Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biomédica
Print version ISSN 0120-4157On-line version ISSN 2590-7379
Biomédica vol.26 suppl.1 Bogotá Oct. 2006
Comparative immunohistological analysis of the Montenegro skin test reaction in asymptomatic infection and in acute and chronic cutaneous leishmaniasis
Nora Guarín 1, Gloria I. Palma 2, Claude Pirmez 3, Liliana Valderrama 1, Rafael Tovar 1, 2, 4, Nancy Gore Saravia 1*
1
Centro Internacional de Entrenamiento e Investigaciones Médicas, Cali, Colombia.2
Facultad de Salud, Universidad del Valle, Cali, Colombia.3
Department of Biochemistry and Molecular Biology, Instituto Oswaldo Cruz, Rio de Janeiro, Brasil.4
Departamento de Ciencias Naturales y Matemáticas, Pontificia Universidad Javeriana, Cali, Colombia.Recibido: 09/06/05; aceptado: 05/09/05
Introduction.
The Montenegro skin test reaction and leishmaniasis lesions share fundamental characteristics of a delayed type hypersensitivity reaction.Objectives. To determine whether the Montenegro skin test reaction (response to leishmanin) might approximate and thereby provide insight into the early inflammatory and immune response to Leishmania infection.
Materials and methods. We compared the inflammatory response in biopsies of acute (evolution time £one month), and chronic lesions (evolution time ³6 months) with the Montenegro skin test reaction in the corresponding patients, and with the Montenegro skin test of asymptomatically infected volunteers.
Results. The proportion of CD4+ and CD8+ T lymphocytes, mononuclear phagocytes and granulocytes were similar in acute lesions and in their corresponding Montenegro skin test reactions. In contrast, CD4+ lymphocytes (32.6%) represented a significantly lower, and B cells (20%) and macrophages (27%) a significantly higher proportion of the cellular infiltrate in chronic lesions as compared to reactions in the corresponding skin test site (CD4+: 43.7%, B cells: 0.9%; macrophages: 17.5%). CD8+ T lymphocytes and macrophages were positively associated ( P=0.038) in the Montenegro skin test of asymptomatically infected individuals whereas CD8+ and CD4+ T cells were positively associated in the Montenegro skin test of chronic patients ( P=0.002). Notably, B cells were markedly more frequent in chronic lesions (20%) than in acute lesions (5.3%) ( P=0.002).
Conclusion. The Montenegro skin test distinguished the cellular immune response to Leishmania in asymptomatic infection and chronic disease and may provide a surrogate of the early response to infection.
Key words: cutaneous leishmaniasis, delayed hypersensitivities, immune response, histopathology, immunoenzyme techniques, B-lymphocytes.
Análisis inmunohistopatológico comparativo de la reacción a la prueba cutánea de Montenegro en infección asintomática y en leishmaniasis cutánea aguda y crónica
Introducción. La reacción a la prueba cutánea de Montenegro (a leishmanina) y la lesión de leishmaniasis comparten características fundamentales de la reacción de hipersensibilidad de tipo retardado.
Objetivos. Determinar si la respuesta cutánea a leishmanina se aproxima y podría modelar la respuesta inflamatoria e inmune temprana a la infección por Leishmania.
Materiales y métodos. Este estudio comparó la respuesta inflamatoria de biopsias de lesiones agudas (tiempo de evolución £1 mes) y lesiones crónicas (tiempo de evolución ³6 meses) con su respectiva reacción a la prueba cutánea de Montenegro, y con la reacción a la prueba de Montenegro de individuos infectados asintomáticos.
Resultados. La proporción de linfocitos T CD4+ y CD8+, células fagocíticas mononucleares y granulocitos fue similar entre lesiones agudas y sus reacciones a la prueba cutánea a leishmanina. En contraste, los linfocitos CD4+ (32,6%) representaron una proporción significativamente mas baja, y los linfocitos B (20%) y macrófagos (27%) una proporción significativamente más alta del infiltrado celular en lesiones crónicas que en sus correspondientes reacciones a la prueba cutánea de Montenegro (CD4+: 43,7%, linfocitos B: 0.9%; macrófagos: 17,5%). Se encontró una asociación positiva entre linfocitos T CD8+ y macrófagos ( P=0,038) en la reacción a la prueba cutánea de Montenegro de los individuos con infección asintomática, mientras que los linfocitos T CD8+ y CD4+ estuvieron asociados positivamente en las reacciones a la prueba cutánea de Montenegro de pacientes crónicos ( P=0,002). Adicionalmente, la proporción de linfocitos B en lesiones crónicas (20%) fue más alta que en lesiones agudas (5,3%) ( P=0,002).
Conclusión. La reacción cutánea de Montenegro permitió diferenciar la respuesta celular inmune entre infección asintomática y enfermedad crónica, y simula la respuesta temprana a la infección.
Palabras clave: leishmaniasis cutánea, hipersensibilidad retardada, inmunología, histopatología, técnicas inmunoenzimaticas, linfocitos B.
Tegumentary leishmaniasis presents a broad clinical spectrum ranging from self-healing lesions, to chronic mucosal involvement and the hyporesponsive diffuse form. Furthermore, a high proportion of individuals are infected but have no clinical manifestations (1,2). Both the Leishmania strain and the immune status of the host influence the development of T-cell mediated specific immune responses. The balance between T helper (Th) 1 and 2 mediators, which exert mutually modulating effects, can become polarized and this may be determined at onset of the infection (3,4). Locally, the interaction between infected antigen presenting cells and T lymphocytes is fundamental for the development and control of the leishmaniasis lesions. Other cells with immunological functions also participate in the in situ response including dendritic cells (5), plasma cells, mast cells, neutrophils, eosinophils, keratinocytes and natural killer cells (6-9).
The delayed hypersensitivity reaction to Leishmania antigens, also known as the Montenegro skin test, is a marker of exposure and specific sensitization to the parasite. Previous descriptions of the histological characteristics of the Montenegro skin test in human leishmaniasis have indicated that cutaneous leishmaniasis lesions and the Montenegro skin test share the fundamental features of a delayed hypersensitivity reaction due to the persistent presence of the parasite or its antigens (10,11). Notably both the Montenegro skin test response and lesions present a predominantly Th1 cytokine profile in localized tegumentary leishmaniasis (12). The intensity of the skin test response has been associated with the clinical presentation of the disease. Hence in the diffuse form there is no inflammatory reaction to the antigen, whereas mucosal leishmaniasis typically presents an exacerbated response (13,14).
Lesions in tegumentary leishmaniasis have a variable duration and are notoriously heterogeneous, even during the early stages of the disease. The initial events in the immune response to Leishmania have not been readily accessible, nor have early markers that might differentiate the response in clinically resistant individuals and susceptible patients. If leishmaniasis lesions and the Montenegro skin test share similar cellular responses, the skin test could be a surrogate for the study of the in situ events occurring during the early stages of cutaneous leishmaniasis.
We have conducted a comparative histopathologic study of biopsies of lesions of cutaneous leishmaniasis with differing time of evolution and their respective Montenegro skin test, and biopsies of the skin test site in individuals having asymptomatic infection and in normal volunteers. The spectrum of individuals included in the study sought to examine the local response of putative susceptible individuals, here operationally defined as patients with chronic lesions, and putative resistant individuals, defined as asymptomatically infected residents of endemic areas (15). In addition to T lymphocytes and macrophages, we evaluated other cell types such as eosinophils and B cells, whose role in human leishmaniasis have not been established.
Materials and methods
Subjects
Participants in the study were recruited among patients consulting the Hospital Regional San Andrés in Tumaco (Colombian Pacific coast), the outpatients in CIDEIM (Cali) and individuals participating in an epidemiological survey conducted in endemic areas of leishmaniasis transmission of the municipality of Tumaco. None of the participants had been previously treated with antimonial drugs. Volunteers residing in Cali, who had never lived in endemic areas of leishmaniasis transmission participated as controls. All protocols were reviewed and approved by the ethical committees of the participating institutions in accordance with the requirements of the Ministerio de la Protección Social de Colombia. A signed informed consent was obtained in all cases.
The study groups were defined as follows:
Chronic lesion group (putative susceptible individuals): individuals presenting active cutaneous leishmaniasis lesions having a duration of 6 months or more, with or without previous history of cutaneous leishmaniasis or presence of scars consistent with prior leishmaniasis.
Acute lesion group: patients presenting active lesions with an evolution time of one month or less, without evidence of previous cutaneous leishmaniasis.
Asymptomatically infected (putative resistant individuals): Montenegro skin test positive individuals residing in an endemic area of leishmaniasis transmission, without clinical history or evidence of leishmaniasis.
Normal controls: healthy subjects who had always resided in areas free of leishmaniasis transmission.
Specificity controls: in order to control the specificity of the inflammatory reaction, Montenegro skin test antigen was applied to one forearm while the diluent of this antigen was applied to the other forearm in two individuals of the healthy control group.
Diagnosis
Parasitological methods (lesion scraping and/or aspiration, culture or biopsy) were utilized for diagnosing most patients (16/18). Diagnosis was achieved in two patients based on clinical signs, histopathological findings, epidemiologic exposure and Montenegro skin testing reactivity.
Montenegro skin testing antigen (leishmanin)
Skin testing was carried out using 0.1 ml of Montenegro antigen (leishmanin). This antigen consisted of a suspension of 1x107 heat killed and thimerosal-fixed Leishmania panamensis and Leishmania amazonensis promastigotes (Instituto Nacional de Salud, Colombia). The test was read at 48 hours using the ballpoint pen method (16); induration of 5 mm or more in diameter was considered positive. The presence of vesiculation or ulceration was also noted.
Biopsies
Two punch biopsies, one from the lesion and one from the Montenegro skin testing site, were obtained using a 4 mm disposable scleral punch. The OCT (Miles Laboratories, Naperville, IL, USA) embedded biopsies were snap-frozen and later transferred to long term storage at –70°C until used. A single biopsy (skin test site) was obtained from individuals in the healthy control and asymptomatically infected groups.
Staining techniques
Five-micrometer sections conventionally stained with hematoxylin and eosin were used to study the morphology of the inflammatory reactions and to evaluate the eosinophil population. Macrophages and lymphocyte subpopulations were evaluated using immunohistochemical methods of characterization (Vector Laboratories, Burlingame, CA, USA). Briefly, the frozen sections were fixed in cold acetone, endogenous peroxidase was blocked with 3% hydrogen peroxide in methanol, and then incubated with the primary antibody at a dilution based on prior titration followed by incubation with biotinylated secondary antibody and a preformed avidin-biotin-horseradish peroxidase complex. Monoclonal antibodies used to distinguish the infiltrating cell populations included anti-Leu (CD4) 1:50, anti-Leu-2a (CD8) 1:50 (Becton Dickinson, CA, USA), anti-human B cell (CD20) 1:50 and antihuman monocyte (CD14) 1:10 (Dako, Carpinteria, CA, USA).
The chromogen reaction was developed with a diaminobenzidene solution.
Evaluation of tissue sections
Quantification of cell populations was carried out blindly: slides were assigned a code number and were read by two different evaluators or by one evaluator performing two independent readings of the same slide. The following determinations were made for each slide: the total area of the section, the area of inflammation and the number of cells staining positive for each particular monoclonal antibody. Readings were carried out using a Nikon Labophot-2 microscope with a grid fitted to the ocular. The grid covered an area of 0.04 mm2, and this area contained approximately 325 inflammatory cells. Three different fields (0.12 mm2, equivalent to approximately 975 cells) were randomly selected in order to count the number of positive cells for each monoclonal antibody. The total number of positive cells in the biopsy was calculated using the following formula:
(Inflamed area x number of positive cells in 3 fields) /0.12 mm2
The inflamed area of the tissue sections varied among the biopsies. In order to compare the inflammatory response of the biopsies, a unit area of 0.19 mm2, corresponding to the mean area of inflammation of the Montenegro skin testing of healthy volunteers, was operationally defined as the basis of comparison of cell densities.
Statistical analysis
The percentage of each cell subpopulation was calculated in order to compare cell densities in the Montenegro skin testing sites and in the lesions. Cell density data were transformed to logarithms (base 10) in order to normalize the distribution for statistical analysis. Descriptive statistics were calculated for each cell population using the variation coefficient as an indicator of internal dispersion in each group. One way ANOVA with contrast tests of Duncan was used for comparing the results obtained independently for the different study groups. Because the CD4+/CD8+ lymphocyte ratio was not normally distributed, a Kruskal- Wallis test was performed to analyze the differences in this variable. Correlations among results of the groups were established with the correlation coefficient of Spearman. All of the analyses were conducted considering a significance level of 0.05.
Results
The general characteristics of the individuals participating in the study are summarized in table 1. There was no significant difference in age of the patients included in the different groups. Females predominated in the active chronic lesion (putative susceptible group), whereas males predominated in the acute lesion and asymptomatically infected groups. The mean time of evolution of lesions was 0.7 months for the group having acute lesions and 26 for the chronic lesions.
Montenegro skin testing in normal controls
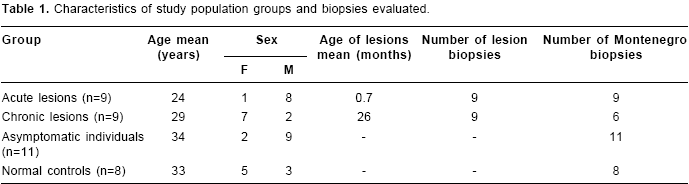
Healthy individuals presented a scarce and superficial inflammatory response to Montenegro skin testing
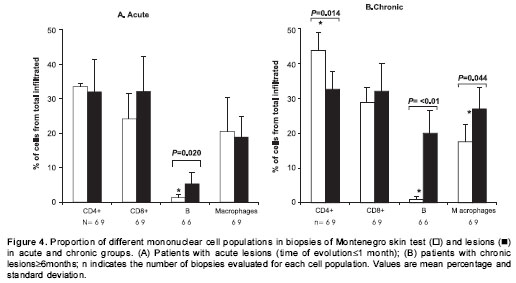
(figure 1A) consisting of a few perivascular macrophage, CD4+ and CD8+ T lymphocytes and polymorphonuclear (PMN) leukocytes (non-specific response) including scarce eosinophils. The diluent of leishmanin containing thimerosal induced an inflammatory response similar to that observed in Montenegro skin testing of normal controls involving the same cell subsets (figure 1B). The number of inflammatory cells was significantly lower in normal controls than in the other groups (mean for control group: 712; acute: 10,057; chronic: 14,884; and asymptomatic individuals: 14,129. P<0.05). The CD4/CD8 T lymphocyte ratio showed a statistically significant difference among groups (median for Montenegro skin testing normal control group: 2.8; Montenegro skin testing asymptomatic group: 1.7; Montenegro skin testing acute group: 1.2; Montenegro skin testing chronic group: 1.5; acute lesion group: 0.7; chronic lesion group:1.2) (Kruskal-Wallis among groups, P=0.045). Although not statistically significant, CD4/CD8 ratio in the Montenegro skin testing of control group was higher than in the patient groups.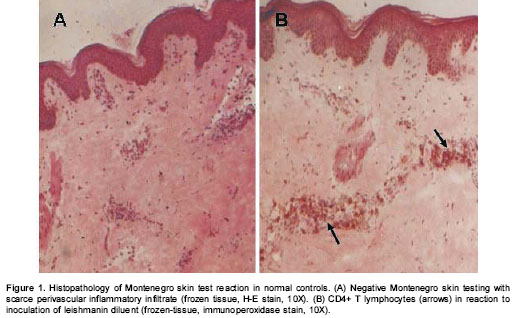
Histopathological characteristics of Montenegro skin testing in acute and chronic patients and resistant individuals
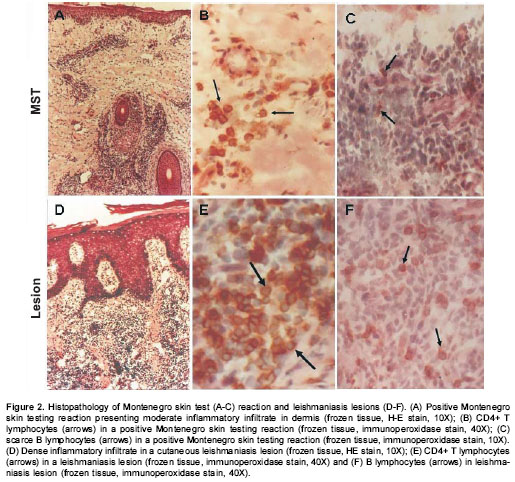
No differences were observed in the size of Montenegro skin testing reaction. The in situ inflammatory response showed a moderate chronic inflammatory infiltrate in the papillary and reticular dermis (figure 2A), with a predominance of CD4+ (figure 2B) and CD8+ T lymphocytes and macrophages. Scarce granulocytes were observed with presence of eosinophils. Occasional B cells were present (figure 2C). Three biopsies of Montenegro skin testing in asymptomatic individuals showed necrosis and vasculitis, and one of these had a severe inflammatory reaction with a subepidermic blister and ulceration (figure 3).
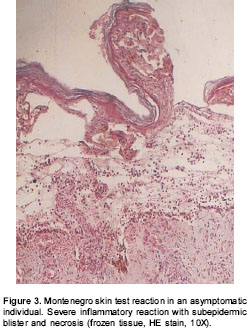
Histopathological characteristics of acute and chronic lesions
Leishmaniasis lesions presented similar cellular constituents to those of Montenegro skin testing. However, the magnitude of the inflammatory response in leishmaniasis lesions was significantly greater than the response to Montenegro skin testing ( P<0.05). Acantosis with ulceration of the epidermis, granulomas and fibrosis were observed in biopsies of both groups (figure 2D). Dermal inflammatory infiltrate was diffuse or multi-focal with a predominance of mononuclear cells including macrophages, CD4+ T (figure 2E) and CD8+ T lymphocytes, and B lymphocytes (figure 2F). PMN leukocytes including eosinophils were present in a smaller proportion.
Montenegro skin testing in asymptomatic individuals and acute and chronic groups compared with leishmaniasis lesions
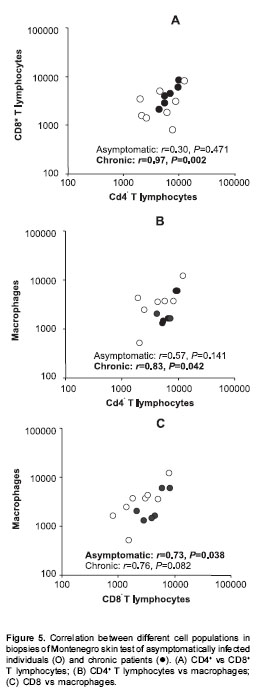
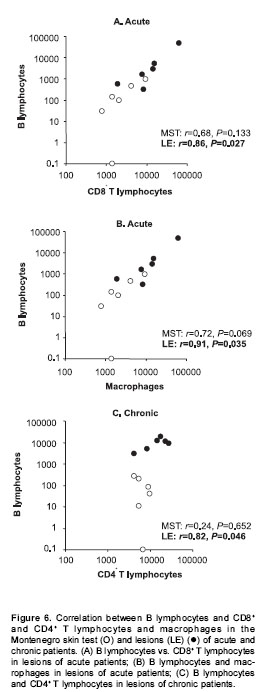
Patients with acute lesions had a similar proportion of CD4+ and CD8+ T lymphocytes and macrophages in both lesions and Montenegro skin testing response (figure 4A). In contrast, the proportion of CD4+ T lymphocytes was lower ( P<0.05) and the proportion of macrophages and B cells was higher ( P<0.05) in lesions of the chronic group compared with their Montenegro skin testing (figure 4B). The lesions of chronic patients presented a significantly greater proportion of B lymphocytes (20%) than the lesions of acute patients (5.3%) ( P=0.002). The Montenegro skin testing of individuals with chronic lesions showed a concomitant increase of CD4+ and CD8+ T lymphocytes ( P=0.002) (figure 5A), as well as a direct association between CD4+ T lymphocytes and macrophages ( P=0.042) (figure 5B). In contrast, a positive association was observed only between CD8+ T lymphocytes and macrophages in the asymptomatic individuals ( P=0.038) (figure 5C). The association between B lymphocytes and other mononuclear cells differed in acute and chronic lesions. A positive association was observed between B lymphocytes, CD8+ T lymphocytes and macrophages in lesions of acute patients (figure 6A, B), whereas B lymphocytes and CD4+ T lymphocytes were associated in lesions of chronic patients (figure 6C).
Discussion
Chronic cutaneous and mucosal lesions of leishmaniasis are generally associated with an intense reaction in the clinical evaluation of Montenegro skin testing (11,14,17), whereas the response is low or absent in the non-healing diffuse presentation of dermal leishmaniasis. Furthermore, Montenegro skin testing response has been found to be significantly lower in patients who experienced recurrent leishmaniasis than in asymptomatically infected residents of endemic areas (1), and this test has been proposed as a predictor of relapse in cutaneous leishmaniasis (18). These observations suggest that the response to this intradermal stimulus could somehow reflect the events occurring in the natural infection.
The comparison of the lesions with the Montenegro skin testing reaction showed that, although tissue responses in both consisted of the same elements, the proportions of the different cell populations were similar only in acute lesions and their Montenegro skin testing. In contrast, in the chronic patients, as might be expected because of the long duration of the inflammatory response at the lesion site, the proportions of the distinct cell populations were significantly different from the corresponding Montenegro skin testing. These results support the possible application of Montenegro skin testing as a surrogate of the early response to infection.
The positive association between CD4+ and CD8+ T lymphocytes in the Montenegro skin testing of acute and chronic patients and the CD4+/CD8+ ratio approximating 1 in the lesions suggest a similar response in both of these inflammatory manifestations and provide circumstantial evidence for the participation of the CD8+ T lymphocytes in the human response to Leishmania infection. This study showed an association between the presence of CD8+ T lymphocytes and macrophages in the Montenegro skin testing reaction of asymptomatic individuals. This association may link the cytotoxic function of the CD8+ T cells to the control of the infections through the release of cytokines and cytotoxins. CD8+ T cell products such as TNF-a y TNF-b could synergize with IFN- g produced by these and other cells to activate macrophages and destroy infected target cells. A cytotoxic response could also partly explain the tissue damage observed in the skin test biopsies of three individuals with asymptomatic infection that presented necrosis. Activated macrophage products IL-18 and IL-12 stimulate a Th1 response and the resolution of several infectious diseases (19). Therefore the association of CD8+ T lymphocytes and macrophages in the recall response to Leishmania (as leishmanin) in asymptomatic individuals may signal an effective response and potentially represent an immune correlate.
Contrary to Montenegro skin testing reaction in asymptomatic individuals, the Montenegro skin testing of chronic patients showed an association between CD8+ T cells and CD4+ T cells. Some activated CD8+ T cells have a suppressive activity mediated through the production of TGF-b and IL- 10, which inhibit the Th1 response and promote and a Th2 response. This CD8+ T cell function may prevail in patients with chronic lesions. However, healing has been associated with an increase in CD8+ cells (20), so these cells are likely to have different functional during pathogenesis and healing.
The presence of B lymphocytes in leishmaniasis lesions has been previously reported (8,21). Yet their significance is not well understood because these cells are not usually observed in the dermis of healthy skin (22), the site of entrance of Leishmania. B lymphocytes, besides being capable of differentiating into antibody producing plasma cells are also antigen presenting cells, especially for soluble antigens. Although tissue macrophages and dendritic cells are considered the primary antigen presenting cells, once specific B lymphocyte clones are developed, they contribute significantly to this function (23). The B lymphocytes from susceptible mice promote increased IL-4 compared to those from resistant mice (24). Depletion of B lymphocytes in this model significantly decreased IL-4 and increased IFN-g production (25). In humans, the increase of B lymphocytes found in lesion biopsies from chronic individuals suggest that B cells could contribute to the development of chronic, non-healing lesions through mechanisms such as preferential antigen presentation, induction of IL-4 production, and the promotion of a Th2 immune response.
Notably, scarce inflammatory cells were observed in biopsies of Montenegro skin testing reactions of normal individuals and those receiving only the diluent of leishmanin (specificity controls). Mercury contained in thimerosal has been shown to induce toxic reactions in the skin of non sensitized animals (26), and both mercury and thiosalicylic acid are involved in allergic contact dermatitis mediated by hapten-specific T lymphocytes (27). In addition, thimerosal produced a high number of false positive Montenegro skin testing in dogs from a leishmaniasis endemic area (28). Similarly, phenol has been shown to induce false positive results in leishmanin tests in humans (29). On the other hand, in vitro reaction to Leishmania antigens by lymphocytes from naïve individuals has been demonstrated (30-32), and could also explain innate and/or cross-reactive recognition of Leishmania antigen without previous exposure. However, the presence of a similar inflammatory response to the diluent of leishmanin indicates that this response is probably due to thimerosal, a widely-used antiseptic and irritant.
Causal relationships cannot be established by circumstantial associations after pathology has occurred. However these associations are useful in generating hypotheses. The association between macrophages and distinct T cell types in the Montenegro skin test reaction of patients with chronic lesions and asymptomatically infected individuals supports the possibility the local response may reveal clues to healing and nonhealing responses and the use of the Montenegro reaction as a surrogate of the early immune response to infection. Experimental approaches including innovative strategies of functional analysis of cells in the skin test and lesion sites and immunomodulatory interventions will be required to establish the roles of the cellular and molecular mediators in the outcome of infection.
Acknowledgments
We thank all the people who donated tissue samples, the staff of the Clinical Unit of CIDEIM for the assistance in the recruitment of patients and Graciela Salinas for technical support in the histochemical and immunohistochemical staining.
Conflict of interest
The authors have no conflict of interest in the conduct or reporting of this study.
Financing
This study was supported in part by the UNDP/ World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) ID 960183, grant I-P50-AI30603-01/05 from the United States National Institute of Allergy and Infectious Diseases, COLCIENCIAS grant 2229-04-001-92.
Corresponding:
Nancy Gore Saravia, CIDEIM, Apartado Aéreo 5390, Cali Colombia.
Telephone: (572) 668 2164; fax: (572) 667 2989
cideim@cideim.org.co
References
1. Bosque F, Saravia NG, Valderrama L, Milon G. Distinct innate and acquired immune responses to Leishmania in putative susceptible and resistant human populations endemically exposed to L. (Viannia) panamensis infection. Scand J Immunol 2000;51:533-41. [ Links ]
2. Weigle KA, Santrich C, Martínez F, Valderrama L, Saravia NG. Epidemiology of cutaneous leishmaniasis in Colombia: a longitudinal study of the natural history, prevalence, and incidence of infection and clinical manifestations. J Infect Dis 1993;168:699-708. [ Links ]
3. Romagnani S. Induction of Th1 and Th2 responses: a key role for the "natural" immune response? Immunol Today 1992;13:379-81. [ Links ]
4. Scott P. IFN-g modulates the early development of Th1 and Th2 responses in a murine model cutaneous leishmaniasis. J Immunol 1991;147:3149-55. [ Links ]
5. Moll H, Flohe S, Rollinghoff M. Dendritic cells in Leishmania major-immune mice harbor persistent parasites and mediate an antigen-specific T cell immune response. Eur J Immunol 1995;25:693-9. [ Links ]
6. Gutiérrez Y, Salinas GH, Palma G, Valderrama L, Santrich CV, Saravia NG. Correlation between histopathology, immune response, clinical presentation, and evolution in Leishmania braziliensis infection. Am J Trop Med Hyg 1991;45:281-9. [ Links ]
7. Magalhaes AV, Moraes MAP, Raick AN, Llanos- Cuentas A, Costa JML, Cuba CC et al. Histopatologia da leishmaniose tegumentar por Leishmania braziliensis braziliensis. Rev Inst Med Trop Sao Paulo 1986; 28:253-62. [ Links ]
8. Palma GI, Saravia NG. In situ characterization of the human host response to Leishmania panamensis. Am J Dermatopathol 1997;19:585-90. [ Links ]
9. Ridley DS, Ridley MJ. The evolution of the lesion in cutaneous leishmaniasis. J Pathol 1983;141:83-96. [ Links ]
10. Mayrink W, Schettini AP, Williams P, Raso P, Magalhaes PA, Lima A de O et al. Histological observations on Montenegros reaction in man. Rev Inst Med Trop Sao Paulo 1989;31:256-61. [ Links ]
11. Pirmez C, Cooper C, Paes-Oliveira M, Schubach A, Torigian V, Modlin RL. Immunologic responsiveness in American cutaneous leishmaniasis lesions. J Immunol 1990;145:3100-4. [ Links ]
12. Pirmez C, Yamamura M, Uyemura K, Paes-Oliveira M, Conceicao-Silva F, Modlin R. Cytokine patterns in the pathogenesis of human leishmaniasis. J Clin Invest 1993;91:1390-5. [ Links ]
13. Castés M, Agnelli A, Verde O, Rondón AJ. Characterization of the cellular immune response in American cutaneous leishmaniasis. Clin Immunol Immunopathol 1983;27:176-86. [ Links ]
14. Saravia NG, Valderrama L, Labrada M, Holguín AF, Navas C, Palma G et al. The relationship of Leishmania braziliensis subspecies and immune response to disease expression in New World leishmaniasis. J Infect Dis 1989;159:725-35. [ Links ]
15. Robledo S, Wozencraft A, Valencia AZ, Saravia N. Human monocyte infection by Leishmania (Viannia) panamensis. J Immunol 1994;152:1265-76. [ Links ]
16. Sokal JE. Measurement of delayed skin-test responses. N Engl J Med 1975;293:501-2. [ Links ]
17. Carvalho EM, Johnson WD, Barreto E, Marsden PD, Costa JL, Reed S et al. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J Immunol 1985;135:4144-8. [ Links ]
18. Passos VM, Barreto SM, Romanha AJ, Krettli AU, Volpini AC, Lima e Costa MF. American cutaneous leishmaniasis: use of a skin test as a predictor of relapse after treatment. Bull World Health Organ 2000;78:968-74. [ Links ]
19. García VE, Uyemura K, Sieling PA, Ochoa MT, Morita CT, Okamura H et al. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J Immunol 1999;162:6114-21. [ Links ]
20. Da-Cruz AM, Bittar R, Mattos M, Oliveira-Neto MP, Nogueira R, Pinho-Ribeiro V et al. T-cell-mediated Immune responses in patients with cutaneous or mucosal leishmaniasis: long-term evaluation after therapy. Clin Diagn Lab Immunol 2002;9:251-6. [ Links ]
21. Lima HC, Vasconcelos AW, David JR, Lerner EA. American cutaneous leishmaniasis: in situ characterization of the cellular immune response with time. Am J Trop Med Hyg 1994;50:743-7. [ Links ]
22. Bos JD, Kapsenberg ML. The skin immune system. Immunol Today 1987;7:235-40. [ Links ]
23. Frohman M, Cowing C. Presentation of antigen by B cells: functional dependence on radiation dose, interleukins, cellular activation, and differential glycosylation. J Immunol 1985;134:2269-75. [ Links ]
24. Rossi-Bergmann B, Müller I, Godinho E. TH1 and TH2 T-cell subsets are differentially activated by macrophages and B cells in murine leishmaniasis. Infect Immun 1993;61:2266-9. [ Links ]
25. Shankar AH, Titus RG. The influence of antigen-presenting cell type and interferon-g on priming and cytokine secretion of Leishmania major-specific T cells. J Infect Dis 1997;175:151-7. [ Links ]
26. Uchida T, Naito S, Kato H, Hatano I, Harashima A, Terada Y et al. Thimerosal induces toxic reaction in non-sensitized animals. Int Arch Allergy Immunol 1994;104:296-301. [ Links ]
27. Lebrec H, Bachot N, Gaspard I, Kerdine S, Guinnepain MT, Laurent J et al. Mechanisms of druginduced allergic contact dermatitis. Cell Biol Toxicol 1999;15:57-62. [ Links ]
28. Paranhos-Silva M, Pontes-de-Carvalho LC, De Sá Oliveira G, Góes Nascimento E, Dos-Santos WL. Skin reactions to thimerosal and Leishmania in dogs from a leishmaniasis endemic area: it is better to keep them apart. Mem Inst Oswaldo Cruz 2001;96:679-81. [ Links ]
29. Pineda JA, Macias J, Morillas F, Fernandez-Ochoa J, Cara J, de la Rosa R et al. False-positive results of leishmanin skin test due to phenol-containing diluent. Trans R Soc Trop Med Hyg 2001;95:173-4. [ Links ]
30. Akuffo H, Alexis A, Eidsmo L, Saed A, Nylén S, Maasho K. Natural killer cells in cross-regulation of IL- 12 by IL-10 in Leishmania antigen-stimulated blood donor cells. Clin Exp Immunol 1999;117:529-34. [ Links ]
31. Kemp M, Hansen MB, Theander TG. Recognition of Leishmania antigens by T lymphocytes from nonexposed individuals. Infect Immun 1992;60:2246-51. [ Links ]
32. Maasho K, Satti I, Nylén S, Guzmán G, Koning F, Akuffo H. A Leishmania homologue of receptors for activated C-kinase (LACK) induces both interferon-g and interleukin-10 in natural killer cells of healthy blood donors. J Infect Dis 2000;182:570-8. [ Links ]