaron los linfocitos de bazo, ganglios linfáticos y sangre periférica. Mediante citometría de flujo se analizó la unión a Fluos-rrGH y los linfocitos CD4+, CD8+ y B se definieron empleando anticuerpos monoclonales específicos marcados con PE. Se encontró que el patrón de expresión de GHR varía con el tejido y tipo celular linfoide. El bazo es el órgano más sensible al déficit de proteína, y la mayor expresión de receptores para GH se presenta en las células B seguidas por las células CD4+. Al reducir el contenido de proteína en la dieta de 20% a 0% se observó un incremento del 12% al 52% en el número de células B positivas para el receptor de GH y del 8% al 17% en las células CD4+ positivas para GHR en el bazo. En contraste, sólo el 10%-13% de linfocitos de los ganglios linfáticos y 2%-4% de la sangre periférica presentaron receptores para GH en su superficie, los cuales no se vieron afectados significativamente por el déficit de proteína. En conclusión, el incremento en los receptores para GH en linfocitos bajo estrés catabólico inducido por la restricción en proteína, apoya la hipótesis del papel modulador del eje GH/IGF-I en la preservación de la homeostasis del sistema inmune.
Palabras clave: receptor de hormona de crecimiento, malnutrición, linfocitos.
In recent years, it has become apparent that a tight communication exists between the endocrine and immune systems. Of particular importance is the role of growth hormone (GH)/insulin-like growth factor-I (IGF-I) axis in immune function. GH and IGF-I contribute to regulate, in concert with cytokines, the development and function of the immune system. Whether these effects are brought about through endocrine, autocrine or paracrine mechanisms remains to be conclusively determined for different immune cell types and tissues (1,2).
Weigent et al. (3) first established the extrapituitary production of GH in human lymphocytes. These researchers used fluorescent-labeled anti- GH antibodies to show that the number of Ghpositive cells doubled in response to mitogenic stimulation. This result was extended to show that GH messenger RNA (mRNA) is expressed in human lymphocytes and translated into a Ghimmunoreactive protein with a molecular weight similar to that of pituitary GH (4). GH receptors have been detected in normal lymphoid cells, as well as in the transformed human lymphoblastoid cell line IM-9 (5,6). In the rat the GH receptor (GHR) has been shown to be widely expressed with comparatively much lower levels of expression in lymphoid tissues (7).
Nutrition is an important determinant of immune responses; deficits in protein and calories have deleterious effects on the host´s defense systems. Both protein and energy intake are critical in the regulation of serum levels of IGF-I, IGF binding protein-3 (IGFBP-3), IGF binding protein-1 (IGFBP-1), GH and GH binding protein (GHBP), as well as the expression levels of the GHR and the IGF-I receptor (IGF-IR) (8-10). It is known that a GH resistant state is induced during nutritional stress, depending on the specific type and length of nutrient deprivation (11). In humans and in several other species, when food intake is reduced, serum GH levels are increased (except in the rat) and circulating levels of IGF-I, IGFBP-3 and GH-BP are reduced (11,13).
Despite the growing knowledge of the actions of GH on cells of the immune system, further studies are needed to understand which specific subsets of lymphocytes are the primary targets for GH action (2). One approach to this question is the quantitative analysis of the GH receptors on subpopulations of lymphocytes using flow cytometry.
The aim of this work was to study the binding characteristics of recombinant rat GH (rrGH), coupled to Fluos [5(6)-carboxyfluorescein-Nhydrosuccinimide ester], to lymphocytes and to determine the relative distribution of growth hormone receptors on the cell subpopulations from some lymphoid tissues in normal growing rats under catabolic stress caused by protein restriction. In addition some elements associated to the GH/IGF-I axis were also determined.
Materials and methods
Animals and dietary protocol
Male Wistar rats (Instituto Nacional de Salud, Bogotá, Colombia) weighing approximately 110 g were individually housed in steel metabolism cages and were given a diet containing 12% protein (i.e., 12 g of crude protein per 100 g of food, ICN Biomedicals, Aurora, OH) for a 5-day preadaptation period. After this period, animals were randomly assigned into five groups of six animals each with diets including 0, 4, 8, 12 or 20% protein for a period of 14 days (ICN Biomedicals, Aurora OH). These five diets are isocaloric and each provides 3.7 kcal/g. Rats were subjected to a lightdark cycle of 12/12 h at a constant temperature of 22±2°C. Animals were given their diets in a powdered form and had free access to tap water. Individual food rations for the whole experimental period were weighed. At the end of the experiment the non-consumed food was weighed and feed consumption calculated. Body weight was measured every third day.
Sampling of blood and tissues
On day 0 blood samples were taken from the orbital plexus under diethyl ether anesthesia and serum samples were stored at -20°C. At the end of the experiment rats were anaesthetized intraperitoneally with 0.4 ml/100 g body weight of RompunTM (20 mg/ml)-KetalarTM (50 mg/ml)-saline (9 g NaCl/l) (1:2:3 v/v) and exsanguinated by cardiac puncture. A sample volume of 1 ml was allowed to clot, the serum was separated and frozen at -20°C until analysis, and 0.5 ml were collected in heparinized tubes and processed for determinations on PBL (peripheral blood lymphocytes) by flow cytometry. Spleen and mesenteric lymph nodes were removed quickly, rinsed and placed in cold Hank's balanced salt solution until processed by flow cytometry on the same day. The animal protocol was approved by the Animal Care and Use Committee of the National University of Colombia (Bogotá, Colombia).
Flow cytometric analysis
Flow cytometric analysis was used to determine the percentage of cells that were reactive for GHR (GHR+) in rats fed various diets. Single cell suspensions of splenocytes and from lymph nodes were prepared in Hank's balanced salt solution. The cells were washed and red blood cells in splenocyte suspensions were lysed using PharM LyseTM lysis solution (BD Pharmingen San Diego, CA). Cells were resuspended in PBS (phosphate buffer saline) containing 0.3% BSA and 0.1% sodium azide. Cell viability was determined by trypan blue exclusion using a hemocytometer.
The following monoclonal antibodies (mAb) were purchased from BD Pharmingen: PE-conjugated mouse anti-rat CD4, CD8, CD45RA and immunoglobulin G1(IgG1) isotype control. The CD4 mAb was used to identify helper T-cells, the CD8 mAb was used to identify suppressor/cytotoxic T cells, and the CD45RA mAb was used to identify B cells.
Fluorescein labeling
Recombinant rat GH (rrGH) was obtained from the National Hormone and Peptide Program, NIDDK (Torrance, CA). Recombinant rGH was conjugated to Fluos [5(6)-carboxyfluorescein-N-hydrosuccinimide ester] using a fluorescein labeling kit (Boehringer Mannheim). Briefly, 1 mg of hormone was dissolved in 1 ml of 100 mM sodium bicarbonate buffer at a pH of 8.5. Fluos was dissolved in dimethylsulfoxide at 20 mg/ml, and 10 µl of this solution was added to the dissolved hormone. The resulting solution was gently stirred for 2 h at room temperature in the dark. Free Fluos was removed by gel filtration with Sephadex G-25 (Pharmacia) in PBS with 0.1% sodium azide. The molar ratio of Fluos:protein (F/P) was approximately 10:1. This ratio results in the incorporation of approximately 3 to 5 molecules of Fluos per molecule of rhGH. BSA-Fluos was used as a control with similar F/P molar ratio. The hormone-Fluos conjugate was mixed 1:1 with glycerol and stored at -20 °C.
The first set of experiments was conducted to investigate whether Fluos-rrGH was able to bind to rat lymphocytes. The human IM-9 lymphoblastoid cell line obtained from ATCC (American Type Culture Collection) was used as a control. For time course and saturation studies, IM-9 cells or splenocytes (1 x 10
6) were incubated with increasing concentrations of fluos-rrGH (0.5 µg -20 µg) at different times (0.5 h - 4 h) and processed as indicated below for cytofluorometry. The binding specificity was performed on both, IM-9 cells and splenocytes; briefly, 1 x 106 cells were incubated for 1 h with 1 µg of fluos-rrGH in the presence of 30 µg or 100 µg of unlabeled rrGH and processed as described previously (10). Fluos-BSA and FITCanti-mouse IgM were used as controls.Cells derived from the corresponding tissues mentioned above (1 x 10
6 cells) and blood (50 µl) were co-labeled in a set of three tubes with FluosrrGH and either PE-anti CD4, PE-anti CD8, Peanti CD45RA in PBS containing 0.3% BSA and 0.1% sodium azide. Fluos-BSA and PE-IgG1 were added to a different tube as control. Recombinant rGH and BSA were used at a concentration of 1 µg/tube. Cells were then washed twice and resuspended in PBS containing 1% paraformaldehyde. Red blood cells from the PBL samples were lysed immediately after labeling using PharM LyseTM lysis solution (BD Pharmingen, San Diego CA) washed and resuspended as indicated above. Cell acquisition was performed in a FACScanTM flow cytometer, and a minimum of 50,000 events was acquired for each test. Data were analyzed using Cell-QuestTM software (Becton Dickinson).Hormone determinations
Serum IGF-I, prolactin and GH levels were measured by radioimmunoassay (RIA) (rat/mouse IGF-I assay system, rat prolactin assay system and rat GH system, National Hormone and Pituitary Program. Harbor-UCLA medical Center, Torrance, CA. Kindly provided by Dr. A. F. Parlow). Serum insulin levels were determined using a highly sensitive rat insulin RIA kit (Linco Research Inc., St.Charles, MO).
SDS-PAGE and Western ligand binding assay
Serum IGFBP levels were measured in serum collected on day 15. Samples containing 2 µl of serum were mixed with 2 µl of 2X nonreducing SDS protein gel loading solution (Quality Biological Inc. Gaithersburg, MD). The resulting samples were then subjected to SDS-PAGE on 4-20% gradient gels (Novex, San Diego, CA). Proteins were then transferred to nitrocellulose membranes (0.2 µm) using standard electroblotting methods. Membranes were blocked with TBS (Tris Buffer Saline) containing 1% BSA and incubated with 1.5 x 10
6 cpm of [125I]-IGF-I (Amersham, Chicago, IL) overnight at 4°C in TBS with 0.1% tween-20. Membranes were washed three times with TBS containing 0.1% tween-20 and exposed to Phosphorimager screens (Fuji Photo Film Co., Ltd., Kanagawa, Japan). Levels of IGFBP-3 and IGFBP-1 were determined by quantifying the levels of [125I]-IGF-I incorporated into bands corresponding to 40-45 kd and 25-28 kd, respectively.Statistics
Statistical analyses were performed using the twotailed t test (SAS Institute Inc., 1999). Protein level was considered the main source of variation and mean comparisons for each variable under study were performed between groups receiving different protein diets. Values were considered to be statistically different when P values were less than 0.05.
Results
Food consumption and body weight
Animals fed 0% and 4% protein diets each lost 40±5.1 g and 8.7±1.6 g of body weight, respectively. At 8%, 12% and 20% protein diets, rats gained 56.3±9.6 g, 64±9.6 g and 75±11.8 g of body weight, respectively. Cumulative food intake in grams over the 14-day period was as follows: animals fed 20% protein diet, 259±5 g; 12%, 244±4 g; 8%, 240±14 g; 4%, 198±25 g and 0%, 143±5 g. Rats fed a 0% or a 4% protein diet consumed significantly less food than did animals fed a 8%, 12% or 20% protein diets (
table 1). However, the animals that received the 0% and 4% protein diets ate significantly more food per gram of body weight than did animals that received 8%, 12% and 20% protein diets (table 1). Thus the 0% and 4% protein diets induced protein deficiency, but not energy deficiency, since these animals consumed equal or greater number of calories that did rats on 8%, 12% and 20% protein diets.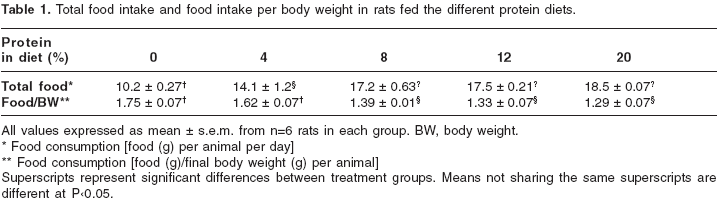
Relative distribution of lymphocytes in lymphoid organs
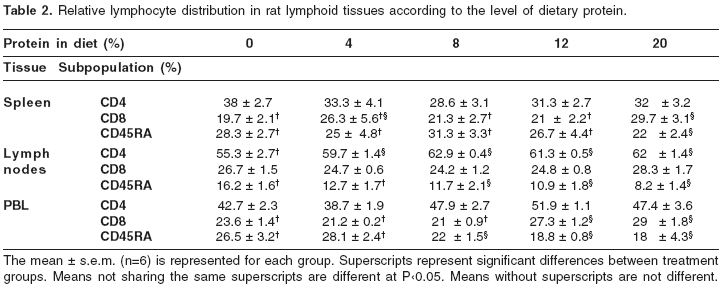
The cellular distribution of CD4+ T cells, CD8+ T cells and B cells in the spleen, mesenteric lymph nodes and peripheral blood of male rats fed various diets is shown in table 2. The percentage of CD4+ T cells in these tissues did not differ among rats fed 4, 8, 12, or 20% protein diets; however a trend to increase was seen in the spleen of rats fed 0% protein diet. Conversely, the percentage of CD4+ T cells was significantly diminished in lymph nodes in rats fed 0% protein diet. The percentage of CD8+ T cells in the spleen and peripheral blood was significantly (P<0.05) lower in rats fed 0% protein diet compared to animals fed with 12 or 20% protein diets. In contrast, the percentage of B cells tended to increase in spleen, mesenteric lymph nodes and peripheral blood as the amount of dietary protein decreased. In rats fed 0 and 4% protein diets, the percentage of B cells in lymph nodes and peripheral blood was significantly higher than in animals fed 8, 12 and 20% protein diets (P<0.05). The percentage of splenic B cells was significantly lower in rats fed 20% protein diet than in animals fed 4 or 0% protein diets (P<0.05).
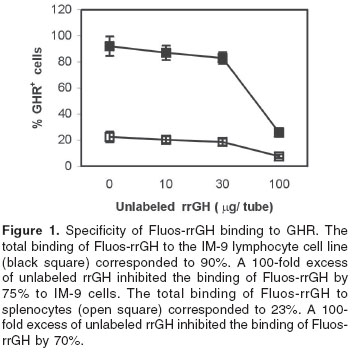
Binding characteristics of Fluos-rrGH to rat splenocytes demonstrated by flow cytofluorometric analysis
Fluos-rrGH was able to bind specifically to GHR in rat splenocytes and the IM-9 cell line. The total binding of Fluos-rrGH saturation occurred with 1 µg of rrGH and 2 h of incubation at 4°C for both cell types (data not shown). Competition analysis with unlabeled rrGH showed that binding to splenocytes and IM-9 cells was specific. Figure 1 shows that a 75% inhibition of binding was obtained with 100 µg of unlabeled hormone on IM- 9 cells; similar results were obtained in splenocytes. No inhibition in both cases was observed using Fluos-BSA.
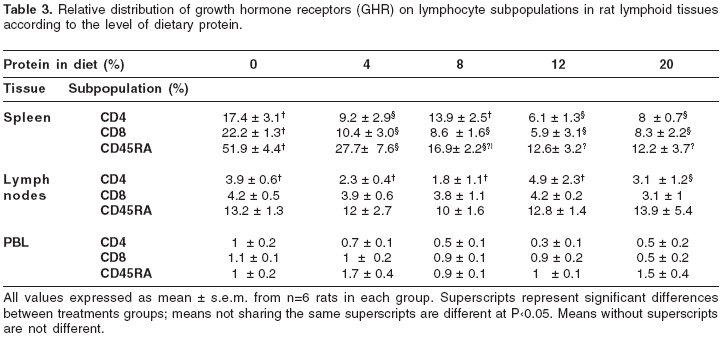
Relative distribution of growth hormone receptors on lymphocytes from lymphoid tissues
Table 3 lists the relative distribution of GHR on the corresponding tissues analyzed. Among the tissues analyzed, splenic lymphocytes were the most responsive to changes in the dietary protein content (figure 2). Rats fed 0 and 4% protein diets had a 4 and 2-fold increase respectively in the percentage of GHR+ B cells, as compared to rats fed diets containing either 12 or 20% protein. Interestingly, dietary protein restriction did not affect significantly the expression of GHR on both mesenteric lymph nodes and peripheral blood lymphocytes.
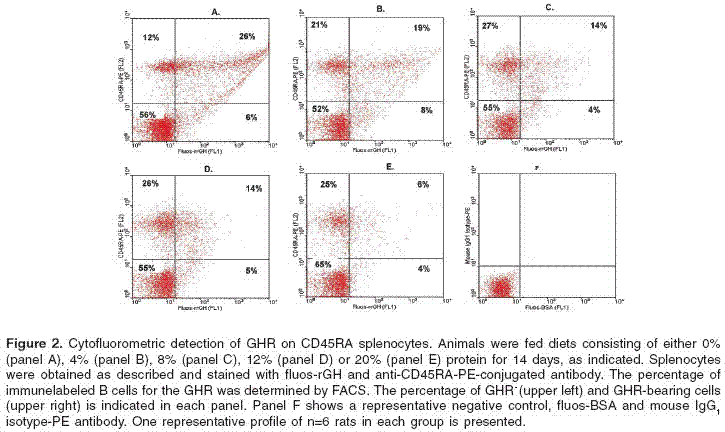
Serum growth hormone levels
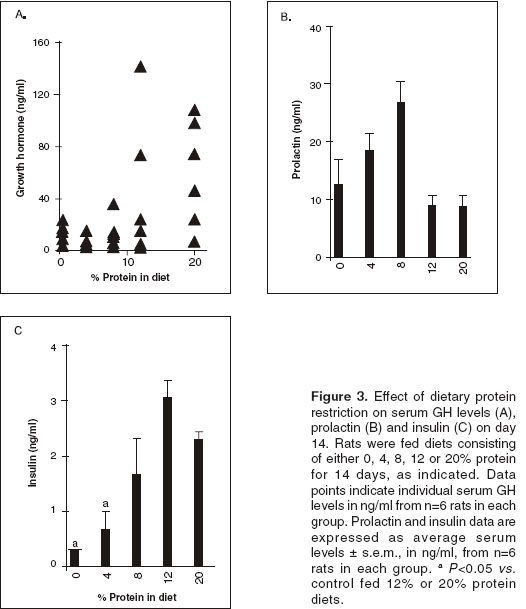
Serum GH levels were markedly decreased in response to decreasing dietary protein content as shown in figure 3A. In the groups that received 20% and 12% protein diets, serum GH levels were similar, whereas in the groups that received 8%, 4% and 0% protein diets were significantly lower (P<0.05) compared to animals fed 20% protein diet.
Serum prolactin levels
Serum prolactin levels increased in response to decreasing the dietary protein content as shown in figure 3B. Prolactin levels on day 0 were 12.5 ± 3 ng/ml. On day 14, serum levels of animals fed with 20% or 12% protein diet remained unchanged, whereas in rats fed 8% and 4% protein diet, prolactin levels showed an increase, 26.3 ± 3.8 ng/ml and 18.6 ± 3.2 ng/ml respectively. Serum levels in rats fed 0% protein diet were similar to the control.
Serum insulin levels
Serum insulin levels progressively decreased in response to decreasing the dietary protein content, as shown in figure 3C. In rats fed 0 and 4% protein diets, serum insulin levels were significantly lower (P<0.05) than those observed in rats fed 8, 12 or 20% protein diets.
Serum IGF-I levels
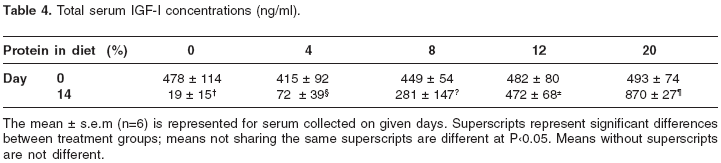
Table 4 lists the changes in serum IGF-I concentrations over the 14-days period. On day 0 circulating levels were similar, but by day 14, similar to serum GH levels, circulating IGF-I levels progressively decreased in response to decreasing the dietary protein content. On day 14 the levels were significantly lower in the group fed 8% protein diet, and further reduced in the groups that received 4% and 0% protein diets compared to animals fed 20% protein diet. Interestingly, in the animals that received 12% protein diet the serum levels remained unchanged over the experimental period, whereas in the group that received 20% protein diet the levels increased significantly (P<0.05) compared to the serum concentrations on day 0.
Serum IGFBP-3 and IGFBP-1 levels
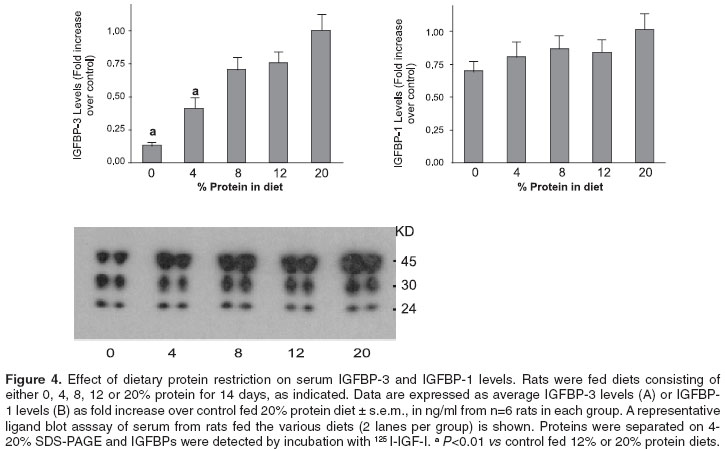
The effect of dietary protein restriction on the serum IGFBPs molecular distribution is shown in figure 4. It is evident that serum IGFBP-3 levels (40-45 kd) progressively decreased as the amount of protein intake was decreased. In rats fed 0 and 4% protein diets, there was a significant reduction (P<0.01) in serum IGFBP-3 levels, 87 and 59% respectively compared to animals fed a 20% protein diet. In rats fed 8 and 12% protein diets a non-significant reduction of 25% was observed. The western ligand blots show that IGFBP-1 levels (25-28 kd) did not change significantly among the animals fed the various protein diets.
Discussion
The effects of hormones on the proliferation and differentiation of immune cells has been widely appreciated and the importance of the GH/IGF-I axis in immune responsiveness is also under study. A recent hypothesis has suggested that the immunomodulatory effects of GH and IGF-I may be explained in terms of their anabolic and somatogenic actions (1, 2).
The purpose of this study was to determine the relative distribution of growth hormone receptors on lymphocytes from some lymphoid tissues as well as some elements associated to the GH/IGFI axis in rats under protein malnutrition. This investigation together with recent reports represents the original studies demonstrating upregulation of the GHR population in lymphoid tissues after protein deprivation (9,10). Growth hormone receptor expression was assayed by flow cytofluorometric analysis using Fluos-rrGH to characterize the distribution of GHR together with specific PE-labeled mAbs defining CD4+ and CD8+ T cells and B lymphocytes from lymphoid cell populations. The binding of Fluos-rrGH to its receptor in splenocytes and IM-9 cells showed similar characteristics; it was saturable and specific. This finding makes this ligand a useful tool in flow cytometry studies of paracrine/autocrine actions of the GH.
It has been shown that GHR is expressed in murine hematopoietic tissues, including primary and secondary lymphoid organs (12). We found that the pattern of expression is different among the different lymphoid tissues studied, as well as, among the various cell subsets. It is well known that fasting or protein calorie malnutrition decreases the GHR expression in the liver causing a resistance to the growth hormone action; less severe malnutrition such as a decreased protein intake, does not affect the GH binding but some post-receptors defects have been described (11, 13). In this study of protein deprivation at various levels, an increase in the GHR binding has been observed. Among the secondary lymphoid organs analysed, the spleen was the most responsive to this phenomenon with the B lymphocytes expressing more GHR followed by CD4+ T cells.
According to our results, as the protein intake decreases, the percentage of splenic CD4+ T cells and B lymphocytes increases and more cells (2-4 fold) become responsive to GH. Although the percentage of CD8+ T cells decreased, upregulation of the GHR was seen in the three splenic cell subsets in response to the nutritional insult. These results were also shown in mice under protein malnutrition (10) and it was further demonstrated that an increase in the mature CD4+CD8- thymocytes might explain the increase in the CD4+ T lymphocytes (14). In contrast, despite the increased percentage of B cells induced by the protein restriction in mesenteric lymph nodes and peripheral blood, only 10-13% in the lymph nodes and 2-4% of the lymphocytes in circulation bind the GH and no significant changes were induced by the protein deprivation. It is more likely that most cells bearing the GHR remain in the tissue due to the anabolic action in order to preserve the homeostasis. The GHR expression in the thymus is much reduced under normal protein intake compared to the other tissues analyzed (<1%, data not shown). Our results are coherent with those of Gagnerault et al. (12), who detected higher levels of GHR expression in B than in T lymphocytes from mice hematopoietic tissues. According to the current information and these results, rat and mouse are very similar in the pattern of GHR expression with higher positive cells in spleen than in mesenteric lymph nodes, peripheral blood lymphocytes and thymus.
The autocrine/paracrine mode of action of the GH/ IGF-I axis in the immune system is currently under study, but the regulatory effect of the dietary protein has not been clarified. In order to have a systemic approach to some elements of this axis serum IGF-I, GH, prolactin, insulin and IGFBP-1 and -3 were determined. Nutritional status is known to regulate these elements (8); as expected, serum IGF-I, GH, insulin and IGFBP-3 levels were progressively reduced in response to decreasing the protein intake.
Prolactin is a lactogenic hormone associated with the response to stress (15). In this study, we found that a low protein intake regulates the serum concentration of prolactin, with a marked increment after 5 days of protein restriction. It has been proposed that prolactin secretion is increased in order to partially counteract the negative suppressive effects on the immune system of glucocorticoid response to stress (16).
In conclusion, among the lymphoid tissues studied, GH receptors in spleen-derived lymphocytes showed to be more responsive to the stress caused by a low or deficient protein intake with a 2-4 fold increase and a highest expression in B lymphocytes, followed by CD4+ T cells. These results support the hypothesis of an immunomodulatory role of the GH/IGF-I axis in preserving the homeostasis of the immune tissues.
Acknowledgements
We thank Leonardo Lareo and María Fernanda Gutiérrez at Pontificia Universidad Javeriana, Colombia, for their help with the animal facility. This study was supported by Colciencias, grant 1101-05-100-68 Colombia and the International Program in the Chemical Sciences, IPICS; Uppsala University, Sweden, Project COL:01.
Correspondencia:
Myriam Sánchez, Laboratorio de Hormonas, Departmento de Química, Universidad Nacional de Colombia, Bogotá, Colombia.
Tel: (571) 316 5000, extensión14466
Recibido: 16/03/04; aceptado: 21/09/04
References
1. Dorshkind K, Horseman N. The roles of prolactin, growth hormone, insulin-like growth factor-I, and thyroid hormones in lymphocyte development and function: insights from genetic models of hormone and hormone receptor deficiency. Endocrine Reviews 2000;21:292-312. [ Links ]
2. Mejia-Naranjo W, Sánchez-Gómez M, LeRoith D. The growth hormone-insulin-like growth factor-I axis and immunity. In: Matera L, Rapaport R, editors. Growth and lactogenic hormones. Amsterdam: Elsevier; 2002. p.9-25. [ Links ]
3. Weigent D, Baxter J, Wear W, Smith L, Bost K, Blalock J. Production of immunoreactive growth hormone by mononuclear leukocytes. FASEB J 1988;2: 2812-8. [ Links ]
4. Weigent D, Riley J, Galin F, LeBoeuf R, Blalock J. Detection of growth hormone and growth hormonereleasing hormone-related messenger RNA in rat leukocytes by the polymerase chain reaction. Proceedings of the Society for Experimental Biology and Medicine 1991;198:643-8. [ Links ]
5. Kiess W, Butenandt O. Specific growth hormone receptors on human peripheral mononuclear cells: expression, identification and characterization. J Clin Endocrinol Metabol 1985;60:740-6. [ Links ]
6. Lesniak M, Gorden P, Roth G. Binding of 125I-human growth hormone to specific receptors in human cultured lymphocytes. Characterization of the interaction and a sensitive radioreceptor assay. J Biol Chem 1974;249: 1661-7. [ Links ]
7. Tiong T, Herington A. Tissue distribution, characterization and regulation of the mRNA for growth hormone receptor and serum binding protein in the rat. Endocrinology 1991;129:1628-34. [ Links ]
8. Ketelslegers J, Maiter D, Maes M, Underwood L, Thissen J. Nutritional regulation of the growth hormone and insulin-like growth factor-binding proteins. Hormone Research 1996;45:252-7. [ Links ]
9. Mejía-Naranjo W, Yakar S, Bernal R, LeRoith D, Sánchez - Gómez M. Regulation of the splenic somatotropic GH/IGF-I axis by dietary protein intake and Insulin - like Growth Factor - I in the rat. Growth Horm IGF Res 2002;13:254-63. [ Links ]
10. Mejía-Naranjo W, Yakar S, Sánchez-Gómez M, Umana A, Setser J, LeRoith D. Protein calorie restriction affects nonhepatic IGF-I production and the lymphoid system: studies using the liver-specific IGFI gene-deleted mouse model. Endocrinology 2002;143: 2233-41. [ Links ]
11. Oster M, Fielder P, Levin N, Cronin M. Adaptation of the growth hormone and insulin-like growth factor-I axis to chronic and severe calorie or protein malnutrition. J Clin Inv 1995;95:2258-65. [ Links ]
12. Gagnerault M, Postel-Vinay M, Dardenne M. Expression of growth hormone receptors in murine lymphoid cells analyzed by flow cytofluorometry. Endocrinology 1996;137:1719-26. [ Links ]
13. Maiter D, Maes M, Underwood L, Fliesen T, Gerard G, Ketelslegers J. Early changes in serum concentrations of somatomedin-C induced by dietary protein deprivation in rats: contributions of growth hormone receptor and post-receptor defects. J Endocrinol 1988;118:113-20. [ Links ]
14. Mejía-Naranjo W, Yakar S, Sánchez-Gómez M, LeRoith D. Role of the local GH IGF-I axis on the maturation process of thymocytes in the LID mouse model under nutritional stress. In: First Joint Symposium GH-IGF 2002. 2002. Boston, MA: Elsevier Science Ltd.; 2002. p.257-8.
15. Ben-Jonathan N, Arbogast L, Hyde J. Neuroendocrine regulation of prolactin release. Prog in Neurobiol 1989;33:399-447. [ Links ]
16. Yu-Lee L. Molecular actions of prolactin in the immune system. Proc Soc Experiment Biol Med 1997;215:35-52. [ Links ]














