Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Biomédica
versión impresa ISSN 0120-4157versión On-line ISSN 2590-7379
Biomédica v.26 supl.1 Bogotá oct. 2006
Photodynamic activity of aluminium (III) and zinc (II) phthalocyanines in Leishmania promastigotes
Patricia Escobar 1, Indira P. Hernández 1, Cesar M. Rueda 1, Fernando Martínez 2, Edgar Páez 2
1 Centro de Investigación de Enfermedades Tropicales (CINTROP), Facultad de Salud, Escuela de Medicina, Departamento de Ciencias Básicas, Universidad Industrial de Santander, Bucaramanga, Colombia.
2
Centro de Investigaciones en Catálisis (CICAT), Escuela de Química, Universidad Industrial de Santander, Bucaramanga, Colombia.Recibido: 01/08/05; aceptado: 10/02/06
Introduction.
Photodynamic therapy is a two-step procedure, involving the use of photosensitizing agents followed by selective illumination of the target lesion with visible light. It produces highly reactive oxygen species and subsequent cellular damage.Objective. This study was designed to determine whether Leishmania chagasi and L. panamensis promastigotes were sensitive to photodynamic therapy in vitro.
Material and methods. Leishmania promastigotes were treated with aluminium phthalocyanine chloride and zinc phthalocyanine photosensitizers before illumination with visible light at 670 nm. The parasite photoactivity was calculated by sigmoidal regression analysis.
Results. Leishmania chagasi promastigotes were highly photosensitive to aluminium phthalocyanine chloride treatment with effective inhibitory dose50 (ED50) concentration values of 0.0033, 0.0083 and 0.0093 µM upon exposure to 10.0, 5.0, and 2.5 J/cm2 light intensities respectively. By contrast, the activity of aluminium phthalocyanine chloride on L. panamensis was significantly lower ( P<0.01) with ED50 values of 0.17, 0.25, 0.34 µM at the same light intensities. Zinc phthalocyanine activity was significantly ( P<0.01) less active than aluminium phthalocyanine chloride on both strains of these two species and no differences in zinc phthalocyanine activity were found between them. A dose-response phototoxic effect with both phthalocyanines was observed. Parasite inhibition was not observed after aluminium phthalocyanine chloride or zinc phthalocyanine treatment in the dark. The reference drugs hexadecylphosphocholine and amphotericin B were not photoactive.
Conclusion. Treatment of Leishmania promastigotes with aluminium phthalocyanine chloride and zinc phthalocyanine followed by illumination with visible light at 670 nm inhibited in vitro growth of promastigotes of L. chagasi and L. panamensis. Photodynamic therapy against Leishmania could be a promising strategy for leishmaniasis treatment.
Key words : Leishmania, photochemotherapy, drug therapy, in vitro.
Actividad fotodinámica de ftalocianina de aluminio (iii) y zinc (ii) en promastigotes de Leishmania
Introducción. La terapia fotodinámica es un procedimiento en dos pasos que usa un agente fotosensibilizador y luz visible produciendo radicales de oxígeno altamente reactivos originando daño celular.
Objetivo. En este estudio se determinó la fotosusceptibilidad de promastigotes de dos cepas de las especies Leishmania chagasi y L. panamensis a la terapia fotodinámica in vitro.
Materiales y métodos. Promastigotes de Leishmania fueron tratados con ftalocianina de aluminio clorada o ftalocianina de zinc antes de la irradiación con luz visible a 670 nm. La fotoactividad fue calculada por regresión sigmoidea.
Resultados. Los promastigotes de L. chagasi fueron altamente fotosensibles al tratamiento con ftalocianina de aluminio clorada con valores de concentraciones inhibitorias50 de 0,0033, 0,0083 y 0,0093 µM utilizando una intensidad de luz de 10,0, 5,0 y 2,5 J/cm2 respectivamente.
La actividad de la ftalocianina de aluminio clorada en promastigotes de la cepa de L. panamensis fue significativamente menor ( P<0,01) que en la cepa L. chagasi con valores de 50 ED50 de 0,17, 0,25, 0,34 µM respectivamente. El tratamiento con ftalocianina de zinc fue significativamente menos fotoactivo ( P<0,01) en ambas especies sin diferencia significativa en la actividad entre ellas. El efecto fototóxico inducido por las ftalocianinas fue dependiente de la dosis utilizada. No se presentó fototoxicidad en condiciones de oscuridad. Los medicamentos de referencia hexadecilfosfocolina y anfotericina B no fueron fotoactivos.
Conclusión. El tratamiento de promastigotes de Leishmania con ftalocianinas seguido de la irradiación con luz visible a 670 nm inhibió el crecimiento in vitro de promastigotes de L. chagasi y L. panamensis. La terapia fotodinámica contra la Leishmania podría ser una estrategia promisoria en el tratamiento de leishmaniasis.
Palabras clave : Leishmania, fotoquimioterapia, quimioterapia, in vitro.
The leishmaniases are a group of diseases caused by trypanosomatid protozoa of the genus Leishmania transmitted to humans by the bite of an infected sandfly. Leishmaniasis occurs in approximately 88 countries in the New and Old World where 350 million individuals are exposed, 12 million are infected and 1.5 to 2 million new cases of cutaneous leishmaniasis and half a million of visceral leishmaniasis are reported annually (1).
Since the 1940s, pentavalent antimonial (SbV) drugs have been the treatment of choice for all forms of human leishmaniasis. Other drugs such as amphotericin B, pentamidine isothionate and aminosidine (paromomycin) constitute the traditional alternatives to SbV for leishmaniasis treatment (2). However, currently available drugs require parenteral administration, many vary in efficacy and are toxic and expensive. Moreover, resistance to the most widely used antileishmanial drugs has been reported (3). The process of drug discovery in leishmaniasis is difficult due to the biological characteristics of the parasite and to the lack of economical incentives for pharmaceutical companies to invest in the development of new treatments. Fortunately, the current situation is more promising than it has been for several years. The duration of treatment has been reduced, oral instead of parenteral administration is now available, and new drugs, formulations or dosage regimens of old drugs with less toxicity are also on clinical trials (4-6).
Photodynamic therapy is a two-step procedure that uses a combination of photosensitizer agents and visible light in the presence of molecular oxygen to obtain a therapeutic effect (7). It has been used as an alternative procedure for detection and treatment of cancer and other skin illnesses like psoriasis and vitiligo (8). Photodynamic therapy has been used to eliminate in vitro microorganisms such as bacteria, yeasts, viruses and parasites and successfully in disinfection of blood products. In addition, it has become a potential low cost treatment for local skin infections (9-12).
Photosensitizer agents such as phenothiazine dyes, porphyrins and phthalocyanines contain macrocyclic structures with accessible sites for triplet excited states under visible light radiation. They have the ability to accumulate in unhealthy tissues and cells exhibiting little or no toxicity; however, after light illumination in the presence of molecular oxygen, they are excited and a lethal effect on infected tissues is induced. Photooxidation reactions are produced by radical intermediates subsequently scavenged by oxygen or by the generation of the highly cytotoxic monovalent oxygen (O.-) after energy transfer from the photoexcited sensitizer (8,13,14).
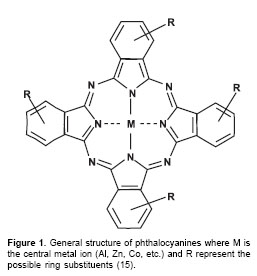
Phthalocyanines (figure 1) are a new generation of photosensitizer agents for photodynamic therapy (15-17). These compounds are good candidates for photodynamic therapy because they are efficiently incorporated into target cells, are non-toxic for healthy cells, exhibit high yield of triplet states generation, and induce an optimal light tissue penetration by their high absorbance coefficients at 650-680 nm (18,19). The photophysical properties of phthalocyanines are strongly dependent on the central metal ion. For application in photodynamic therapy, Zn (II) and Al (III) complexes exhibit the most favourable properties. Unfortunately most phthalocyanines are insoluble in water or in biologically compatible solvents. However, water-soluble phthalocyanines derivatives can be readily synthesised through substitution of the ring with moieties such as sulphonic acid, carboxylic acid and amino groups (15).
While the use of the photodynamic therapy in cancer treatment is well accepted, its use against microorganisms is a relatively new area of research and, at least in the case of Leishmania, it is in exploratory stages (20,21). Early in vitro investigations showed that some electron carriers and porphyrins in combination with menadione induced a selective destruction of intracellular amastigotes (22-24). Further experiments demonstrated that transgenic Leishmania parasites, expressing the second and third enzymes involved in the synthesis of heme metabolic pathway such as aminolevulinate dehydratase and porphobilinogen deaminase respectively, become highly susceptible to ultraviolet light exposition after aminolevulinic acid treatment (20). Recently, the efficacy of photodynamic therapy treatment was demonstrated on human skin lesions caused possibly by Leishmania donovani parasites, using aminolevulinic acid as photosensitizer (21).
In order to investigate for new chemotherapeutic strategies on leishmaniasis, the aim of this study was to determine the in vitro photodynamic activity of aluminium chloride and zinc phthalocyanines in promastigotes of Leishmania panamensis and L. chagasi parasites.
Materials and methods
Phthalocyanines and reference drugs
Aluminium phthalocyanine chloride (AlPc) and zinc phthalocyanine (ZnPc) were purchased from Sigma-Aldrich (St. Louis, USA). Stock solutions were prepared in dimethylformamide (Fluka). Working solutions were made in culture medium immediately before the assays. Dimethylformamide was not toxic for the parasites at the dilution used. Hexadecylphosphocholine, kindly provided by Professor Simon Croft (London School of Hygiene and Tropical Medicine, UK), and amphotericin B purchased from Sigma were used as reference drugs.
Parasites
L. panamensis (MHOM/PA/71/LS94) and L. chagasi parasites (MHOM/BR/74/PP75) were kindly donated by the Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM), Cali, Colombia. Parasite promastigotes were cultured at 28°C in minimal essential medium (MEM, Gibco, USA) supplemented with 10% of heat inactivated foetal calf serum (hiFCS, Gibco).
Phototoxic assays on Leishmania promastigotes
Parasites were harvested in the late exponential growth phase, diluted to 1 x 106 parasites/ml and incubated with control drugs, AlPc or ZnPc using a three-fold dilution series (from 0 µM to 15 µM), in 96 microwell plates (Becton Dickinson, New Jersey, USA) for 24 hours at 28°C. The parasites were illuminated using light intensities of 10.0 J/ cm2, 5.0 J/cm2 and 2.5 J/cm2 at 670 nm with a non-ionic red laser light system (BFW, Edmund Industrial Optics). Control cells were not illuminated. Twenty-four hours after illumination, inhibition of promastigotes growth was microscopically determined by counting parasite numbers in a haemocytometer. Inhibition of parasite growth was determined by comparison to untreated controls. The phototoxic effect was demonstrated by comparing the activity of phthalocyanines with and without illumination. Each experiment was repeated by three times.
Analysis
ED50 and ED90 values were calculated by sigmoidal regression analysis (MSx/fitTM; ID Business Solution, Guildford, UK). Results were expressed as mean ± SEM and statistical significance was determined by Students t-test.
Results
Photosensitivity of L. chagasi and L. panamensis to aluminium and zinc phthalocyanines
L. chagasi promastigotes were 30 to 50 times more photosensitive than L. panamensis after AlPc treatment with ED50 values of 0.0033, 0.0093 and 0.0083 µM versus 0.17, 0.25, 0.34 µM of AlPc at light intensities of 10.0 J/cm2, 5.0 J/cm2 and 2.5 J/cm2 respectively (
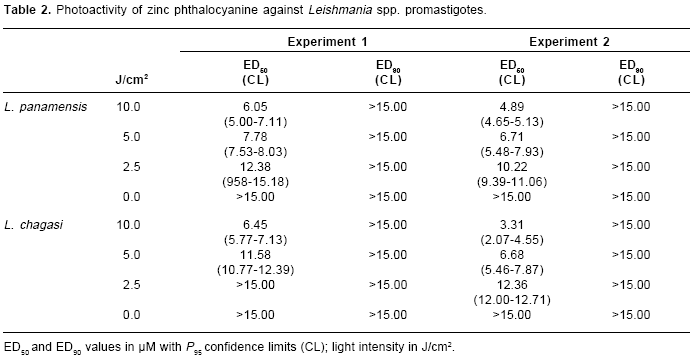
table 1). A dose response was observed in the activity of AlPc on the strains of both parasites species. In contrast, both species of Leishmania showed the same range of photoactivity after ZnPc treatment (table 2). No significant parasite inhibition was induced on nonphthalocyanine treated parasites after illumination.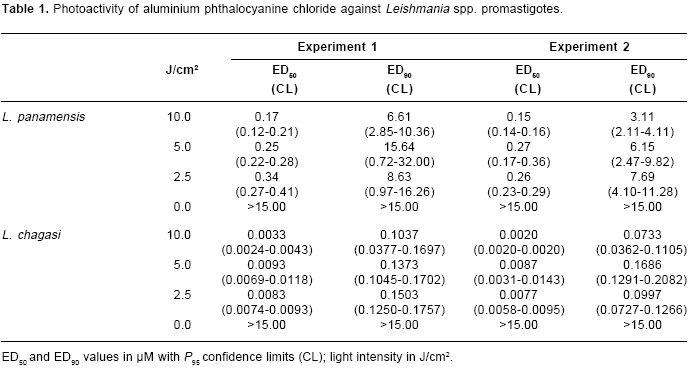
This result indicates that the parasite inhibition was due to the photosensitization effect of the phthalocyanine compound under visible light.
Level of photoactivity induced by aluminium and zinc phthalocyanine
In all assays, AlPc treatment induced more phototocixity than ZnPc. Aluminium phthalocyanine was 30 to 40 times and 1,500-2,000 times more photoactive than ZnPc on L. panamensis and L. chagasi promastigotes respectively (
table 1 and table 2).No inhibition on both parasites species was induced by AlPc or ZnPc treatment on the dark (
tables 1 and tables 2). At the maximal dose of any of the Pc used (15 µM), only up to 5% of parasite inhibition was observed without illumination.Photoactivity induced by hexadecylphosphocholine and amphotericin B
No photoactivity was induced by the reference drugs after illumination at 5.0 J/cm2 and 2.5 J/ cm2 (data not shown). Without illumination, HPC was active against L. panamensis and L. chagasi promastigotes with ED50 values of 4.15 µM ( P95 confidence limits 3.85-4.45 µM) and 1.80 µM (P95 confidence limits 1.56-2.04 µM) respectively after 3 days of incubation. In addition, AmB was active against L. panamensis and L. chagasi with ED50 values of 0.11 µg/mL ( P95 confidence limits 0.10- 0.12 µg/mL) and 0.025 µg/mL (P95 confidence limits 0.021-0.028 µg/mL) respectively.
Discussion
The phototoxic effect of phthalocyanines against strains of two Leishmania species was determined for the first time in this work. The antileishmanial activity induced by the photodynamic treatment in vitro was strongly dependent on both the Leishmania species and the type and concentration of phthalocyanine used.
Leishmania chagasi promastigotes were highly susceptible to photodynamic therapy in vitro using AlPc treatment and red light illumination at 670 nm. The range of activities induced by AlPc (between ED50 of 0.0033 to 0.0093 µM) was lower than other antileishmanial drug activities such as hexadecylphosphocholine and amphotericin B (25-27).
The efficacy of photodynamic therapy on trypanosomatids has been demonstrated in previous studies. The ability to inactivate Trypanosoma cruzi parasites from blood components was observed using photosensitizers such as amotosalen or psoralen and ultraviolet illumination (11,28) and cationic silicon phthalocyanines and red light illumination (28). In addition, a high photosensitivity was showed by transgenic Leishmania promastigotes after ultraviolet illumination previously treatment with aminolevulinic acid (20).
There are two types of mechanism that could be involved in the parasites inhibition after photodynamic therapy. The type I mechanisms due to the production of hydroxyl radicals and other active oxygen species that react with biomolecules in situ with subsequent cytotoxic results. The type II mechanism induced by the reactions between singlet oxygen with molecules involved in the maintenance of cell-wall/membrane structures such as phospholipids, peptides and sterols. Because it is known that Leishmania promastigotes are very susceptible to active oxygen species (29-31), it is possible to suggest that the low concentration of AlPc used in this study induces the release of activated oxygen species to inhibit the parasite. The mechanisms involved in Leishmania photodamage are under study.
The difference in drug sensitivity between the two strains pertaining to two species of Leishmania such as L. chagasi and L. panamensis was notable. Over fifteen species of Leishmania are known to cause disease in humans with a wide clinical spectrum from visceral to cutaneous manifestations. Each species of Leishmania has specific biochemical and molecular characteristics that provide the basis for the taxonomy of this genus. These differences are reflected in the variable sensitivity of Leishmania species to drugs, for example, pentavalent antimonials (32), the aminoglycoside antibiotic paromomycin (aminosidine) (33), several azoles (34), the pyrazolopyrimidines allopurinol and allopurinol riboside (35) and hexadecylphosphocholine (27). But why the strain of L. chagasi used was almost 50 times more sensitive than L. panamensis to photodynamic therapy it is not known. Almost all the literature about the in vitro photodynamic therapy activities is coming from the cancer models. In these models, the cell toxicity induced by phthalocyanines in vitro has been shown to be dependent on factors such as type of tumour cell, metal phthalocyanine and its derivatives, cell uptake, phthalocyanine incubation time, light intensity and/or phthalocyanine localization and distribution in the cells (16,36-40).
In this work two types of phthalocyanines were compared. Aluminium phthalocyanine treatment showed a higher phototoxic effect than ZnPc. This result could be attributed to the amphiphilic properties of AlPc that could facilitate cell uptake and intracellular localization, contrary to the hydrophobicity of ZnPc. The AlPc used in this study was soluble in culture medium, whereas ZnPc was less soluble. In general, phthalocyanines are prone to self-aggregation and dimers are reported to be inactive or much more inefficient than monomers as photosensitizers (17). In water, ZnPc displays a strong tendency to form aggregates as a result of the propensity of the large hydrophobic skeleton to avoid contact with the aqueous medium. On the other hand, it is known that the central metal ligand plays a crucial role in the photobiological activity influencing the excited triplet state yield and lifetime (16,17). Another important reason which could justify the difference in AlPc versus ZnPc activity against parasites was the type of illumination system used in this study. It could be possible to obtain a higher ZnPc activity using a wide range of wavelength for illumination.
In this paper, we have demonstrated that AlPc and ZnPc treatment after red light illumination at 670 nm effectively inhibits L. chagasi and L. panamensis promastigotes. In order to confirm the useful of photodynamic therapy in leishmaniasis, it is imperative to continue this study testing the phothoactivity of these compounds in axenic or intracellular amastigotes and further in animal models. However the results shown in this paper, using only the promastigote free form of the parasite, give us an idea about the effects of photodynamic therapy on Leishmania and open a new perspective for an alternative treatment against this insidious parasite.
Acknowledgements
The authors would like to thank Professor Raymond Bonnett, Emeritus Professor of Organic Chemistry at Queen Mary, University of London, for his scientific support and advice during the development of the project.
Conflict of interest
The authors declare that there are no conflicts of interest on the results published in this paper.
Financial support
This work was supported by the Instituto Colombiano para el Desarrollo de la Ciencia y la Tecnologia "Francisco Jose de Caldas" COLCIENCIAS (Grant 1102-04-14130; RC No 480- 2003), by the Universidad Industrial de Santander, Bucaramanga, Colombia and by the Program "Apoyo a los doctorados nacionales", from COLCIENCIAS.
Correspondence:
Patricia Escobar, CINTROP, Escuela de Medicina, Departamento de Ciencias Básicas, Universidad Industrial de Santander, Bucaramanga, Colombia.
Phone/fax: (00576) 563 971
References
1. Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 2004;27:305-18. [ Links ]
2. Croft SL, Coombs GH. Leishmaniasis-current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol 2003;19:502-8. [ Links ]
3. Ouellette M, Drummelsmith J, Papadopoulou B. Leishmaniasis: drugs in the clinic, resistance and new developments. Drug Resist Updat 2004;7:257-66. [ Links ]
4. Berman JD. Human leishmaniasis: clinical, diagnostic, and chemotherapeutic developments in the last 10 years. Clin Infect Dis 1997;24:684-703. [ Links ]
5. Croft SL, Karbwang J. Antiparasitic drugs: the current status. Curr Opinion Anti Infec Inves Drugs 2000;2:21-3. [ Links ]
6. Murray HW. Clinical and experimental advances in treatment of visceral leishmaniasis. Antimicrob Agents Chemother 2001;45:2185-97. [ Links ]
7. Bonnett R. Metal complexes for photodynamic therapy En: McCleverty JA, Meyer TJ, editors. Comprehensive inorganic chemistry II. Oxford: Elsevier Pergamon; 2004. p.945-1003. [ Links ]
8. Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M et al. Photodynamic therapy. J Natl Cancer Inst 1998;90:889-905. [ Links ]
9. Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) . J Antimicrob Chemother 1998;42:13-28. [ Links ]
10. Ben-Hur E, Barshtein G, Chen S, Yedgar S. Photodynamic treatment of red blood cell concentrates for virus inactivation enhances red blood cell aggregation: protection with antioxidants. Photochem Photobiol 1997;66:509-12. [ Links ]
11. van Voorhis WC, Barrett L K, Eastman RT, Alfonso R, Dupuis K. Trypanosoma cruzi inactivation in human platelet concentrates and plasma by a psoralen (amotosalen HCl) and long-wavelength UV. Antimicrob Agents Chemother 2003;47:475-9. [ Links ]
12. Zeina B, Greenman J, Corry D, Purcell WM. Antimicrobial photodynamic therapy: assessment of genotoxic effects on keratinocytes in vitro. Br J Dermatol 2003;148:229-32. [ Links ]
13. Moan J, Berg K, Bommer JC, Western A. Action spectra of phthalocyanines with respect to photosensitization of cells. Photochem Photobiol 1992;56:171-5. [ Links ]
14. Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumours. J Photochem Photobiol B 1997;39:1-18. [ Links ]
15. Nunes SM, Sguilla FS, Tudesco AC . Photophysical studies of zinc phthalocyanine and chloroaluminum phthalocyanine incorporated into liposomes in the presence of additives. Braz J Med Biol Res 2004;37:273-84. [ Links ]
16. Rosenthal I, Ben-Hur E. Role of oxygen in the phototoxicity of phthalocyanines. Int J Radiat Biol 1995;67:85-91. [ Links ]
17. Bonnett R, Martinez G. Photobleaching of sensitisers used in photodynamic therapy. Tetrahedron 2001;57:9513-47 [ Links ]
18. Foley MS, Beeby A, Parker AW, Bishop SM, Phillips D. Excited triplet state photophysics of the sulphonated aluminium phthalocyanines bound to human serum albumin. J Photochem Photobiol B 1997;38:10-7. [ Links ]
19. Oda K, Ogura S, Okura I. Preparation of a watersoluble fluorinated zinc phthalocyanine and its effect for photodynamic therapy. J Photochem Photobiol B 2000;59:20-5. [ Links ]
20. Sah JF, Ito H, Kolli BK, Peterson DA, Sassa S, Chang KP. Genetic rescue of Leishmania deficiency in porphyrin biosynthesis creates mutants suitable for analysis of cellular events in uroporphyria and for photodynamic therapy. J Biol Chem 2002;277:14902-9. [ Links ]
21. Gardlo K, Zuzana H, Claes DE, Rauch L, Megahed M, Ruzicka T et al. Treatment of cutaneous leishmaniasis by photodynamic therapy. J Am Acad Dermatol 2003;48:893-6. [ Links ]
22. Mauel J, Schnyder J, Baggiolini M. Intracellular parasite killing induced by electron carriers. II. Correlation between parasite killing and the induction of oxidative events in macrophages. Mol Biochem Parasitol 1984;13:97-110. [ Links ]
23. Croft SL, Evans AT, Neal RA. The activity of plumbagin and other electron carriers against Leishmania donovani and Leishmania mexicana amazonensis. Ann Trop Med Parasitol 1985;79:651-3. [ Links ]
24. Abok K, Cadenas E, Brunk U. An experimental model system for leishmaniasis. Effects of porphyrincompounds and menadione on Leishmania parasites engulfed by cultured macrophages. APMIS 1988;96:543-51. [ Links ]
25. Yardley V, Croft SL. Activity of liposomal amphotericin B against experimental cutaneous leishmaniasis. Antimicrob Agents Chemother 1997;41:752-6. [ Links ]
26. Sereno D, Lemesre JL. Axenically cultured amastigote forms as an in vitro model for investigation of antileishmanial agents. Antimicrob Agents Chemother 1997;41:972-6. [ Links ]
27. Escobar P, Matu S, Marques C, Croft SL. Sensitivities of Leishmania species to hexadecylphophocholine (miltefosine), ET-18-OCH3 (edelfosine) and amphotericin B. Acta Trop 2002;81:151-7. [ Links ]
28. Gottlieb P, Shen LG, Chimezie E, Bahng S, Kenney M E, Horowitz B et al. Inactivation of Trypanosoma cruzi trypomastigote forms in blood components by photodynamic treatment with phthalocyanines. Photochem Photobiol 1995;62:869-74. [ Links ]
29. Murray HW. Susceptibility of Leishmania to oxygen intermediates and killing by normal macrophages. J Exp Med 1981;153:1302-15. [ Links ]
30. Gantt KR, Goldman TL, McCormick ML, Miller MA, Jeronimo SM, Nascimento ET. Oxidative responses of human and murine macrophages during phagocytosis of Leishmania chagasi. J Immunol 2001;167:893-901. [ Links ]
31. Channon JY, Blackwell JM. A study of the sensitivity of Leishmania donovani promastigotes and amastigotes to hydrogen peroxide. I. Differences in sensitivity correlate with parasite-mediated removal of hydrogen peroxide. Parasitology 1985;91:197-206. [ Links ]
32. Grogl M, Thomason TN, Franke ED. Drug resistance in leishmaniasis: its implication in systemic chemotherapy of cutaneous and mucocutaneous disease. Am J Trop Med Hyg 1992;47:117-26. [ Links ]
33. Neal RA, Allen S, McCoy N, Olliaro P, Croft SL. The sensitivity of Leishmania species to aminosidine. J Antimicrob Chemother 1995;35:577-84. [ Links ]
34. Rangel H, Dagger F, Hernandez A, Liendo A, Urbina JA. Naturally azole-resistant Leishmania braziliensis promastigotes are rendered susceptible in the presence of terbinafine: comparative study with azole-susceptible Leishmania mexicana promastigotes. Antimicrob Agents Chemother 1996;40:2785-91. [ Links ]
35. Avila JL, Casanova MA. Comparative effects of 4- aminopyrazolopyrimidine, its 2'-deoxyriboside derivative, and allopurinol on in vitro growth of American Leishmania species. Antimicrob Agents Chemother 1982;22:380-5. [ Links ]
36. Paquette B, Ali H, Langlois R, van Lier JE. Biological activities of phthalocyanines-VIII. Cellular distribution in V-79 Chinese hamster cells and phototoxicity of selectively sulfonated aluminum phthalocyanines. Photochem Photobiol 1988;47:215-20. [ Links ]
37. Margaron P, Madarnas P, Quellet R, van Lier JE. Biological activities of phthalocyanines. XVII histopathologic evidence for different mechanisms of EMT-6 tumor necrosis induced by photodynamic therapy with disulfonated aluminum phthalocyanine or photofrin. Anticancer Res 1996;16:613-20. [ Links ]
38. Gomes ER, Cruz T, Lopes CF, Carvalho AP, Duarte CB. Photosensitization of lymphoblastoid cells with phthalocyanines at different saturating incubation times. Cell Biol Toxicol 1999;15:249-60. [ Links ]
39. Gijsens A, Derycke A, Missiaen L, De Vos D, Huwyler J, Eberle A et al. Targeting of the photocytotoxic compound AlPcS4 to Hela cells by transferrin conjugated PEG-liposomes. Int J Cancer 2002;101:78-85. [ Links ]
40. Choi CF, Tsang PT, Huang JD, Chan EY, Ko WH, Fong WP et al. Synthesis and in vitro photodynamic activity of new hexadeca-carboxy phthalocyanines. Chem Commun (Camb) 2004;19:2236-7. [ Links ]














