Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO  Similares em Google
Similares em Google
Compartilhar
Biomédica
versão impressa ISSN 0120-4157versão On-line ISSN 2590-7379
Biomédica v.25 n.1 Bogotá mar. 2005
Anti-glucuronoxylomannan IgG1 specific antibodies production
in Cryptococcus neoformans resistant mice
Claudia Parra 1, John Mario González 2, Elizabeth Castañeda 3, Susana Fiorentino 1
1 Grupo de Inmunobiología, Departamento de Microbiología, Facultad de Ciencias, Pontificia Universidad Javeriana, Bogotá, D.C., Colombia.
2
Department of Neurology, Keck School of Medicine, University of South California, Los Angeles, U.S.A.3
Grupo de Microbiología, Instituto Nacional de Salud, Bogotá, D.C., Colombia.Background.
Cryptococcus neoformans is a widely disseminated fungus shown to be responsible for infections in individuals with impaired cell mediated immunity, such as patients with human immunodeficiency virus (HIV). Cryptococcus neoformans has a polysaccharide capsule composed of glucuronoxylomannan (GXM), which acts as a major virulence factor and is considered to be a thymus independent type-2 antigen (TI-2).Objective. In the current study, the production kinetics were evaluated for IgG subclasses specific for GXM, and assessed with the cross reactive antibodies to Streptococcus pneumoniae polysaccharide. In addition, spleen B cell subpopulations were quantified in murine models of cryptococcosis with different susceptibilities to the infection.
Material and methods. Antibodies were detected by ELISA at different time intervals after C. neoformans infection in moderately resistant (Balb/c), highly resistant (CBA/j) and susceptible (C57BL/6) mouse strains. B cells subpopulations were determined by flow cytometry analysis.
Results. Early production of IgG1, described as protector antibodies, coincided with a decrease of the number of C. neoformans colony forming units in the lungs. Polysaccharide cross-reactive antibodies were detected in each of the three mouse strains. Antibody titers were highest in the susceptible strain (C57BL/6), a strain which also showed the highest proportion of splenic CD5+ B lymphocytes. In contrast, CBA/J mice showed the highest levels of CD43+ B.
Conclusions. These findings suggest that IgG1 antibodies specific for GXM, are implicated in host protection against C. neoformans infection and may be regulated by CD43+ cells. They also suggest that cross reactivity antibodies are not important in the protection against C. neoformans infection.
Keywords: Cryptococcus neoformans, glucuronoxylomannan, cross-reactive antibodies, IgG subclasses.
Producción de anticuerpos IgG1 anti-glucuronoxilomanana en ratones resistentes a la criptococosis
Introducción. Cryptococcus neoformans es un hongo ubicuo que está relacionado con infecciones oportunistas en pacientes inmunocomprometidos, especialmente los pacientes infectados con el virus de la inmunodeficiencia humana. C. neoformans posee una cápsula que contiene principalmente glucuronoxilomanana que actúa como factor de virulencia y es considerado como antígeno timo independiente tipo-2.
Objetivos. Determinar la cinética de producción de las subclases de IgG específicas para la glucuronoxilomanana, medir los anticuerpos de reactividad cruzada contra el polisacárido de Streptococcus pneumoniae y determinar las subpoblaciones esplénicas de linfocitos en tres modelos de criptococosis en ratones con diferentes susceptibilidades a la infección.
Materiales y métodos. Los anticuerpos fueron detectados por la técnica inmunoenzimática ELISA después de la infección con C. neoformans en ratones moderadamente resistentes (Balb/c), resistentes (CBA/j) y susceptibles (C57BL/6). Las subpoblaciones de linfocitos B se determinaron por citometría de flujo.
Resultados. Se observó la producción temprana de anticuerpos IgG1, que coincidía con una disminución en el número de unidades formadoras de colonias de C. neoformans en pulmón.
Los anticuerpos de reactividad cruzada se detectaron en las tres cepas de ratones después de la infección; fueron mayores en la cepa susceptible C57BL/6, la cual mostró el porcentaje más alto de linfocitos B, CD5+ en el bazo. En contraste, los ratones CBA/J presentaron los mayores niveles de linfocitos B, CD43+.
Conclusiones. Estos hallazgos sugieren que los anticuerpos IgG1 específicos para la glucuronoxilomanana están implicados en la protección del hospedero contra la infección por C. neoformans y podrían estar regulados por células CD43+. También sugiere que los anticuerpos de reactividad cruzada no poseen un papel relevante en el control de la infección.
Palabras claves: Cryptococcus neoformans, glucuronoxilomanana, anticuerpos de reactividad cruzada, subclases de IgG.
Cryptococcus neoformans is an ubiquitous fungus which has been shown to be responsible for infections in individuals with impaired cell mediated immunity, such as patients infected with human immunodeficiency virus (HIV) (1). C. neoformans has a polysaccharide capsule composed of glucuronoxylomannan (GXM), which acts as a major virulence factor (2) and is considered to be a thymus independent type-2 antigen (TI-2). A murine model in which C. neoformans is inoculated via intratracheal route has allowed the definition of different levels of susceptibility among the various mouse strains (3,4). It has been proposed that these differences are attributed mainly to the presence of a Th1 cellular immune response (3).
The role of the humoral response in protection against cryptococcal infection has been well demonstrated. Passive serotherapy with monoclonal IgG1 antibodies directed against GXM offers a possible alternative therapy for cryptococcal infection in immunosupressed patients (5). Studies in lethally infected mice have shown that passive administration of monoclonal antibodies reduced serum polysaccharide levels and lung fungal burden and prolonged survival. In addition, these studies suggest that the protective efficacy depends on the isotype of antibodies used; the conclusion was drawn that anti-GXM specific IgG1 antibodies were more effective than IgM and IgG3 antibodies (5-8).
Isotype switching of a non-protective IgG3 antibody to a protective IgG1 antibody confirmed the isotype importance for antibody efficacy against C. neoformans (9,10). Interestingly, the immune response to purified cryptococcal capsular polysaccharide is influenced by the genetic background of the animal (11-13). In spite of the relevant role of this anti-polysaccharide antibody in the control of in vivo infection, to date, only the kinetics of the humoral response to cryptococcal proteins has been studied (14). The relationship between the isotype of the antibody produced after cryptococcal infection and the antigen specificity in vivo is thus far only partially understood. In other infections with microorganisms such as Streptococcus pneumoniae that express antigenic polysaccharide moieties, the presence of crossreactive anti-polysaccharide antibodies has been demonstrated (15). However, its protective or deleterious role in host defense is incompletely understood.
Cells implicated in the proliferative responses to TI-2 antigens, including polysaccharides, are CD5+ lymphocytes (16-18) that are characterized phenotypically as B220lowIgMlow cells normally found in the spleen (18). These cells are able to undergo self-renewal upon stimulation with TI-2 antigens. The paucity of CD5+ B cells in mice is correlated with an inability to respond to TI-2 antigens (19). However, in vivo activation of CD5+ lymphocytes seems to be deleterious and is strongly implicated in the generation of nonprotective humoral responses (20). CD5+ lymphocyte expansion is controlled by the presence of CD43+ cells, suggesting that deleterious responses might be naturally regulated (21).
In this study, we wanted to analyze IgG subclass production after intratracheal infection with C. neoformans of three mouse strains, and observed differential production of IgG1 depending on the strain of mouse. Our results provide additional evidence for the efficacy of the antibody response to C. neoformans, which is dependent on the subclass of antibody elicited and on the specificity of recognition.
Materials and methods
Animals
Six 8 week-old male CBA/J (H2k), BALB/c (H2d) and C57BL/6 (H2b) mice were purchased from Jackson Laboratories and maintained in the animal facilities of the Instituto Nacional de Salud (INS). Animals were maintained on food and water ad libitum, and treated in accordance with the international guidelines at the INS in Bogotá, Colombia.
Maintenance of C. neoformans, and purification of capsular polysaccharide and GXM
C. neoformans var. grubii, serotype A (ATCC 90113), was used in all experiments. The fungus was cultured in Sabouraud dextrose agar medium at 27°C and passaged weekly in vitro. To maintain virulence, C. neoformans was passed in vivo in C57BL/6 mice, recovered from spleen and cultured in Sabouraud agar; capsular polysaccharide was obtained as previously described (22). Briefly, purified polysaccharide was stored at 4°C, and the carbohydrate content was determined by Dubois technique (22). To purify the GXM, polysaccharide was diluted in 0.2M NaCl and sonicated for 15 minutes, after which 3.0 mg of cetyltrimethylammonium (CTAB) per mg of polysaccharide was added. Precipitation was carried out using two volumes of 0.05% CTAB and centrifuged at 12,000g at 23°C for 1 hour. The precipitate was washed with 10% ethanol and diluted in 1M NaCl. The GXM was dialyzed, dried and stored at 4°C. The presence of LPS in GXM preparation was determined by the Lymulus amebocyte lysate assay (LAL). Only samples in which LPS was less than 0.25 µg/ml were used. Protein content was determined by Bradford technique (Biorad) and revealed to be consistently less than 10 µg/mL.
Mice infection and blood sampling
Mice were infected by intratracheal inoculation. Briefly, aseptic exposure of the trachea was done under anesthesia with ketamine (150 mg/kg) and xylazine (10 mg/kg), followed by inoculation with 50 µL of PBS containing 106 colony-forming units (CFU) of C. neoformans. Uninfected mice were used as negative controls. Animals were bled via cardiac puncture on day 0, 4, 7, 11, 17, 23 and 45 post-infection. Blood was allowed to coagulate at room temperature and serum was obtained after centrifugation. Lungs and spleens were extracted aseptically and weighed, after which the lungs and spleens were minced in 1 mL 0.9% sterile saline solution. The number of CFU in each organ was determined after culturing in Sabouraud and cafeic acid agar by incubation at 27°C for 72 h.
Kinetics of the antibody anti-GXM response in C. neoformans-infected mice
Sera were evaluated for the presence of specific antibodies to GXM by ELISA technique. Briefly, a total of 2.5 µg GXM in 100 µl of PBS (pH 7.2) was used to coat 96-well flat-bottom plates (NUNC, MaxiSorp, Naperville, IL, U.S.A), which were incubated overnight at 4°C. Plates were washed 5 times with PBS, 0.1% of BSA and 0.05% Tween 20 (ICN, Cappel, USA) and blocked with PBS and 1% BSA for 1 hour at 37°C. After blocking, plates were washed again and serum samples were added. Sera and controls were diluted 1/10 in PBS (pH 7.2) and 0.1% BSA. Samples were incubated for 1 hour at 37°C and washed again. Goat antimouse isotype antibodies (anti-IgG1, anti-IgG2a, anti-IgG2b, anti-IgG3) (ICN) were diluted in PBS (pH 7.2) and 0.1% BSA and used either at 2.5 µg/ ml or (IgG2b) 3.5 µg/ml concentration (ICN). After incubation for 1 hour at 37°C plates were washed and conjugated antibody rabbit anti-goat IgG labeled with alkaline phosphatase (100 µl of diluted 1/9000 in PBS and 0.25% BSA per well) was added (ICN) and incubated for an additional 1 hour at 37°C. After washing, 100 µl of substrate (1 mg of P-nitrophenyl phosphate (ICN) diluted in 100 ml of buffer 2-amino-2-methy-propaneidiol [pH 10.3]) was added to each well and incubated for 45 minutes at room temperature. Reaction was stopped using 100 µl of 3N NaOH per well. Plates were read at 405 nm using a spectrophotometer (Multi-Scan 340, Biosystems). As positive controls anti-GXM mAb of isotype IgG1 (provided by F. Drommer, Institute Pasteur, Paris) IgG3, IgG2a and IgG2b (provided by A. Casadevall, Albert Einstein Medicine College, New York) were used.
Cross-reactivity assay
The cross-reactivity of sera from mice infected with C. neoformans to the capsular polysaccharide from Streptococcus pneumoniae serotype 14 (ATCC, USA) was determined using the ELISA technique as described above with minor modifications. Briefly, a mouse polyclonal antibody was used as a positive control for antipneumococcal polysaccharide and antibody titres were obtained against polysaccharide from S. pneumoniae serotype 14 and cross reactivity against GXM. For the ELISA test, the cutoff was established by determining optical density plus 3 standard deviations, of serum from non-infected mice tested in plates covered with GXM.
In some experiments, cross reactivity was measured in soluble competition ELISA. Briefly, sera from C. neoformans infected mice were adsorbed with 0.5, 5.0 and 50 µg/mL of S. pneumoniae polysaccharide (serotype 14) for 2 hours at 37°C. Supernatants were withdrawn, centrifuged to remove debris and tested using GXM as antigen. Results are shown as percentage of inhibition: OD adsorbed serum x 100/OD total serum – 100.
Flow cytometric analysis
Mononuclear cells (MNC) obtained from spleens of C57BL/6 or CBA/j mice were used to determine the relative percentage of B cells subpopulations. Fc receptors were blocked before staining with normal mice serum and then cells were washed twice in PBS (supplemented with FCS 2% and 0.01% sodium azide). MNC (3x105) were added with fluorescent antibodies anti-CD5-FITC, anti- CD43 FITC, anti-IgM-PE and anti-B220-Biotin (BD Pharmingen, San Diego, CA, U.S.A). After 30 min of incubation at 4°C, MNC were washed and streptavidin-APC (BD, Pharmingen) was added for 30 min at 4°C. Finally, MNC were washed and a three-color analysis was performed using a flow cytometer (Becton Dickinson, FACScan, San Jose, CA).
Statistical analysis
Unpaired Student t-test was applied for statistical analysis of the results. Values were considered statistically significant when p value was £ 0.05.
Results
Differential infection profile and humoral antibody response of mice to infection with C. neoformans
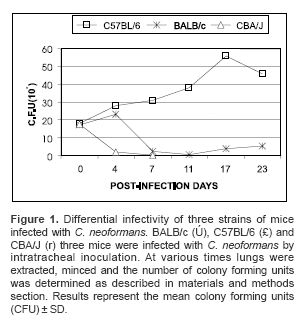
Intratracheal inoculation of C. neoformans had the highest rate of infectivity in susceptible C57BL/6 mice, with CFU values reaching 5.6x106 and 4.6x106 by day 14 and 23, respectively (figure 1; open squares). In moderately resistant BALB/c mice, a gradual decrease in the number of CFU from 1.7x106 at day 14 to 5.1x105 at day 23 was observed (figure 1; filled crosses), while in highly resistant CBA/J mice no CFU were detected after 7 days post-infection (figure 1; open triangles). These results demonstrate that there is indeed a differential pattern of infectivity that is dependent on the strain of mice infected.
Measurement of total anti-GXM IgG antibodies after infection with C. neoformans showed that the highest levels of anti-GXM antibodies could be found in CBA/J and BALB/c mice, which significantly increased from day 7 until day 23 (p<0.05) as compared to C57BL/6 mice (
figure 2A). Infection of C57BL/6 mice with C. neoformans resulted in significantly lower levels of anti-GXM IgG from day 4 as compared to either BALB/c or CBA/J mice (figure 2A). We next determined the production of various anti-GXM IgG isotypes in the various mouse strains following intratracheal infection with C. neoformans. Measurement of IgG1 antibodies revealed that this was the predominant isotype in both CBA/J and BALB/c mice, and was significantly higher than C57BL/6 mice from day 7 to day 45 (p<0.05) as compared to CBA/J mice (figure 2B). By day 45, IgG1 antibody production diminished in both BALB/c and CBA/J mice, whereas, levels of IgG1 in C57BL/6 mice had returned to baseline levels (figure 2B). In contrast, the highest level of IgG3 production was measured in C57BL/6, starting from day 4 to day 11, as compared to either CBA/J or BALB/c (**p<0.05, *p<0.01) (figure 2C). By day 45, levels of IgG3 in C57BL/6 mice had diminished. Measurement of specific anti-GXM IgG2a antibodies did not reveal any significant differences amongst the different species of mice, with the exception of day 45 postinfection with C. neoformans, when levels of anti-GXM IgG2a were significantly higher in CBA/J and BALB/c as compared to C57BL/6 mice (figure 2D). Measurement of anti-GXM IgG2b levels revealed that only CBA/J mice exhibited significantly higher anti- GXM IgG2b levels at day 45 post-infection with C. neoformans than either BALB/c or C57BL/6 (**p<0.05, *p<0.01) (figure 2E).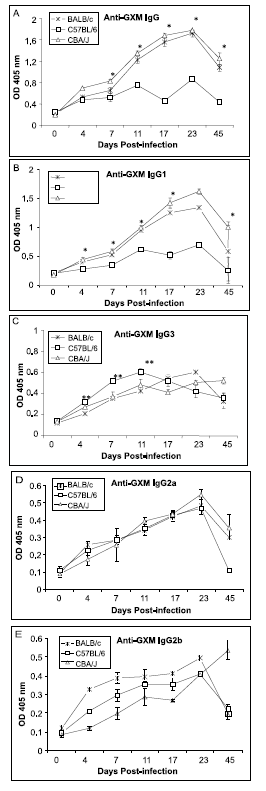
Examination of cross-reactive IgG directed against pneumococcal polysaccharide after infection of mice with C. neoformans
We next examined whether cross-reactive antipneumococcal polysaccharide antibodies could be detected after infection of the various mouse strains with C. neoformans. By days 4 and 7 post-infection with C. neoformans, detectable levels of antipneumococal antibodies could be measured in both BALB/c and CBA/J mice (figure 3A). However, from day 11 to day 23 post-infection, C57BL/6 mice produced more IgG directed against pneumococal polysaccharide than either CBA/J or BALB/c mice (
figure 3A). To test whether these antipneumococcal polysaccharide antibodies were GXM cross-reactive antibodies, a competition assay was performed by mixing the serum from each mouse strain obtained after 17 days postinfection with various amounts of pneumococcal polysaccharide. A dose dependent inhibition of anti-GXM antibodies with pneumococcal polysaccharide was observed in all the mouse strains, although, inhibition was significantly higher in susceptible C57BL/6 mice as compared to DBA/J or BALB/c mice (figure 3B). Next, normal serum obtained from non-infected BALB/c mice and inoculated with pneumococal polysaccharide was tested. Cross reactive antibodies recognizing GXM polysaccharide were detected in a 1/40 dilution, as compared with specific antibodies to pneumococal polysaccharide that were detected at a 1/320 dilution (figure 3C).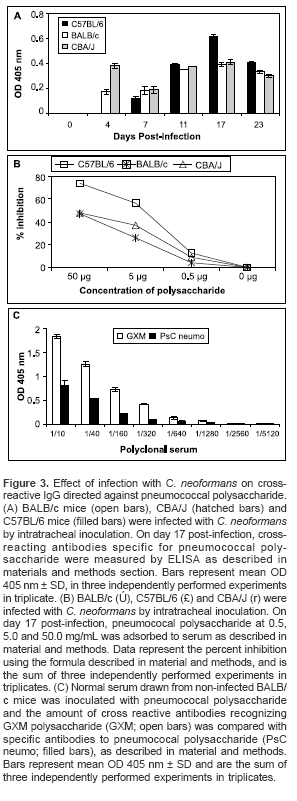
Characterization of B cell subpopulations after infection of mice with C. neoformans
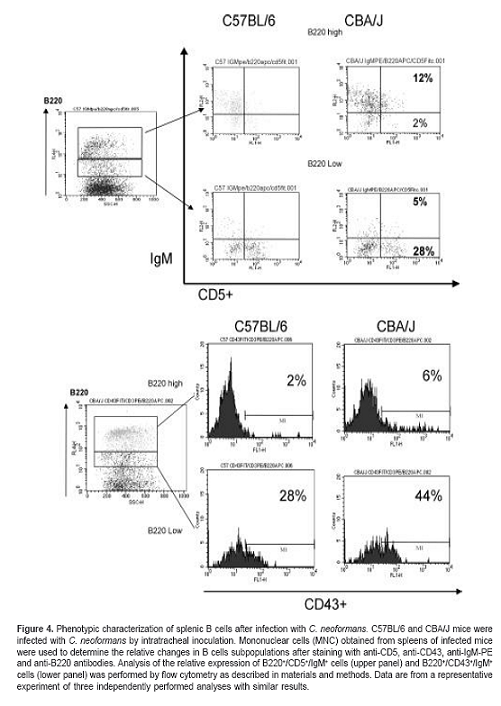
To study whether resistant and susceptible mice present different B cell subpopulations in response to infection with C. neoformans, we analyzed the percentage of CD5+ and CD43+ B cells using flow cytometric analysis. B cells could be divided into B220high and B220low populations; C57BL/6 mice exhibited a higher percentage of CD5+ B cells coexpressing the B220low phenotype (figure 4; upper panel). In contrast, CBA/J mice exhibited a higher percentage of CD43+ cells with the B220low phenotype (figure 4; lower panel).
Discussion
The present work constitutes the first study of the kinetics of the humoral response to GXM in susceptible and resistant mice infected with C. neoformans. We show that BALB/c and CBA/J mice, defined as moderately and highly resistant to C. neoformans infection (4), produce higher amounts of total IgG (mainly of the IgG
1 isotype) than the susceptible C57BL/6 mice. Protection mediated by exogenous IgG1 anti-GXM specific mAbs has been shown in several studies (6,10); in contrast to non-protective IgG3 mAbs directed to the same epitope (10), IgG1 antibodies have been previously reported as protective in experiments of passive transfer in the mouse model (23-25). In these studies, it was observed that the three strains of infected animals produced both IgG2a and IgG2b but at relatively lower levels, and there were no significant differences between strains (23-25). Taken together, these results suggest that IgG2a and IgG2b do not play an important role in murine resistance or susceptibility to cryptococcosis in vivo.The potent humoral response and resistance to cryptococcosis that is observed in the murine system remains to be established in humans. However, a close correlation has been observed between hypogammaglobulinemia (26), or HIV infected patients (11,27) and the risk of infection with C. neoformans. It has been suggested that a correlation exists between the decrease of certain IgG subtypes and C. neoformans meningitis in HIV patients (28). This is relevant, since HIV patients show deregulation in Ig rearrangement of heavy variable chains (VH) (28), and absence of V(H)3 segment is commonly associated with a low antibody response to polysaccharides (29). These patients have a deficient response to polysaccharide-based vaccines such as S. pneumoniae (29-31). It is important to emphasize that the specificity of the epitopes recognized by the protective antibodies is different from that of the non-protective antibodies (8,32).
These observations, together with our results, indicate that the level of susceptibility in humans and mice to cryptococcosis may be regulated by similar mechanisms, suggesting that the antibody response is as important as the cellular response. Classically, defects in cellular immunity, particularly those that require T lymphocytes, are considered as the main risk factor for the mycosis, both in humans and mice (3,4). In the present study, it was observed that the susceptible mouse strain produced the highest amount of crossreactive antibodies. Polysaccharides, due to their biochemical characteristics, may contribute to the kind of cross-reactivity observed with GXM (33), and cross-reactive antibodies are considered as critical elements for pre-immune resistance to other encapsulated pathogens (34).
The lack of an effective specific immune response might be intrinsically related to the type of B lymphocytes stimulated early during the infection process. In our present study, it was observed that C57BL/6 susceptible mice had a high percentage of B220
low/CD5+ cells. In contrast, a high percentage of B220low/CD43+ cells were detected in CBA/J resistant mice. Although CD5+ B lymphocytes or B1 subpopulations are classically implicated in the proliferative response to polysaccharides (15-17), antibodies produced by the B1 population are characterized by low affinity, and self-reactivity (35). Some of these responses are associated with a non-protective humoral response in humans (20). On the other hand, the CD43+ population is generally implicated in the negative control of CD5+ cells growth (21). The presence of a B220low/CD43+ population in CBA/J mice supports our hypothesis on the role of different B cell populations in the development of a protective or non-protective humoral response to C. neoformans.In conclusion, this study demonstrates that production of anti-GXM antibodies of the IgG
1 isotype correlates with resistance to infection with C. neoformans. The presence of the lowest levels of specific anti-GXM antibodies in susceptible C57BL/6 mice together with the higher levels of cross-reactive anti-pneumococcal antibodies in these mice suggests that cross-reactivity antibodies are less protective. It is important to consider that this non-protective humoral response is developed in a non-immunosuppressed mouse strain, normally considered as a good Th1 cytokine producer (36). The fine specificity of these cross-reactive antibodies and the mechanisms which control their production should be further studied to better understand this anti-fungal immune response. Taken together, our findings suggest that the development of IgG1 antibodies specific for GXM might participate in the protection against C. neoformans, and that this response is dependent on the B lymphocyte subpopulation stimulated early during infection.Acknowledgements
The authors thank Armelle Regnault, Alexander Asea and Sophie Tourdeau for helpful discussions.
Interest conflict declaration
None declared
Financing
This work was supported in part by a grant 1203- 05-706-96 from the Instituto Colombiano para el desarrollo de la Ciencia y la Tecnología, Francisco José de Caldas, (Colciencias), Colombia.
Correspondencia:
Susana Florentino, Carrera 7 No. 43-82, Bogota, D.C., Colombia.
Phone: (571) 320 8320, extension 4020; fax: (571) 320
8320, extension 4021
susana.fiorentino@javeriana.edu.co
Recibido: 19/10/04; aceptado: 01/02/05
References
- Currie BP, Casadevall A. Estimation of the prevalence of cryptococcal infection among patients infected with the human immunodeficiency virus in New York City. Clin Infect Dis 1994;19:1029-33.
- Rodriguez ML, Alviano CS, Travassos LR. Pathogenicity of Cryptococcus neoformans: virulence factors and immunological mechanisms. Microbes Infect 1999;1:293-301.
- Hoag KA, Street NE, Huffnagle GB, Lipscomb MF. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am J Respir Cell Mol Biol 1995;13:487-95.
- Huffnagle GB, Yates JL, Lipscomb MF. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. J Exp Med 1991;173:793-800.
- Mukherjee S, Lee S, Mukherjee J, Scharff MD, Casadevall A. Monoclonal antibodies to Cryptococcus neoformans capsular polysaccharide modify the course of intravenous infection in mice. Infect Immun 1994;62:1079-88.
- Mukherjee J, Scharff MD, Casadevall A. Protective murine monoclonal antibodies to Cryptococcus neoformans. Infect Immun 1992;60:4534-41.
- Mukherjee J, Scharff MD, Casadevall A. Cryptococcus neoformans infection can elicit protective antibodies in mice. Can J Microbiol 1994;40:888-92.
- Mukherjee J, Nussbaum G, Scharff MD, Casadevall A. Protective and nonprotective monoclonal antibodies to Cryptococcus neoformans originating from one B cell. J Exp Med 1995;181:405
 9.
9. - Yuan R, Casadevall A, Spira G, Scharff MD. Isotype switching from IgG3 to IgG1 converts a nonprotective murine antibody to Cryptococcus neoformans into a protective antibody. J Immunol 1995;154:1810-6.
- Yuan R, Spira G, Oh J, Paizi M, Casadevall A, Scharff MD. Isotype switching increases efficacy of antibody protection against Cryptococcus neoformans infection in mice. Infect Immun 1998;66:1057-62.
- Breen JF, Lee IC, Vogel FR, Friedman H. Cryptococcal capsular polysaccharide-induced modulation of murine immune responses. Infect Immun 1982;36:47-51.
- Fagarasan S, Honjo T. T-Independent immune response: new aspects of B cell biology. Science 2000;90:89-92.
- Kozel TR, Gulley WF, Cazin J. Immune response to Cryptococcus neoformans soluble polysaccharide: immunological unresponsiveness. J Infect Immun 1977;18:701-7.
- Lary O, Dromer F. Do kinetics of the humoral response to Cryptococcus neoformans proteins during murine cryptococcosis reflect outcome? Infect Immun 2000;68:3724-6.
- Wang D, Wells SM, Stall AM, Kabat EA. Reaction of germinal centers in the T-cell-independent response to the bacterial polysaccharide alpha(1-->6) dextran. Proc Natl Acad Sci USA 1994;91:2502-6.
- Fagarasan S, Honjo T. T-Independent immune response: new aspects of B cell biology. Science 2000;290:89-92.
- Vos QA, Lees ZQ, Wu CM, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev 2000;176:154-70.
- Kantor AB, Stall AM, Adams S, Watanabe K, Herzenberg LA. De novo development and self-replenishment of B cells. Int Immunol 1995;7:55-68.
- Hayakawa K, Hardy RR, Herzenberg LA. Peritoneal Ly-1 B cells: genetic control, autoantibody production, increased lambda light chain expression. Eur J Immunol 1986;16:450-6.
- Antall PM, Meyerson H, Kaplan D, Venglarcik J, Hostoffer RW. Selective antipolysaccharide antibody deficiency associated with peripheral blood CD5+ B-cell predominance. J Allergy Clin Immunol 1999;103:637-41.
- Ostberg JR, Dragone LL, Borrello MA, Phipps RP, Barth RK, Frelinger JG. Expression of mouse CD43 in the B cell lineage of transgenic mice causes impaired immune responses to T-independent antigens. .Eur J Immunol 1997;27:2152-9.
- Cherniak R, Sundstrom JB. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect Immun 1994;62:1507-12.
- Dromer F, Charreire J, Contrepois A, Carbon C, Yeni P. Protection of mice against experimental cryptococcosis by anti-Cryptococcus neoformans monoclonal antibody. Infect Immun 1987;55:749-52.
- Sanford JE, Lupan DM, Schlageter AM, Kozel TR. Passive immunization against Cryptococcus neoformans with an isotype-switch family of monoclonal antibodies reactive with cryptococcal polysaccharide. Infect Immun 1990;58:1919-23.
- Shapiro S, Beenhouwer DO, Feldmesser M, Taborda C, Carroll MC, Casadevall A et al. Immunoglobulin G monoclonal antibodies to Cryptococcus neoformans protect mice deficient in complement component C3. Infect Immun 2002;70:2598-604.
- Neto RJ, Guimaraes MC, Moya MJ, Oliveira FR, Louzada PL Jr, Martinez R. Hypogammaglobulinemia as risk factor for Cryptococcus neoformans infection: report of 2 cases. Rev Soc Bras Med Trop 2000;33:603-8.
- Houpt DC, Pfrommer GS, Young BJ, Larson TA, Kozel TR. Occurrence, immunoglobulin classes, and biological activities of antibodies in normal human serum that are reactive with Cryptococcus neoformans glucuronoxylomannan. Infect Immun 1994;62:2857
 64.
64. - Fleuridor R, Lyles RH, Pirofski L. Quantitative and qualitative differences in the serum antibody profiles of human immunodeficiency virus-infected persons with and without Cryptococcus neoformans meningitis.J Infect Dis 1999;180:1526-35.
- Pirofski LA. Polysaccharides, mimotopes and vaccines for fungal and encapsulated pathogens. Trends Microbiology 2001; 9:445-51.
- Abadi J, Friedman J, Mageed RA, Jefferis R, Rodriguez-Barradas MC, Pirofski L. Human antibodies elicited by a pneumococcal vaccine express idiotypic determinants indicative of V(H)3 gene segment usage. J Infect Dis 1998;178:707-16.
- Chang Q, Abadi J, Alpert P, Pirofski L. A pneumococcal capsular polysaccharide vaccine induces a repertoire shift with increased V(H)3 expression in peripheral B cells from human immunodeficiency virus (HIV)-uninfected but not HIV-infected persons. J Infect Dis 2000;181:1313-21.
- Zhang H, Zhong Z, Pirofski L. Peptide epitopes recognized by a human anti-cryptococcal glucuronoxylomannan antibody. Infect Immun 1997;65:1158
 64.
64. - Casadevall A, Cleare W, Feldmesser M, Glatman-Freedman A, Goldman DL, Kozel TR et al. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother 1998;42:1437-46.
- Robbins JB, Schneerson R, Glode MP. Cross-reactive antigens and immunity to diseases caused by encapsulated bacteria. J Allergy Clin Immunol 1975;56:141
 51.
51. - Hardy RR. Variable gene usage, physiology and development of Ly-1+ (CD5+) B cells. Curr Opin Immunol 1992;4:181-5.
- Sathiyaseelan J, Jiang X, Baldwin CL. Growth of Brucella abortus in macrophages from resistant and susceptible mouse strains. Clin Exp Immunol 2000;121:289-94














