Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biomédica
Print version ISSN 0120-4157On-line version ISSN 2590-7379
Biomédica vol.26 suppl.1 Bogotá Oct. 2006
Leishmania panamensis
transmission in the domestic environment: the results of a prospective epidemiological survey in Santander, ColombiaGerardo Muñoz 1, Clive R. Davies 2
1 Departamento de Ciencias Básicas, Facultad de Salud, Universidad Industrial de Santander, Bucaramanga, Santander, Colombia.
2
Department of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, London, United Kingdom.Recibido: 10/08/05; aceptado: 24/02/06
Introduction.
Domestic transmission now appears to be the principal route of Leishmania panamensis infection in deforested regions characterized by the replacement of primary forest by permanent plantations, i,e coffee or cacao crops. This paper presents the results of the disease patterns in a representative population of the Opón focus, in Santander, Colombia.Objective. The principal aims were: 1) to measure the incidence rate in a representative population of the Opón focus; 2) to identify demographic risk factors for infection; 3) to estimate the proportion of infections which cause disease; 4) to estimate the protection against disease from acquired immunity; 5) to estimate the frequency of reactivations, and 6) to estimate the risk of mucosal leishmaniasis.
Material and methods. A 19 month prospective survey of leishmaniasis caused by Leishmania panamensis was carried out amongst 1380 people in a cacao growing region of Santander Department, Colombia. The population was diagnosed clinically and by the Montenegro skin test (at two time points).
Results: The incidence rate was 0.19 infections/person-year, with 31% of infections apparently subclinical. The risk of acquiring cutaneous leishmaniasis decreased with age even in the absence of apparent previous infections. Protective immunity followed both clinical and subclinical infections, persisting for at least 10 years after a primary lesion. Mucocutaneous leishmaniasis was detected in 12% of the population with cutaneous lesions, of which 77% had mild symptoms, and 23% perforated nasal septa. The risk of mucosal leishmaniasis was greatest for males, and for people whose primary cutaneous lesion was on the head.
Conclusion. The average age of infection in Opón, 7.7 years (1/l), and the absence of gender as a risk factor is highly indicative of intradomiciliary or peridomiciliary transmission.
Key words: Leishmania, epidemiology, cutaneous leishmaniasis, mucocutaneous leishmaniasis.
Transmisión de Leishmania panamensis en ambientes domésticos: resultados de un estudio epidemiológico prospectivo en Santander, Colombia
Introducción. La transmisión doméstica de Leishmania panamensis parece ser la fuente de infección mas frecuente en regiones deforestadas, caracterizadas por el reemplazo del bosque primario por plantaciones permanentes como cacao y café. Este papel presenta los resultados de los patrones de enfermedad en una población representativa del foco del Opón, en Santander, Colombia.
Objetivo. Los objetivos principales fueron: 1) cuantificar la tasa de incidencia en una población representativa de la población del foco del Opón; 2) identificar los factores de riesgo demográficos para la infección; 3) estimar la proporción de infecciones que causan enfermedad; 4) estimar la protección contra la enfermedad según la inmunidad adquirida; 5) estimar la frecuencia de reactivaciones, y 6) estimar el riesgo de leishmaniasis mucosa.
Materiales y métodos: Se llevó a cabo un estudio prospectivo de leishmaniasis causada por Leishmania panamensis durante 19 meses, entre 1.380 personas habitantes de una región cacaotera del departamento de Santander, Colombia. La población fue diagnosticada por clínica y por la prueba de Montenegro (en dos tiempos).
Resultados: La tasa de incidencia fue de 0,19 infecciones/persona-año, 31% de los cuales tuvieron una infección aparentemente subclínica. El riesgo de adquirir leishmaniasis cutánea decrece con la edad aún en ausencia de infecciones previas aparentes. Una inmunidad protectiva subsiguió a las infecciones clínicas y subclínicas, persistiendo por lo menos durante 10 años posterior a una infección primaria. La leishmaniasis mucocutánea se detectó en 12% de la población con lesiones cutáneas, de las cuales 77% tuvieron síntomas no severos, y 23% perforación del tabique nasal. El riesgo de leishmaniasis mucosa fue más grande para hombres y para personas cuyas lesiones primarias se localizaron en la cabeza.
Conclusión. El promedio de edad de infección en el Opón, 7,7 años (1/l), y la ausencia de factores de riesgo relacionados con el género indican que la transmisión es intra o peri domiciliaria.
Palabras clave: Leishmania, epidemiología, leishmaniasis cutánea, leishmaniasis mucocutánea.
Leishmania (Viannia) panamensis is responsible for zoonotic cutaneous leishmaniasis and occasionally mucocutaneous leishmaniasis in eight countries in Central and South America (1,2). The total number of human cases caused by L. panamensis annually is unknown, as i) most cutaneous leishmaniasis cases are not reported (3), ii) L. panamensis is often sympatric with other Leishmania species responsible for cutaneous leishmaniasis (e.g. Leishmania braziliensis), and iii) the Leishmania species responsible for the reported cases are rarely identified.
However, on the basis of those few human isolates which have been characterized, it appears that about half of all cutaneous leishmaniasis cases reported each year within the eight endemic countries are due to L. panamensis (2) . This percentage varies from less than 1% in Guatemala to over 90% in Panama and Costa Rica. In Colombia, about 50% of all cutaneous leishmaniasis cases reported are due to L. panamensis; but this represents the greatest number of reported cases caused by L. panamensis in any country (an estimated 3,000/ year) (2).
The geographic distribution of L. panamensis coincides with the overlapping distribution of the twotoed sloth Choloepus hoffmanni thought to be the principal reservoir host, and the sandfly species Lutzomyia trapidoi and Lutzomyia ylephiletor, thought to be the principal vectors (4). Hence, the classical risk factors for human infection, as described in Panama (5) and on the Pacific coast of Colombia (6) involve occupational exposure to infectious sandfly bites when adult male humans enter the forest.
Indeed, the requirement of forest habitat for the survival of sloth populations led Herrer and Christensen to write optimistically in 1976 that the "increased deforestation activities by new immigrants to provide more farm land may soon disrupt the transmission cycle of Leishmania which will lead to the disappearance of the disease." In fact, the history of the last 25 years is that while the deforestation of endemic areas has continued unabated, the incidence rate of L. panamensis has increased steadily. How has this been possible?
Part of the answer lies in the changing patterns of L. panamensis transmission. Persistently endemic communities, characterised by a high prevalence of active lesions in children and apparent domestic transmission, were occasionally detected in Panama in the 1970s, e.g. in El Aguacate (7).
However, this epidemiological pattern has only recently become widespread, with all household members now apparently at risk of infection in many endemic zones of L. panamensis. For example, domestic transmission now appears to be the principal route of L. panamensis infection in the provinces of Esmeraldas and Pichincha on the Pacific coast of Ecuador (8), a deforested region characterized by the replacement of primary forest by coffee plantations, and by relatively high densities of Lutzomyia trapidoi and Lutzomyia gomezi in and around houses. Domestic transmission of L. panamensis is also suspected in Acosta, Costa Rica, where the presumed sandfly vectors, L. ylephiletor and L. gomezi, are abundant in the coffee plantations that have replaced the primary forest (9,10).
Because the course of cutaneous leishmaniasis infection depends on both the age and immune status of patients (11,12) the change in L. panamensis transmission from sylvatic to domestic could also affect the characteristic clinical symptoms of an exposed population.
This paper describes the results of the first prospective survey to investigate the course of infection in a population apparently exposed to L. panamensis in the domestic environment (Angulo and others, unpublished data): the inhabitants of the Opón focus in Colombia. The principal aims were (1) to measure the incidence rate in a representative population of the Opón focus, (2) to identify demographic risk factors for infection, (3) to estimate the proportion of infections which cause disease, (4) to estimate the protection against disease from acquired immunity, (5) to estimate the frequency of reactivations, and (6) to estimate the risk of mucosal leishmaniasis.
Materials and methods
Study site
The study was carried out in 12 villages in the Opón area, Landázuri municipality (6° 20' N; 73°, 43' W), Santander Department at altitudes ranging from 400 to 1,200 m above sea level. It is a mountainous region where the principal economic activities are silviculture of tropical hard woods (in the rain forest), cattle ranching, and the cacao crops. The relative abundance (in percentage) of the vegetation around houses was estimated by eye within concentric circles with radiuses from the house of 50 m, 100 m, 200 m, 300 m, and 800 m, respectively. Most houses adjoin pasture land (ca. 80%) and/or cacao plantations (ca. 40%), and are some distance away from the surviving patches of primary or secondary forest. About 35% and 85% of houses are within 800m from primary and secondary forest, respectively.
Study design and population
During the first visit in January, 1995, a scaled map for each village was completed and all 527 occupied houses recorded. A unique identification number was allocated to each person in every household and the Colombian national identity card was requested in order to verify the date of birth.
If possible, the whole family attended the interviews in order to minimise recall bias. Demographic data (age, sex, and date of immigration, when appropriate) and clinical status (including age when infected) were recorded, and a proportion of the population (those giving consent) were given a Montenegro skin test (April- June, 1995).
All households were visited five times at about three month intervals following the initial crosssectional survey, checking for changes in the study population (due to immigration, emigration, birth or death) and looking for new cutaneous or mucocutaneous leishmaniasis cases.
During the final visit (February-March, 1997) the study population was given a second Montenegro skin test an average of 19 months after the first one using the same batch of antigen as before. In 1995 the Montenegro skin test was applied to 51% of the Opón population (1,380/2,704), of which 55.5% (766) received a second one.
There were no differences by gender between the group remaining in the project and the emigrants/ refusal group; nevertheless, the former were slightly older (mean=20.8 years old) than the latter (mean=18.2 years old) (Kruskal-Wallis H: 7.303, P=0.006).
The great majority of refusals were adults with a positive result in the first Montehnegro skin test. Thus, the main possibility of bias due to the refusal would apply to the measurement of recovery rate (r) rather than the measurement of transmission rate. During the prospective study, 163 emigrants, 10 deaths, 126 immigrants and 37 births were recorded in the study population. Analysis incorporating age were carried out on a reduced data set of 1,333 persons, because of missing data from 47 people.
Clinical diagnosis
Past cases of cutaneous leishmaniasis were identified by characteristic scars, which were detected using the following criteria: no history of trauma, duration for more than 2 weeks, central depressed surface and contours with no sharp angles. Suspected leishmaniasis lesions were defined by the absence of prior history of trauma and more than two weeks of evolution. Confirmation of the aetiology of suspected lesions was made by a positive result from one or more diagnostic tests (13), with the informed written consent of the patients. These included: 1) microscopic examination of Giemsastained slides made from dermal scrapings; 2) PCR using the B1 and B2 primers as described by de Bruijn (1992) (14), and 3) the in vitro culture of parasites from aspirates or biopsies in NNN biphasic medium. The sensitivity of microscopy diagnosis was 40% for all suspected patients and 60% for the 55 patients with a positive culture, while the equivalent values for PCR were 43% and 87%.
All confirmed patients were provided with free treatment (Glucantime®) and followed-up on a monthly basis. Characterisation of Leishmania isolates was carried out by IEA in thin-layer starch-gel electrophoresis, using 8 isoenzymes (PEPD, NH, MPI, ME, GPI, PGM, 6PGD, and ES) as described by Godfrey and Kilgour (1976) (15). All 25 parasites isolated during the survey were characterised as L. panamensis; 23 were indistinguishable from the reference strain MHOM/PA/71/LS94, and two isolates demonstrated the same variant of PEPD .
After clinical inspection and following the results of the diagnostic tests, the study population was classified into two groups: people with scar or lesions (L+) and people with no history of leishmaniasis (L-). The upper respiratory tract was visualised with the help of a nasal speculum, lantern and a tongue depressor by a trained clinician (16) on the L+ population. Lesions compatible with mucosal leishmaniasis were defined as septal erosion or perforation; erythema or ulcers in the septum, turbinate, oral or nasal mucosa. In order to exclude acute lesions the clinician visited the same population three times in three consecutive months. Persistent lesions were included as suspected a mucocutaneous leishmaniasis patient but biopsy was not taken because there was not hospital aid in such remote areas.
Montenegro skin tests
The Montenegro skin test was applied (with their informed written consent) to some or all of the residents of 331 households following the technique recommended by the World Health Organization (17), i.e. using a mixture of L. panamensis and L. braziliensis at a concentration of 5x106 ml-1 heat-killed promastigotes for each species. A pressurised intradermal injector (Dermo-jet model G, Robbins Instruments, USA) was used to inject 0.1 ml of leishmanin intradermally on the external surface of one arm, and the diameter of induration was measured 48 hours later. The choice of cut-off point, 3 mm, was made empirically, and the study population with a Montenegro skin test was classified into two groups according to their skin test response: Montenegro negative (M-) and Montenegro positive (M+).
Ethical aspects
Written informed consent was obtained from every person or family included in the present study, following the indications of the Ministerio de Salud de Colombia (Resolution 008430, 1993). In addition, approval was requested and obtained from the ethical committee of the Industrial University of Santander
Analysis
The transmission rate was measured using the results of both the cross-sectional and prospective studies. The analysis of the cross-sectional data focused on the comparison of prevalence rates (active or cumulative; Montenegro skin test responsiveness or clinical) according to sex and age. In addition, a simple infection-recovery model (11) was fitted to the age prevalence curves (i.e. the proportion that were infected at age "a") by maximum likelihood (18). The model assumes that susceptibles became infected at a constant rate "l" (the force of infection), and infected recovered and returned to the susceptible class at a constant rate "r" (the recovery rate). It follows that the proportion infected at age "a" can be predicted from the equation:
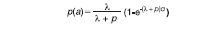
The assumptions of these models were then tested by analysing the results of the prospective survey, which permitted direct estimates of incidence (in relation to age or gender) and recovery rate during the 19 month follow-up period.
Explanatory variables for variation in infection rate or in the clinical consequences of infection were mostly sought using general linearised models in GLIM ( v. 4.07) (19). When the outcome data were counts (e.g. the number of scars), Poisson errors were specified; and when the outcome data were proportions (e.g. prevalence) or binary (e.g. healthy versus with disease) these were treated as binomial variables in logistic regression. When no suitable error structure could be identified, a nonparametric method was adopted.
For multivariate analyses model simplification to the minimal adequate model was achieved by backward stepwise elimination. The absolute or relative change in residual deviance caused by the removal of each explanatory term was examined for significance ( P<0.05). Interaction terms were assessed and removed in order of diminishing complexity, i.e. before the main effect terms. This procedure was repeated until the minimal adequate model retained only significant explanatory variables. For minimal adequate models with binomial or Poisson error structures, changes in residual deviance were compared to
c2 distribution. The F-ratio statistic was employed to test models with a normal error distribution. Finally, the fit of each model was checked by inspecting a plot of the residuals against the fitted values.Results
Population infection rate
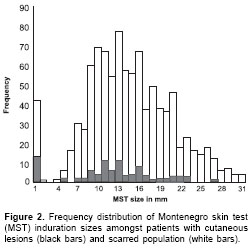
The distribution of induration sizes suggest a bimodal- like shape (
figure 1, figure 2) indicating a differential response between uninfected and infected people. In order to define a cut-off point for M+, the distribution of Montenegro skin test induration sizes was compared between L+ and L- people. It is clear from these data that there is no empirical reason for choosing the textbook cut-off point of 5 mm, as the majority of the 10 people with induration sizes between 1-4 mm also had scars. In contrast, clinical cases were a small minority amongst those with a zero skin test response. Given the few data, the choice of cut-off point between 1-4 mm must be somewhat arbitrary. In the analysis described here, we used a relatively conservative cut-off point of 3 mm, i.e., induration sizes of 3 mm or greater are treated as a positive skin test response or "M+".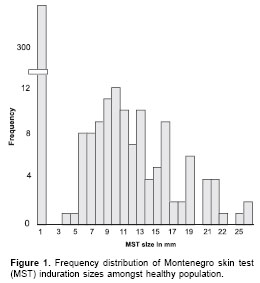
The cumulative Montenegro skin test prevalence of infection amongst the whole study population in 1995 was 0.75 (1,044/1,380) (
table 1). The induration sizes of the skin test response according to clinical status was as follows: 1) people with active cutaneous lesions (no mucosal), n=80; geometric mean (gm)=8.0 mm (95% Confidence Intervals [CI] 7.8-8.2 mm); 2) cutaneous scarred population (no mucosal), n=782; gm=12.2 mm (95%CI 12.1-12.2 mm); 3) people with mucocutaneous lesions, n=108 (concurrent active cutaneous lesions, 8; concurrent cutaneous scars, 100); gm=15.6 mm (95%CI 14.2-17 mm), and 4) "healthy" people, n=410; gm=1.0 mm (95%CI 0.9- 1.1 mm).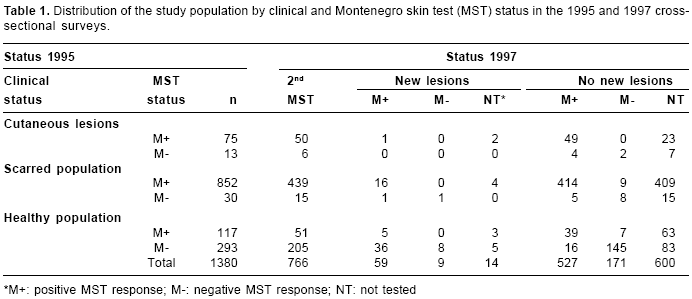
In order to determine the average incidence rate in recent years, the force of infection (
l) was calculated by fitting a simple infection-recovery model to the cross-sectional age prevalence data for M+ (18), making the assumption of constant transmission and recovery rate with time and age. The model estimates a mean recovery rate of 0.01/ year, and l to be 0.14 (95%CI 0.12-0.15) cases/ person-years (figure 3), which is significantly smaller than the Montenegro skin test conversion rate detected during the prospective survey. The mean number of person-months at risk between the first and second skin test date was 19 months.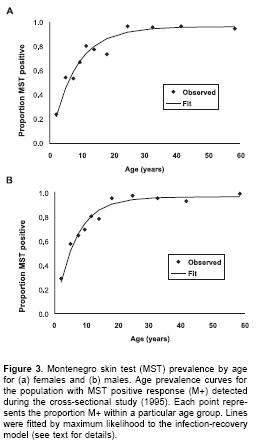
Amongst the 226 M- people who were re-tested, 62 converted to M+, resulting in an incidence of 0.21 (95%CI 0.17-0.24) conversions/person-year (Only 5 people in the second skin test presented size of 3 or 4mm). Evidence of past infections were detected in 10 people (6 scars, 4 lesions) who converted from M-1995 to M+1997 (
table 1), suggesting that these conversions reflect infections already prevalent in 1995 but with an extended incubation period prior to skin test conversion, rather than incident infections during the prospective study. Thus, our most reliable assessment of incidence rate is 0.19 (95%CI 0.15-0.23) conversions/ person-year which is calculated from the 52/205 M-L-1995 who converted during the 19 month prospective study.During the prospective survey, loss of positive skin test responsiveness was detected in 16 people at a rate, r, of 0.02 (95%CI 0.01-0.03)/ person-year. Recovery rate was significantly greater in people without clinical symptoms, 0.13 (7/51), than amongst L+ people
, 0.02 (9/439) (c2=16.2, degrees of freedom [df]=1, P<0.0001).The assumption of constant transmission rate with time was validated by a cross-sectional survey carried out on the same population in 2004 (n=1,127) , i.e. seven years after the first survey (1997). The force of infection (
l) calculated by fitting a simple infection-recovery model to the cross-sectional age prevalence data in 2004 was 0.12 (95%CI 0.11-0.14), i.e. remarkably close to the previous estimate.Risk factors for infection
The cumulative prevalence of infection for males, 0.77 (535/692)/person was not significantly greater than that for females, 0.73 (509/688)/person:
c2=2.08, df=1, P=0.15. In the follow-up study, incidence of infection in males, 0.24 (26/109 M-1995 re-tested)/person-year, was also not significantly different from the incidence of infection in females, 0.30 (36/117)/person-year (c2=1.35, df=1, P=0.24). Similarly, l estimated from the age MST prevalence curves for males, 0.14 (95%CI 0.11-0.16)/ person-year was not significantly different from l calculated for females, 0.12 (95%CI 0.10-0.13)/ person-year (figure 3). Thus, there is no evidence of any difference in the risk of infection between males and females.Because of the relatively high transmission rate in the Opón focus, the majority of M-1995 (224/ 336) are concentrated amongst children less than 10 years old. This explains the relatively low mean age of 8.6 (95%CI 6.7-10.5) years amongst the 62 people who converted during the study. However, there was no significant difference between the mean age of the people who remained M- in 1997, 10.2 (95%CI 8.4-11.9) years, compared to the mean age of "converters" (t test on log transformed data t=1.55, df=1, P>0.05). The absence of any relationship between age and risk of infection is illustrated in
figure 4, which demonstrates that there is no trend between the incidence rates calculated for eight age groups (with equal denominators) (F=0.05, df=1, P>0.05). Thus, new infections occurred irrespective of age, validating the key assumption made in the infection- recovery model to calculate l from age prevalence data. 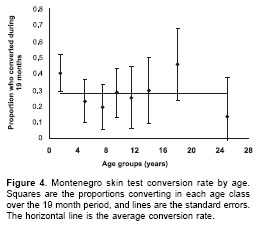
The most reliable estimate of the proportion of infections leading to clinical disease comes from the prospective data. The proportion of skin test conversions which lead to disease (a) was 0.69 (95%CI 0.56-0.82) [36/52]. The possibility that some of the "subclinical" skin test conversions were cross-reactions cannot be discounted. However, no significant difference was detected in the induration sizes of the skin test response following "subclinical" infections (6.7 mm; 95%CI 5.3- 8.6 mm) compared to the response following clinical incident infections (6.5 mm; 95%CI 5.8-7.2 mm) (t-test on log transformed data: t=0.29; df=51; P=0.76).
The significance of age and gender was tested by comparing the two subpopulations of people with incident subclinical (SC) or clinical (C) infections. The geometric mean age for C, 3.8 years (95%CI 2.8-5.1 years), was significantly smaller than for SC 8.7 years (95%CI 6.6-11.3 years) (t-test on log transformed data: t=3.3; df=51; p=0.0021). The sex ratio amongst SC cases, 43.7% male (7/16), was not significantly different from that amongst C cases, 47.2% male (17/36) (
c2=0.05, df=1, P>0.05).Acquired immunity against disease
During the follow up, 82 cutaneous leishmaniasis patients were clinically and laboratory diagnosed (
table 1). In order to determine the role of acquired immunity for protecting against new episodes of clinical leishmaniasis, risk factors for cutaneous leishmaniasis were sought by testing for significant associations with the presence of cutaneous leishmaniasis (as a binary response) amongst the population of 1,333. The explanatory variables tested in the multivariate model were village, age, gender, Montenegro skin test induration size, and previous lesion (intercept estimate -1.439). Significant risk factors were age (c2=14.5, P<0.001; estimate -0.05635), skin test size (c2=4.2, P<0.05; estimate -0.246), and previous lesions (c2=10.7, P<0.01; estimate -2.019), and there was a significant interaction effect between MST size and clinical status (c2=4.2, P<0.05).The main conclusions are as follows: 1) irrespective of Montenegro skin test and clinical status, the odds of a new lesion decreased significantly with age by 5.4% per year; 2) amongst both the healthy and scarred population, the odds of a new lesion decreased with skin test size; this effect was significantly greater amongst the healthy population, for which the odds of a new lesion decreased by 22% per 1 mm increase in skin test size; this compares to an equivalent reduction of 9% for the scarred population; 3) irrespective of age and Montenegro skin test status, the odds of a new lesion was significantly less for the scarred than for the healthy population; but this effect was less marked for people with a greater skin test size. For example, for a person with no skin test response (0 mm), the odds of a new lesion decreased by 88%, whereas for people with skin test responses of 10 mm the odds decreased by only 43% .
Recurrent leishmaniasis
Full clinical histories were recorded for 125 of the 170 cutaneous leishmaniasis patients in the study (i.e. the 88 patients detected in the first crosssectional survey, plus the 82 new cases detected during the prospective survey) . Previous leishmaniasis scars were detected in 80 (64%) of these patients. Incidence decreased from 0.12 to 0.016 cases/person-years in the first 10 years after the primary lesion (
figure 5), indicating that these early secondary infections were relapses rather than reinfections.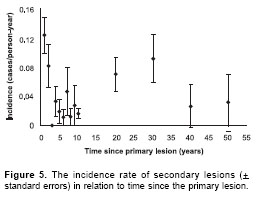
Mucocutaneous leishmaniasis
Clinical inspections for mucocutaneous leishmaniasis were carried out on 881 persons with current or past cutaneous leishmaniasis symptoms, of which 12% (108) presented lesions clinically compatible with mucocutaneous leishmaniasis. The majority (77%) of the mucocutaneous leishmaniasis symptoms were mild, i.e. with erythema, ulcers or erosion detected on single mucosa, usually the nasal septum but, also, the nasal mucosa (n=18), oral mucosa (n=2) and turbinate (n=2). The remaining 23% (n=25) had moderate symptoms (perforated nasal septa) (
table 2).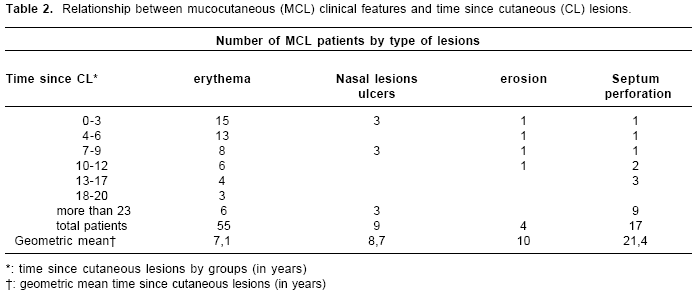
There was some evidence that severity increased with time since cutaneous leishmaniasis episode, as the geometric mean time for mild mucocutaneous leishmaniasis was 7.5 years (95%CI: 5.3- 9.8 years) compared to 21.4 years (95%CI: 19- 23.7 years) for moderate cases (t=4.11, df=1, P<0.001). Risk factors for mucocutaneous leishmaniasis were sought by testing for significant associations with the presence of mucocutaneous leishmaniasis (as a binary response) amongst the 881 cutaneous leishmaniasiscases observed.
The explanatory variables tested in the multivariate model were: 1) whether the cutaneous leishmaniasis lesions were active or healed, and the time since the cutaneous leishmaniasis episode; 2) the area, number and localisation of the cutaneous leishmaniasis lesions; 3) the Montenegro skin test induration size; 4) whether the cutaneous leishmaniasis lesion was treated with antimonials; and 5) age and gender. The significant risk factors for mucocutaneous leishmaniasis were gender ( P<0.0001; odds ratio [OR] for males=2.2 [95%CI 1.4-3.4]), location of cutaneous leishmaniasis lesion on the head ( P=0.002; OR=2.0 [95%CI 1.3-3.0]), Montenegro skin test size ( P=0.001; OR for unit increase in log transformed Montenegro skin test induration in mm=2.4 [95%CI 1.4-3.9]) and previous treatment ( P<0.001; OR=2.3 [95%CI 1.5-3.7]).
Discussion
Personal risk factors for infection are most accurately estimated from prospective studies with two assessments of Montenegro skin test status in the population. Correlates with infection rate can then be identified either by cohort studies (as in this study) or by nested case-control studies (Llanos-Cuentas, unpublished data). In contrast, retrospective studies of infection, whether by casecontrol studies (20) or cohort studies, are plagued by potential bias and other inaccuracies associated with faulty recall or changes in circumstance. Thus, here we focus on the comparison of our results with those from the only two previously published large scale prospective Montenegro skin test surveys of New World cutaneous leishmaniasis: in Tumaco, Colombia (6) and in the Peruvian Andes (11).
New infections occurred irrespective of age and gender in Opón and in Perú, whereas in Tumaco incidence of infection increased with age, and infection was three times greater in males than in females. These differences are presumably the result of differences in exposure rather than differences in susceptibility. The average age of infection in Opón, 7.7 years (1/l), and the absence of gender as a risk factor is highly indicative of intradomiciliary or peridomiciliary transmission, as in the Peruvian Andes. In Tumaco, infection mainly occurs during farming activity, an occupation in Colombia carried out principally by males. The epidemiological contrast with Tumaco is striking, given the similarities between Opón and Tumaco both in the agent of disease ( L. panamensis) and in the presumed principal vectors ( Lu. trapidoi, and Lu. gomezi).
Clinical infection is determined by the host-parasite interaction which includes host genetic factors, acquired resistance to infections, and parasite pathogenicity. Our estimates of pathogenicity from the cross-sectional and prospective surveys are surprisingly different. The explanation appears to lie in the significant difference between the recovery rate of Montenegro skin test responsiveness following subclinical or clinical infections. Subclinically infected people apparently revert to M- relatively quickly, so that despite a relatively high transmission rate, only a small proportion of people sampled in the cross-sectional survey retain their M+L- status. Hence, our best estimate for the proportion of subclinical infections in the Opon focus was 31%. This is higher than the rate reported for L. peruviana in Peru (17%) but lower than that reported in Tumaco (88%) (6,11).
This result is consistent with the hypothesis that L. panamensis in Opón is more pathogenic than in Tumaco, and that L. peruviana is more pathogenic than both L. panamensis populations studied in Colombia. Alternatively, the differences may represent methodological inconsistencies between the three research groups, i.e. different sensitivities of the clinical or Montenegro skin test diagnosis (due to different field workers or different leishmanin batches), including the hypothetical possibility of a booster effect from serial skin testing. In addition, geographical differences could be explained by different levels of cross-reacting parasitic infections in the three regions such as lizard Leishmania (21).
The proportion of subclinical infections may also be influenced by factors related to human susceptibility. In Tumaco the majority of the community are negros who are infected when adults, whilst in Opón the people are whites and "mulatos" (i.e. a mixture of indigenous, Spaniards and negros) infected in childhood. Evidence that people who developed chronic disease in Tumaco were innately more susceptible than those who were subclinically infected came from an experimental study, where the in vitro infection rate of macrophages differentiated from peripheral blood monocytes with L. panamensis was higher in cutaneous leishmaniasis patients (from Tumaco) than in asymptomatically infected persons (22). Further evidence for genetic variation in susceptibility comes from a comparison of leishmaniasis symptoms in Amerindians and mixed race people in Bolivia (23).
In the Opón focus, the geometric mean age for clinical infections (3.8 years) was significantly smaller than that for subclinical infections (8.7 years); but there was no significant gender association with clinical symptoms. A positive relationship between age and the proportion of subclinical infections was also observed in Perú where the geometric mean age for clinical infection was significantly lower than those with subclinical infections (7.7 and 15.0 years, respectively) and in Tumaco too the odds that a skin test conversion was associated with a lesion decreased significantly with age: gender adjusted OR=0.2 for adults (>30 year old) as compared with children (<10 years old). This consistent pattern of a decreasing incidence of clinical infection with age could be related to undetected acquired immunity following infection (12). Alternatively, adults may be innately less susceptible to cutaneous leishmaniasis (23) as they are to visceral leishmaniasis (24), even in the absence of prior exposure to leishmaniasis.
A novel hypothesis to explain the reduced susceptibility to cutaneous leishmaniasis with age comes from the recent findings that pre-exposure to sandfly saliva reduces the clinical outcome of L. major infection in experimental mice (25). Antibodies to salivary gland antigens are thought to cancel the enhancing immunomodulatory effects from subsequent co-inoculation of saliva with the Leishmania parasites (26). As adults living in endemic areas have had a longer exposure to sandfly bites than children, their presumed higher levels of antibodies to sandfly antigen could feasibly reduce their risk of clinical symptoms. This speculative hypothesis is consistent with the finding of a three-fold higher risk of clinical disease for migrants compared to native inhabitants in Alto Beni, Bolivia (23).
In Opón, people with previous clinical episodes (i.e. scars) on average had 80% protection against subsequent clinical infections, although this effect was more marked amongst people who had a negative skin test response. Skin test responsiveness was also a significant indicator of acquired protection, even amongst those with no clinical symptoms. Within the healthy population, the odds of a subsequent clinical infection decreased by 22% for each 1 mm increase in skin test size in Opón. This corresponds with an equivalent measurement of 18% in a Peruvian study of acquired immunity following subclinical infections. Hence, the Opón results confirm the conclusions from the Peruvian study that protective immunity can be acquired following a Leishmania infection even in the absence of clinical symptoms. This result provides hope for the future development of vaccines. In addition, it provides a rationale for using the Montenegro skin test response as an indirect indicator of the protection which may follow a putative vaccine within the context of an intervention trial.
The recurrence rate of leishmaniasis in Opón (3%/year) in the prospective study, was similar to recurrence rates previously detected in Colombia ( L. panamensis), Perú ( L. peruviana) and Brazil ( L. braziliensis): 2.0%/year (6), 2.9%/year (11), and 2.7%/year (16) respectively. The consistency of these data is remarkable given the differences in the treatment regimes, parasite species, and even in the definition of recurrence used by the different research teams. On the Pacific coast of Colombia, where L. panamensis was the most common parasite isolated (27), the group of recurrent patients (80/498) required higher doses of Glucantime® and showed a lower skin test response compared with non-recurrent patients, suggesting that the risk of reactivation is associated with a low-cell mediated immune response.
In Opón, as in the Peruvian Andes, the incidence rate of recurrence decreased dramatically during the first 10 years following the primary lesion, indicating that the majority of recurrent leishmaniasis in this period is due mainly to relapses rather than reinfections. On the Colombian Pacific coast the cumulative frequency of recurrences in a group of 498 patients also increased rapidly in the first year after the first lesion, with few recurrences detected during the following 42 months of the follow up (12,27). From this group of recurrent cases, 50% were suggested to be caused by relapses based on the apparent genetic identity of parasites isolated from the same patient in both leishmaniasis episodes (primary and secondary infections) (27). In all patients with identical parasites in consecutive episodes, the secondary lesions were located on the same part of the body on the primary lesions. In the Opón focus, 36% of recurrent lesions were located on the same part of the body as the previous scars, and this percentage decreased significantly with time since primary lesion, indicating that the early recurrences are more likely to be relapses and the later recurrences are more likely to be reinfections (presumably due to the loss of acquired immunity).
Prior to the Opón study there were remarkably few descriptions of the frequency and severity of mucosal leishmaniasis following L. panamensis infection. Most reports come from hospital/health post admissions, which are notoriously biased, or fail to take out account of the potential confounding effect of time since the primary cutaneous leishmaniasis episode. Nevertheless, the inclusion of suspected mucocutaneous leishmaniasis patients based only on clinical diagnosis may have selection bias and the results have to be taken with precaution. Active search of rural populations are likely to encounter milder mucocutaneous leishmaniasis symptoms than are generally seen in mucocutaneous leishmaniasis admissions, but may include more severe mucocutaneous leishmaniasis symptoms than are detected amongst cutaneous leishmaniasis admissions (as the ratio of moderate: mild mucocutaneous leishmaniasis symptoms increases significantly with time since the cutaneous leishmaniasis episode). With these caveats, it seems that mucocutaneous leishmaniasis due to L. panamensis in Colombia lesions may be more severe than in Panamá: 23% (25/108) of mucocutaneous leishmaniasis cases in Opón and 17% (4/23) on the Colombian Pacific Coast (28) had perforated nasal septa, compared to 9% (3/33) in Panama (29). The cumulative prevalence of mucocutaneous leishmaniasis amongst cutaneous leishmaniasis patients was also greater in Opón, 12%, than on the Colombian Pacific Coast, 9% (10/114) (30).
Geographic variability in symptoms could be due to genetic variability in parasite pathogenicity (31), human susceptibility (32), or sand fly saliva immunomodulatory activity (33). Alternative explanations could involve variability in availability of treatment. Prior to 1995 there were no medical facilities available in Opón. While this could explain the high frequency of chronic mucocutaneous leishmaniasis lesions, we found no evidence that treatment of cutaneous leishmaniasis lesions reduced the risk of mucocutaneous leishmaniasis. Our data indicated the reverse association, presumably because the odds of treatment was confounded by the severity of the cutaneous leishmaniasis episode (a known correlate of mucocutaneous leishmaniasis risk following L. braziliensis infection) (30). Previous studies of mucocutaneous leishmaniasis due to L. braziliensis suggest that risk of mucocutaneous leishmaniasis may decrease with age of primary cutaneous leishmaniasis episode (31). Hence, variability in the age distribution of cutaneous leishmaniasis cases could also impact on the frequency of mucocutaneous leishmaniasis symptoms. In Opón cutaneous leishmaniasis patients tend to be younger than on the Colombian Pacific coast or in Panama, but we were unable to detect any association between age and mucocutaneous leishmaniasis risk.
The remaining three risk factors for mucocutaneous leishmaniasis which we detected in Opón were consistent with previous analyses of mucocutaneous leishmaniasis risk associated with L. braziliensis, but had not been previously shown for L. panamensis. In particular, we found that, as in Bahia, Brazil (34), people with cutaneous leishmaniasis lesions on the head had significantly greater risk of mucocutaneous leishmaniasis. Secondly, we found that, as in Bolivia (35,36), mucocutaneous leishmaniasis is a greater risk for male than female cutaneous leishmaniasis patients. While we cannot discount possible confounders, it seems likely that this reflects innate gender differences in susceptibility, mediated by sex-associated hormones (37), as demonstrated in laboratory mice infected with various Leishmania species (38). Finally, we found that, as in Belo Horizonte, Brazil (39), mucocutaneous leishmaniasis patients had a greater Montenegro skin test response than cutaneous leishmaniasis patients. This is presumably because mucocutaneous leishmaniasis, whether caused by L. braziliensis or L. panamensis, is a herergic form of cutaneous leishmaniasis with an exaggerated antigenspecific, cell-mediated immune response (40).
Acknowledgements
We want to thank Elsa Morales and Reynaldo Gutiérrez for their technical assistance, Santiago Nicholls from the Instituto Nacional de Salud de Colombia for providing leishmanin; and to Douglas Barker, Sharon McCann, Debbie Nolder and Víctor Angulo for their support.
Conflict of interest
The authors declare that they have no conflict of interest.
Financial support
This study was sponsored by COLCIENCIASColombia (code 11020412926).
Corresponding:
Gerardo Muñoz M., Departamento de Ciencias Básicas, Facultad de Salud, Universidad Industrial de Santander, Carrera 32 Nº 29 - 31, Bucaramanga, Santander, Colombia.
Phone number and fax: (577) 645 5693.
germun@uis.edu.co
References
1. Desjeux P. Human leishmaniasis: epidemiology and public health aspects. World Health Stat Q 1992;45:267- 75. [ Links ]
2. Davies CR, Reithinger R, Campbell-Lendrum D, Feliciangeli D, Borges R, Rodriguez N. The epidemiology and control of leishmaniasis in Andean countries. Cad Saude Publica 2000;16:925-50. [ Links ]
3. Yadon ZE, Quigley MA, Davies CR, Rodrigues LC, Segura EL. Assessment of leishmaniasis notification system in Santiago del Estero, Argentina, 1990-1993. Am J Trop Med Hyg 2001;65:27-30. [ Links ]
4. World Health Organization. Control of the leishmaniasis. Report of a WHO Expert Committee. No. 793. Geneve: WHO Technical Report Series; 1990. [ Links ]
5. Christensen HA, Fairchild GB, Herrer A, Johnson CM, Young DG, Vazquez AM. The ecology of cutaneous leishmaniasis in the republic of Panama. J Med Entomol 1983;20:463-84. [ Links ]
6. Weigle KA, Santrich C, Martinez F, Valderrama L, Saravia NG. Epidemiology of cutaneous leishmaniasis in Colombia: environmental and behavioral risk factors for infection, clinical manifestations, and pathogenicity. J Infect Dis 1993;168:709-14. [ Links ]
7. Herrer A, Christensen HA. Epidemiological patterns of cutaneous leishmaniasis in Panama III: Endemic persistence of the disease. Am J Trop Med Hyg 1976;25:54-8. [ Links ]
8. Mouchet J, Le Pont F, Leon R, Echeverria R, Guderian RH. Leishmaniasis in Ecuador. 5. Leishmaniasis and anthropization on the Pacific coast. Ann Soc Belg Med Trop 1994;74:35-41. [ Links ]
9. Rojas JC, Zeledon R, Murillo J, Urbina A. Identification of risk factors associated with cutaneous leishmaniasis in Costa Rica. En: IDRC. Proceedings of leishmaniasis control strategies. A critical evaluation of IDRC supported research. Ottawa: IDRC; 1992. [ Links ]
10. Herrero MV, Rojas JC, Jimenez AE, Zeledon R, Dobles A, Pereira R et al. Phlebotominae sandflies (Diptera: Psychodidae: Phlebotominae) associated to human houses in an endemic area for cutaneous leishmaniasis in Costa Rica. En: IDRC. Proceedings of leishmaniasis control strategies. A critical evaluation of IDRC supported research. Ottawa: IDRC; 1992. [ Links ]
11. Davies CR, Llanos Cuentas A, Pyke SD, Dye C. Cutaneous leishmaniasis in the Peruvian Andes: an epidemiological study of infection and immunity. Epidemiol Infect 1995;114:297-318. [ Links ]
12. Weigle K, Saravia NG. Natural history, clinical evolution, and the host-parasite interaction in New World cutaneous leishmaniasis. Clin Dermatol 1996;14:433-50. [ Links ]
13. Weigle KA, de Davalos M, Heredia P, Molineros R, Saravia NG, DAlessandro A. Diagnosis of cutaneous and mucocutaneous leishmaniasis in Colombia: a comparison of seven methods. Am J Trop Med Hyg 1987;36:489-96. [ Links ]
14. de Bruijin MHL, Barker DC. Diagnosis of New World leishmaniasis: specific detection of species of the Leishmania braziliensis complex by amplification of kinetoplast DNA. Acta Trop 1992;52:45-58 [ Links ]
15. Godfrey DG, Kilgour V. Enzyme electrophoresis in characterizing the causative organism of Gambian trypanosomiasis. Trans R Soc Trop Med Hyg 1976;70:219-24. [ Links ]
16. Jones TC, Johnson WD Jr, Barretto AC, Lago E, Badaro R, Cerf B et al. Epidemiology of american cutaneous leishmaniasis due to Leishmania braziliensis. J Infect Dis 1987;156:73-83. [ Links ]
17. World Health Organization. Control of the leishmaniasis. Geneve: World Health Organization Series; 1993. p.793. [ Links ]
18. Williams BG, Dye C. Maximum likelihood for parasitologists. Parasitol Today 1994;10:489-93. [ Links ]
19. Crawley MJ. Glim for ecologists. First edition. Oxford: Blackwell Science; 1993. [ Links ]
20. Yadon ZE, Rodrigues LC, Davies CR, Quigley MA. Indoor and peridomestic transmission of American cutaneous leishmaniasis in northwestern Argentina: a retrospective case-control study. Am J Trop Med Hyg 2003;68:519-26. [ Links ]
21. Manson-Bahr PC. Diagnosis clinical aspects and control. In: Peters W, Killick-Kendrick R, editors. The leishmaniasis in biology and medicine. Vol II. London: Academic Press; 1987. p.703-29. [ Links ]
22. Robledo S, Wozencraft A, Valencia AZ, Saravia N. Human monocyte infection by Leishmania (Viannia) panamensis. Role of complement receptors and correlation of susceptibility in vitro with clinical phenotype. J Immunol 1994;152:1265-76. [ Links ]
23. Alcais A, Abel L, David C, Torrez ME, Fandre P, Dedet JP. Evidence for a major gene controlling susceptibility to tegumentary leishmaniasis in a recently exposed Bolivian population. Am J Hum Genet 1997;61:968-79. [ Links ]
24. Davies CR, Mazloumi Gavgani AS. Age, acquired immunity and the risk of visceral leishmaniasis: a prospective study in Iran. Parasitology 1999;119:247-57. [ Links ]
25. Belkaid Y, Kamhawi S, Modi G, Valenzuela J, Noben-Trauth N, Rowton E et al. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med 1998;188:1941-53. [ Links ]
26. Titus RG, Ribeiro JM. Salivary gland lysates from the sand fly Lutzomyia longipalpis enhance Leishmania infectivity. Science 1988;239:1306-8. [ Links ]
27. Saravia NG, Weigle K, Segura I, Giannini SH, Pacheco R, Labrada LA et al. Recurrent lesions in human Leishmania braziliensis infection, reactivation or reinfection? Lancet 1990;336:398-402. [ Links ]
28. Osorio LE, Castillo CM, Ochoa MT. Mucosal leishmaniasis due to Leishmania (Viannia) panamensis in Colombia: clinical characteristics. Am J Trop Med Hyg 1998;59:49-52. [ Links ]
29. Saenz RE, Paz HM, de Rodriguez GC, de Vasquez AM, Mata RE, Johnson CM. Mucocutaneous leishmaniasis in Panama. Etiologic agent, epidemiologic and clinical aspects. Rev Med Panama 1989;14:6-15. [ Links ]
30. Weigle KA, Saravia NG, de Davalos M, Moreno LH, DAlessandro A. Leishmania braziliensis from the Pacific coast region of Colombia: foci of transmission, clinical spectrum and isoenzyme phenotypes. Am J Trop Med Hyg 1986;35:722-31. [ Links ]
31. Saravia NG, Segura I, Holguin AF, Santrich C, Valderrama L, Ocampo C. Epidemiologic, genetic, and clinical associations among phenotypically distinct populations of Leishmania (Viannia) in Colombia. Am J Trop Med Hyg 1998;59:86-94. [ Links ]
32. Alcais A, Abel L, David C, Torrez ME, Flandre P, Dedet JP. Risk factors for onset of cutaneous and mucocutaneous leishmaniasis in Bolivia. Am J Trop Med Hyg 1997;57:79-84. [ Links ]
33. Donnelly KB, Lima HC, Titus RG. Histologic characterization of experimental cutaneous leishmaniasis in mice infected with Leishmania braziliensis in the presence or absence of sand fly vector salivary gland lysate. J Parasitol 1998;84:97-103. [ Links ]
34. Llanos-Cuentas EA, Marsden PD, Cuba CC, Barreto AC, Campos M. Possible risk factors in development of mucosal lesions in leishmaniasis. Lancet 1984;2:295. [ Links ]
35. David C, Dimier-David L, Vargas F, Torrez M, Dedet JP. Fifteen years of cutaneous and mucocutaneous leishmaniasis in Bolivia: a retrospective study. Trans R Soc Trop Med Hyg 1993;87:7-9. [ Links ]
36. Dimier-David L, David C, Munoz M, Vargas F, Bustillos R, Valda L et al. Epidemiological, clinical and biological features of mucocutaneous leishmaniasis in Bolivia after a 221 patient sample. Bull Soc Pathol Exot 1993;86:106-11. [ Links ]
37. Walker W, Roberts CW, Ferguson DJP, Jebbbari H, Alexander J. Innate immunity to Toxoplasma gondii is influenced by gender and is associated with differences in interleukin-12 and gamma interferon production. Infect Immun 1997;65:1119-21. [ Links ]
38. Roberts CW, Walker W, Alexander J. Sex-associated hormones and immunity to protozoan parasites. Clin Micr Review 2001;14:476-88. [ Links ]
39. Passos VM, Barreto SM, Romanha AJ, Krettli AU, Volpini AC, Gontijo CM et al. Cutaneous leishmaniasis in the Metropolitan Region of Belo Horizonte: clinical, laboratorial, therapeutic and prospective aspects. Rev Soc Bras Med Trop 2001;34:5-12. [ Links ]
40. Tapia FJ, Caceres-Dittmar G, Sanchez MA. Inadequate epidermal homing leads to tissue damage in human cutaneous leishmaniasis. Immunol Today 1994;15:160-5. [ Links ]














