Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Biomédica
versão impressa ISSN 0120-4157versão On-line ISSN 2590-7379
Biomédica v.26 supl.1 Bogotá out. 2006
A multifaceted intervention to prevent American cutaneous leishmaniasis in Colombia: results of a group-randomized trial
Carlos A. Rojas 1, 2, Kristen A. Weigle 3, Rafael Tovar 4, 2, Alba L. Morales 5, 6, Bruce Alexander 7, 2
This paper is dedicated to the memory of Jaime Becerra Calle
1
Grupo de Epidemiología, Facultad Nacional de Salud Pública, Universidad de Antioquia, Medellín, Colombia.2
Centro Internacional de Entrenamiento e Investigaciones Médicas, Cali, Colombia.3
Department of Epidemiology, School of Public Health, University of North Carolina, Chapel Hill, United States of America4
Departamento de Ciencias Naturales y Matemáticas, Facultad de Ingeniería, Pontificia Universidad Javeriana, Cali, Colombia5
Programa para la Eliminación de la Oncocercosis en América, Ciudad de Guatemala, Guatemala.6
Centro de Investigaciones Multidisciplinarias en Desarrollo, Cali, Colombia.7
Molecular and Biochemical Parasitology Group, Liverpool School of Tropical Medicine, Liverpool, UKResearch institution: This investigation was conducted when the principal author was working at the Centro Internacional de Entrenamiento e Investigaciones Médicas
Recibido: 10/10/05; aceptado: 24/02/06
Introduction.
American cutaneous leishmaniasis is endemic in Colombia, where approximately 6.000 new cases are reported every year. Current prevention and control measures are restricted to the diagnosis and treatment of cases.Objective. To evaluate the efficacy of a multifaceted intervention to prevent the transmission of Leishmania in the endemic focus of Tumaco, on the Pacific Coast of Colombia.
Materials and methods. A group-randomized trial was conducted. Twenty villages were matched according to prevalence of Leishmania infection, number of inhabitants and level of community participation, and then randomly assigned to intervention or control. The intervention included deltamethrin-impregnated bednets, repellent (20% diethyltoluamide and 0.5% permethrin), modification of sand fly resting sites, and health education. Villages were under surveillance for one year and the use of the intervention measures monitored. The incidence of American cutaneous leishmaniasis and Leishmania infection in the two groups were compared, adherence to the intervention and adverse events were monitored, and the results were adjusted for village intraclass correlation.
Results. Ten cases of American cutaneous leishmaniasis were confirmed in the intervention and 23 in the control group, OR = 0.42, 95% CI 0.14-1.26. The intervention had a greater effect in children < 10 years old, in people living on the periphery of the village and in villages with a prevalence of infection in small children > 1%. Adverse events associated with the use of the bednets and the repellent were reported in 2% of the participants and were always mild.
Conclusion. Incident cases of American cutaneous leishmaniasis were reduced by 58% in the intervention group. However, the small number of cases renders the effect estimate imprecise and precludes us to claim a protective effect for the intervention. Specific populations could be the targets of simpler and more cost-effective interventions in the future.
Key words: Leishmaniasis, cutaneous, /prevention & control, vector control, randomized controlled trials, effect modifiers (Epidemiology), Colombia
Prevención de leishmaniasis cutánea americana en Colombia mediante una intervención múltiple: resultados de un ensayo de grupos aleatorios
Introducción. La leishmaniasis cutánea americana es endémica en Colombia, donde cada año son notificados aproximadamente 6000 casos nuevos. En la actualidad las medidas de prevención y control están limitadas al diagnóstico y tratamiento de los casos.
Objetivo. Evaluar la eficacia de una intervención múltiple para prevenir la transmisión de Leishmania en el foco endémico de Tumaco, costa Pacífica de Colombia.
Materiales y métodos. Se realizó un ensayo de grupos aleatorizados. Veinte veredas fueron pareadas según prevalencia de Leishmania, habitantes y participación comunitaria y luego asignadas aleatoriamente a intervención o control. La intervención incluyó toldillos impregnados con deltametrina, repelente (N, N-dietil-m-toluamida 20% y Permetrina 0,5%), modificación de lugares de reposo para los vectores y educación. Al cabo de un año se comparó la incidencia de infección y enfermedad producida por Leishmania en los dos grupos, se monitorearon la adherencia a la intervención y la aparición de efectos adversos. Los resultados finales fueron ajustados por el efecto de correlación intra-grupo.
Resultados. Se presentaron 10 casos de leishmaniasis cutánea americana en el grupo que recibió la intervención y 23 en el grupo control, OR=0,42, IC95% 0,14-1,26. La intervención tuvo un mayor efecto en los niños menores de 10 años, en aquellos que residían en la periferia de la vereda y en veredas con una prevalencia de infección en niños pequeños mayor del 1%. Se reportaron eventos adversos leves asociados con el uso de los toldillos impregnados y el repelente en 2% de los participantes.
Conclusión. Los casos nuevos de Leishmaniasis cutánea americana se redujeron en un 58% en el grupo que recibió la intervención. Sin embargo, el número pequeño de casos hace que la estimación de la medida de efecto sea imprecisa y no nos permite afirmar que la intervención tiene un efecto protector. Poblaciones específicas podrían ser el blanco de futuras intervenciones más simples y costo-efectivas.
Palabras claves: Leishmaniasis cutánea, /prevención & control, control vectorial, ensayos controlados aleatorios, modificadores del efecto (Epidemiología), Colombia
American cutaneous leishmaniasis is an infectious disease caused by parasites of the genus Leishmania that affects the skin and the upper respiratory mucosa (1). It is transmitted by the bite of infected phlebotomine sand flies (Diptera: Psychodidae) and sylvatic and domestic mammals serve as reservoirs for the parasite (2). The disease is widespread in the Americas, ranging from southern Texas to northern Argentina (3), and the annual number of cases and people who live in areas where American cutaneous leishmaniasis is transmitted has been estimated at 59,300 and 59 million, respectively (4).
Control of American cutaneous leishmaniasis has been restricted principally to case management (3,5). However, both diagnosis and treatment are challenging. Accurate diagnosis, based on laboratory techniques, requires trained personnel and adequate equipment (6). On the other hand, treatment with antimonial derivates such as Glucantime ® is expensive, requires a parenteral route of administration and has frequent adverse effects (7).
Although sand flies can be eliminated by spraying with residual insecticides in many areas of the world where leishmaniasis occurs (8-9), most species involved in American cutaneous leishmaniasis transmission are associated with forested habitats where such interventions are not feasible. However in these areas many species of sand flies can be found resting on the bases of tree trunks during the day and attempts have been made to control the insects in these microhabitats (10). The application of DDT on the walls of houses, used since the 1950s to control mosquito vectors of malaria, produced a temporary reduction in the number of American cutaneous leishmaniasis cases in places where sand flies entered houses to bite (11). A clinical trial recently showed positive results in the control of cutaneous leishmaniasis in Peru using a similar strategy (12).
Control measures targeted against the wild reservoirs of American cutaneous leishmaniasis is also impractical (2), and whether or not domestic animals participate in the transmission of American cutaneous leishmaniasis remains debatable (13-14).
Personal protection measures against insect bites such as insect repellents have been used for many years and have been recommended for the prevention of American cutaneous leishmaniasis (8). However, such measures are only suitable for individuals such as military personnel and tourists who are exposed to sand fly bites for only short periods of time. Insecticide-impregnated bednets are effective in the prevention of malaria and well accepted by communities in Latin America (15). However, the efficacy of this strategy in the prevention of American cutaneous leishmaniasis has not been evaluated. Deltamethrin-impregnated bednets and a repellent containing 20% diethyltoluamide (DEET) and 0.5% permethrin reduced biting by sand flies in Colombia (16-17) although both studies were too small to assess the effect of these interventions on Leishmania transmission.
To understand whether sustainable measures such as the above could reduce American cutaneous leishmaniasis transmission when used by inhabitants of Leishmania-endemic areas, we evaluated the efficacy of an intervention package that incorporated several methods: deltamethrinimpregnated bednets, repellent, painting sand fly resting sites with whitewash and health education. Exposure to infected sand flies in endemic areas may occur in intra-, peri-or extra-domiciliary situations or various combinations of the three and an intervention package of the type evaluated here was felt to be preferable to any single control measure.
Materials and methods
Study area and population
The study was conducted between October, 1994, and June, 1997, in 20 villages located on the banks of four rivers in Tumaco, Nariño department, Colombia. This is an area of active American cutaneous leishmaniasis transmission that has been the site of multidisciplinary research conducted since 1982 by the Centro Internacional de Investigaciones Médicas (CIDEIM) (18-24). All previous Leishmania isolates have been identified as Leishmania (Viannia) panamensis (89%) and Leishmania (Viannia) braziliensis (11%) (19-20).
The predominant insect vectors are Lutzomyia trapidoi (Fairchild & Hertig) and Lutzomyia gomezi (Nitzulescu) (21), and no cases of visceral leishmaniasis due to Leishmania (Leishmania) infantum have ever been diagnosed in the area.
The Tumaco area is classified ecologically as humid tropical rain forest. Most human residences are constructed on wooden platforms with wooden walls and zinc or thatch roofs. Although the number of residences per village varies from 19-122, all communities have two clearly distinguishable zones: a center with houses located around the school and connected by sidewalks and a periphery, consisting of dwellings spread along the riverbank on either side of the center. Inhabitants of the study area are the descendants of African slaves, with 46% less than 15 years old and a slight preponderance of males (55%). The principal occupation is subsistence farming, supplemented in some of the villages with fishing or lumbering.
The intervention
To protect participants in all possible transmission settings, a multifaceted intervention was designed to include protective measures for the residence (impregnated bednets), residence-surroundings (painting of tree trunks with whitewash), and the forest (use of a repellent). All measures were introduced and accompanied by an educational intervention, and actively involved community members and local health Institutions.
New polyester bednets (11.6 m2 and 35 holes per cm2 ) were provided to all the participants after being impregnated with K-Othrine E-25® (deltamethrin). The impregnation was done with the participation of community members following standard procedures (15). Two bars of the repellent Nopikex® (20% DEET and 0.5% permethrin) were delivered to each residence. Participants were instructed by demonstration on how to use the bednets and the repellent (16-17). They were especially encouraged not to wash the nets. Tree trunks that could serve as resting sites for sand flies and were located <50 m from an inhabited residence were painted with whitewash to a height of 1.5 m from the ground. Every three months the bednets were impregnated, additional repellent supplied, and the tree trunks repainted.
An educational program designed and implemented by the Centro de Investigaciones Multidisciplinarias en Desarrollo (CIMDER) that included information about American cutaneous leishmaniasis, its mode of transmission and how to use the different preventive measures accompanied the preventive measures.
Study design and data collection
The study protocol and the consent forms were reviewed and approved by CIDEIMs Institutional Review Board. Inhabitants of the 20 villages were invited to participate in a group-randomized trial and written consent was obtained from all the adults and from parents or guardians of minors. Initially a baseline census and exams were conducted. Participants were examined for scars or active skin lesions suspected to be American cutaneous leishmaniasis, using clinical criteria defined in a previous study (22). The leishmanin skin test was applied to detect prior Leishmania infection (23). The status of community participation in each village was assessed and quantified using a community participation score (Morales AL, unpublished data).
Before randomization, villages were paired according to prevalence of leishmanin skin test positive in children <5 years old, number of inhabitants, and community participation score. One village in each pair was randomly assigned to receive the intervention; the other remained as a control. Control villages did not receive any of the studied interventions, but like the intervention villages, they were subject to active surveillance and case management of American cutaneous leishmaniasis cases. Randomization was performed using a lottery system and was carried out with the participation of delegates from the 20 villages.
After randomization, all the participants were examined and those with a previous negative leishmanin skin test tested again. New residents, willing to participate, were enrolled even if they did not participate in the baseline assessment. The intervention measures were begun shortly after this pre-intervention exam and the intervention and control villages were followed during approximately 12 months (July, 1996-June, 1997). The use of bednets and repellent was monitored in a representative sample of residences (17%) in each study village. At the end of the study, the number of impregnations per bednet was recorded, as well as the number of times they were washed between two consecutive impregnations. Community health volunteers trained to detect suspected American cutaneous leishmaniasis cases searched their villages for new cases and reported to the projects staff during their monitoring visits to the villages. At the end of the follow-up period all the participants were examined (post-intervention exam), and those with a negative leishmanian skin test during pre-intervention were tested again.
Study outcomes
An incident case of American cutaneous leishmaniasis was defined as a person without active skin lesions during the pre-intervention exam who was later found to have lesions that were parasitologically confirmed as American cutaneous leishmaniasis (6). Since re-infection with American cutaneous leishmaniasis has been documented (19), the appearance of new lesions in participants with history of American cutaneous leishmaniasis, were also considered as incident cases.
As in previous studies, we used skin test conversion as a marker for newly acquired infections (22- 23). An incident Leishmania infection was defined as leishmanin skin test conversion from non-reactive in the pre-intervention exam to reactive in the post-intervention. We used the leishmanin skin test produced by the Instituto Nacional de Salud de Colombia. Throughout the study we used the same lot of leishmanin skin test (No. 001, March, 1994).
Covariates
Information about risk factors for American cutaneous leishmaniasis (24) and potential effect modifiers was collected during the baseline assessment (1994-1995). Variables were screened by contingency tables, and those that were associated with either of the study outcomes in the control group were selected for the analysis. Variables with >30% missing values were excluded. A prioridefined effect modifiers were included in the analysis independently of their association with the study outcomes.
The following variables were included in the analysis:
Individual level: age, gender, farming occupation, history of American cutaneous leishmaniasis, presence of typical American cutaneous leishmaniasis scars, farming activities, daily forest hours, and entering the forest;
Household level: residence located on the periphery of the village, residence borders the forest, residence borders the river, roof made of thatch, external walls made of bamboo, incomplete number of external walls (living area not completely enclosed by walls), latrine located outside the residence, distance from residence to the forest, distance from the next residence and total number of animals per residence;
Village level: community participation score, prevalence of Leishmania-infection in children <5 years old during the baseline exam, and number of inhabitants.
Data analysis
Cumulative incidences of American cutaneous leishmaniasis and Leishmania-infection were calculated for the intervention and control group. For American cutaneous leishmaniasis only those who did not have active skin lesions at the pre-intervention exam and who were examined during postintervention were included in the analysis. For infection we included only those with a non-reactive leishmanin skin test in the pre-intervention exam who were tested during post-intervention. The effect of the intervention was calculated using risk ratios (RR) (intervention/control) and risk differences (RD) (intervention-control), and 95% confidence intervals (CI) were computed for descriptive purposes using standard methods (25).
The distribution of the risk factors between the two study groups was compared and the strength of the association measured with the odds ratio (OR). Those risk factors that were associated with study group were considered potential confounders of the intervention-outcome association. Logistic regression was used to adjust for the effect of multiple confounders (26). Separate models were constructed for each of the study outcomes. Nested models were constructed to identify the least biased estimate of the intervention effect (25). A variable that produced a change in the interventions OR>10% after been removed from the model, was considered a confounder and retained in the final model. Once the final model was defined, the generalized estimating equations method (GEE) (27) was used to estimate the parameters while taking into account the correlation of observations within villages (nonindependence). The type of correlation used was exchangeable.
A priori-defined effect modifiers included age, gender, farming occupation, history of American cutaneous leishmaniasis, residence proximity to the forest, residence located on the periphery of the village, roof made of thatch, incomplete number of exterior walls, prevalence of infection in children <5 years old, community participation score, and village size. For each effect modifier we calculated stratum-specific RR of the effect of the intervention. We then used logistic regression and evaluated effect modification using interaction terms in models that included all the confounders already identified. All the analyses were done using SAS version 6.12 (SAS Institute, Cary, NC).
Results
Study populations
During the pre-intervention assessment, 5,009 persons resided in the study area. A total of 4,630 (92%) participated in the exam. Most of the nonparticipants were absent during the days of the exam. Twenty-seven participants were found with skin lesions suggestive of American cutaneous leishmaniasis and were excluded from the study population. The rest (4,603) constitute the population considered susceptible to American cutaneous leishmaniasis. A total of 2,738 participants had a negative skin test and constituted the population susceptible to Leishmania infection. Refusal to be tested was small (3.6%).
Seventy-nine percent of those susceptible to American cutaneous leishmaniasis had a second physical examination during the post-intervention exam and 77% of those susceptible to infection were skin tested again. These rates were the same for the intervention and control groups. Loss to follow-up due to emigration was somewhat greater in the control group, while absence from the village on the days of the post-intervention exam was somewhat more common in the intervention group. Refusal rates were less than 2% in the two study groups. Those who were not examined or tested during the post-intervention survey included a larger proportion of teenagers and farmers compared to the studied groups; however, they did not differ by gender or history of American cutaneous leishmaniasis. Characteristics of nonparticipants were the same in the intervention and control group (data not presented).
Thirteen persons from the control group were excluded because they moved to an intervention village during the follow-up period. No movements in the opposite direction were documented. The study groups to evaluate the interventions effect on the incidence of American cutaneous leishmaniasis included 1,791 in the intervention group and 1,840 in the control group. The study groups to evaluate the interventions effect on the incidence of infection included 1,066 and 1,034 persons, respectively.
The mean follow up time for the intervention villages (12.6 months) was slightly longer than for the control villages (12.2 months). The number of person-years observed for those susceptible to American cutaneous leishmaniasis was 1,891 and 1,873 person-years in the intervention and control group, respectively. The number of person years observed for those susceptible to infection was 1,130 and 1,051 person-years, respectively.
Identification of potential confounders
Characteristics of the residence (distance to the forest <50 m, RR=12.5, 95% CI 1.7-93.5 and roof made of thatch, RR=3.5, 95% CI 1.4-8.6) and the village (prevalence of infection in children <5 years old, RR=13.4, 95% CI 3.9-45.9 and community participation score <50, RR=5.1, 95%CI 2.0-2.9) were strongly associated with American cutaneous leishmaniasis in this setting. However, no association was observed between behavioral and occupational activities and American cutaneous leishmaniasis. On the other hand, several behavioral and occupational activities were moderately associated with infection, as were characteristics of the residence (roof made of thatch, RR=1.9, 95% CI 1.1-3.3 and walls made of bamboo, RR=1.9, 95% CI 1.2-3.2) and the village (prevalence of infection in children <5 years old, RR=3.1, 95% CI 2.0-4.9 and community participation score <50, RR=2.0, 95% CI 1.3-3.1).
The distribution of these risk factors between the two study groups are compared in table 1. We present the information for those susceptible to American cutaneous leishmaniasis. In general, the study groups were comparable in the distribution of behavioral and occupational risk factors, but differed in the distribution of those factors related with the residence and the village. Residences in the control group were more likely to be located at the periphery, close to the forest, have roof made of thatch, have incomplete external walls and have more animals. Also, control villages had lower community participation scores. On the other hand, villages in the intervention group had a greater prevalence of infection in children <5 years old, had a larger number of inhabitants and had slightly more males.
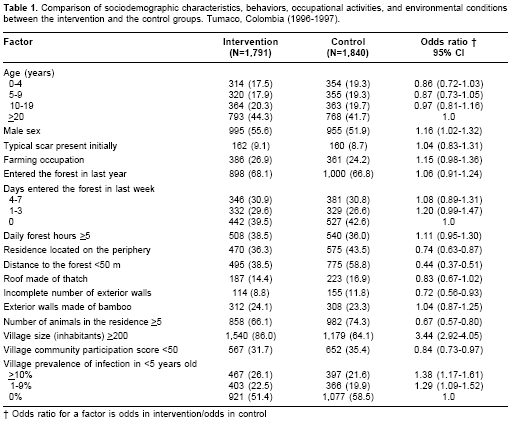
Based on associations with the outcome and study group assignment, potential confounders of the effect of the intervention on American cutaneous leishmaniasis were: residence located on the periphery of the village, roof made of thatch, distance to the forest <50 m, number of animals in the residence, community participation score and prevalence of infection in children <5 years old. Potential confounders of the effect of the intervention on infection were; sex, roof made of thatch, community participation score and prevalence of infection in children <5 years old.
Incidence of American cutaneous leishmaniasis and infection
Thirty-three new cases of American cutaneous leishmaniasis were detected during the study, 23 in the control group and 10 in the intervention group. Cases in the control group appeared throughout the follow-up period, compared to cases in the intervention group that were absent at the beginning and concentrated at the middle and end of the follow up period (
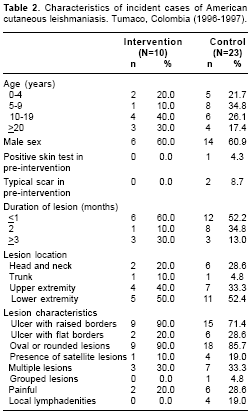
figure 1). Cases in the control group were somewhat younger, on average, than those in the intervention group, though the difference was well within the range of chance expectation (p=0.47). Cases in the two study groups were comparable in the rest of the characteristics. Most lesions were of short duration, located on the extremities and had the typical appearance of a rounded ulcer with raised borders (table 2).
The cumulative incidence of American cutaneous leishmaniasis was 0.56% in the intervention and 1.25% in the control group, RR=0.44, 95% CI 0.21- 0.94 (
table 3). The risk ratio did not change significantly after adjusting for confounding and village intraclass correlation. The cumulative incidence of infection (skin test conversion) was similar in the two study groups, 7.69% in the intervention and 7.74% in the control, RR=0.99 (table 3). The risk ratio changed slightly after adjusting for confounding and intraclass correlation, but did not suggest any intervention effect, OR=1.06, 95% CI 0.54-2.08.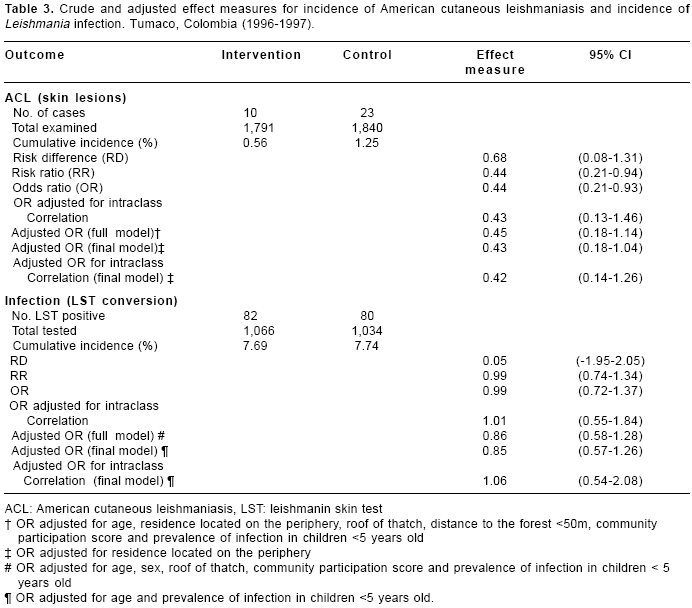
Effect modification
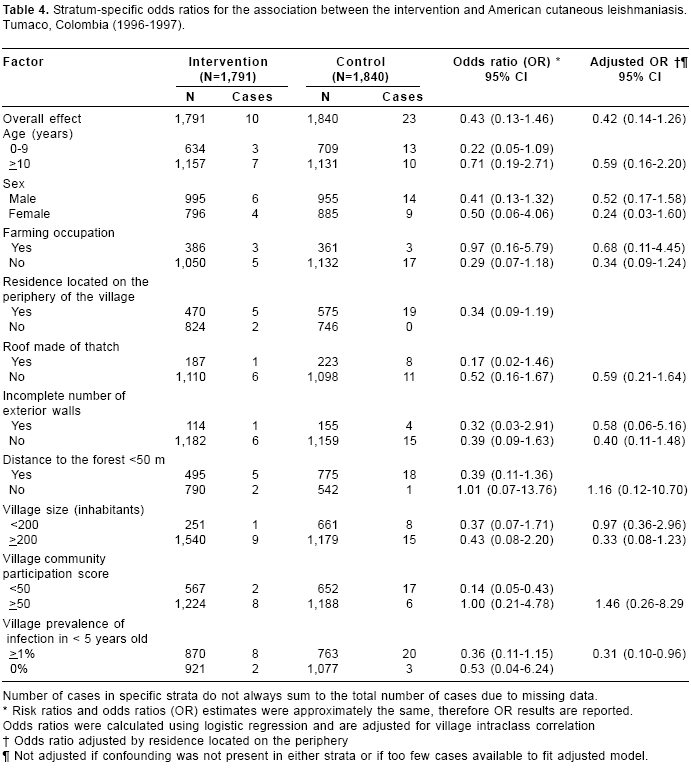
Table 4 presents stratum-specific ORs for the association between the intervention and American cutaneous leishmaniasis, adjusted for village intraclass correlation. Compared to the overall effect, a stronger effect of the intervention was observed in children under 10, non farmers, persons living on the periphery of the village, persons living in houses that facilitate the entrance of sand flies (roof made of thatch, incomplete external walls), persons living in villages with low community participation score. After adjusting for residence located on the periphery of the village, which we considered the most important confounder for this association in this study, a stronger effect was observed in those who live in villages with a prevalence of infection in small children >1% (OR=0.31, 95%CI 0.10 - 0.96) and in women (OR=0.24, 95% CI 0.03-1.60). Because of the small number of cases, not all the stratum-specific effect estimates could be adjusted for confounding.
Stratum-specific ORs for the association between the intervention and Leishmania infection are presented in
table 5. A stronger effect of the intervention was observed among those who lived in residences with roof made of thatch (OR=0.36, 95% CI 0.07-1.78). After adjusting for age and prevalence of infection in children <5 years old, a moderate effect was observed for males (OR=0.58) and children (OR=0.54); however, the 95% CI included the null value for both of these subgroups. Similar to what we observed with American cutaneous leishmaniasis, the intervention seemed to be more effective in larger villages (OR=0.67, 95% CI 0.38-1.17).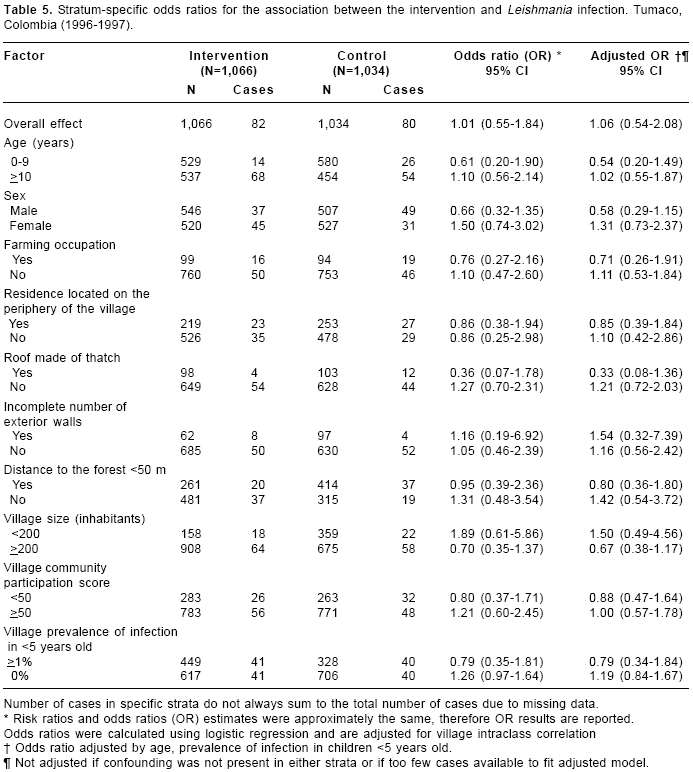
Adherence of participants to the intervention measures
Frequency of bednet use was high and consistent during the study. Among the participants who were interviewed during the first and second monitoring visits, 93% and 96% respectively reported sleeping under the bednet every night. This was confirmed during the two unannounced visits to the residences, where approximately 85% of the bednets were in use by the participants. Because there was not enough variation we could not evaluate dose effect for bednet use.
Four of the intervention villages only had three impregnation sessions due to logistical constraints. Complete adherence to the impregnation schedule, defined as the percentage of bednets that received all the impregnations (4 or 3 depending on the village), varied among villages (17%-100%) (data not presented). Very few participants abstained from washing their bednets between two impregnations. Seventythree percent of the participants reported they washed the bednets three or more times during that period (approximately 3 months). The frequency of adverse effects with the impregnated bednets reported during the monitoring visits was 2%. The most common adverse effect was headache. Among the villages that were offered the four impregnations, no significant differences were observed in the incidence of American cutaneous leishmaniasis and the incidence of Leishmania infection between those who had four and those that had less than four impregnations.
Participants with less than four impregnations washed more often their bednets, OR=2.0, 95%CI 1.4-2.9, adjusted by residence located on the periphery of the villages and by community participation score.
Use of the repellent decreased over time. At the beginning of the intervention, 42% of the interviewed sample reported using the repellent everyday. During the last exam, only 5% (40/781) used it every day; however, 55% were using it between 4 and 6 days per week. The repellent was used principally during the day in the forest (61%) and during the night at home (23%). Differences were reported by gender. More women (11%) reported they never used the repellent, and more men (63%) reported they used it between 4 and 6 days per week. Women used the repellent most frequently at home during the night (39%), whereas men used it in the forest during the day (84%). Almost 100% of the interviewed participants liked the repellent (acceptance). No significant differences were observed in the incidence of Leishmania infection in those who used the repellent >4 days per week compared to those who used it less frequently, 18% (23/131) and 11% (13/113), respectively, (p>0.1). The frequency of adverse effects with the repellent was also 2% and the most common was itching.
Since the modification of the tree trunks with whitewash was not a personal intervention, adherence to this component of the intervention was evaluated in terms of the participation of community members in its implementation and sustainability. Community members participated in large numbers in painting the tree trunks the first time. However, participation decreased gradually during the follow-up period in most villages. Because of this, not all the trees that were part of this intervention could be repainted every 3 months as planned.
Discussion
We found a moderate, not statistically significant, reduction in the overall cumulative incidence of american cutaneous leishmaniasis in the intervention group (OR=0.42, 95% CI 0.14-1.26). Although the small number of cases in our study renders the estimates imprecise, the intervention effect was stronger in subgroups such as children, women, those who lived in large villages (>200 inhabitants) and those who lived in villages with low community participation score.
The community participation score findings contradict our original hypothesis that the intervention would have a greater effect in better organized and in villages with a higher degree of community participation (score>50). To determine whether the score was a surrogate for something else, we measured its association with other factors. The community participation score was strongly associated with residence located on the periphery (OR=11.3, 95% CI 9.3-13.6), villages with a greater proportion of residents who lived on the periphery had lower scores; and prevalence of infection in children <5 years old (OR=8.2, 95% CI 7.0-9.7), villages with a higher prevalence of infection in young children have lower score. Community score seems to be a correlate of less centralized dense village, with a greater risk for American cutaneous leishmaniasis because more residences are located near the forest. On the other hand a high score does not seem to be a requirement for a successful intervention.
We examined other possibilities beside the intervention that could explain the reduction in the incidence of American cutaneous leishmaniasis. There was an unbalanced distribution of potential risk factors between the study groups; however, after controlling for these differences in the analysis our estimation of the intervention effect did not change significantly.
To see if American cutaneous leishmaniasis was more endemic in the control villages we compared the prevalence of American cutaneous leishmaniasis in the two study groups before the intervention. During the baseline exam (1994-1995), no significant differences were observed between the two groups in terms of suspected lesions, history of American cutaneous leishmaniasis and presence of scars. During the pre-intervention exam (1996) the prevalence of participants with suspected lesions was 0.48% (11/2,283) in the intervention villages and 0.68% (16/2,347) in the control villages (p = 0.37) (Rojas CA, unpublished data).
The observed difference in the incidence of American cutaneous leishmaniasis could have resulted from other type of intervention that decreased the contact with sand flies in the intervention villages. Sand flies are susceptible to the residual insecticides used to control malaria (8) and American cutaneous leishmaniasis transmission could also be affected by the permethrin-impregnated bednets used for malaria prevention. Malaria vector-control activities were interrupted in the 20 study villages during the year of follow-up. Instead, detection and treatment of malaria cases was increased, paying special attention to the control group. No outbreaks of malaria were reported during the study. It is also unlikely that participants in the intervention group used additional protective measures. According to information collected before the intervention, inhabitants of the study villages do not use insecticides or repellents at home. These are too expensive for most of the participants (Rojas CA, unpublished data).
Case detection was conducted in the same way in both study groups. Surveillance activities based on community volunteers were implemented in the study villages before randomization, and all the volunteers were selected, trained, and followed in the same way. Personnel who were not aware of the patients study group did the laboratory confirmation of the suspected lesions. It is unlikely that bias in the detection of cases accounted for the observed difference. Neither our surveillance system, nor our physical examination protocol during the post-intervention exam was exhaustive, and it is possible that we missed some cases. This was likely to have affected the two study groups in the same way, and hence would have produced non-differential misclassification, biasing the effect measure estimate towards the null value and underestimating the effect of the intervention.
It was interesting that the intervention did not reduce the incidence of Leishmania infection. A possible explanation for the discrepancy in the results between disease and infection is that the intervention had an effect on the pathogenicity of American cutaneous leishmaniasis. In the natural history of the infectious diseases, infection precedes the appearance of clinical disease and pathogenicity is the transition from infection to disease (1). The pathogenicity of American cutaneous leishmaniasis is affected among other factors by the number of Leishmania parasites (28) and by the hosts immune response.
The intervention measures could have decreased the number of sand fly bites, and therefore the number of parasites introduced in the human host, to a degree that was insufficient to promote the development of clinical disease, but still enough to generate an immune response in the host (leishmanian ski test conversion). This could explain why the same proportion of participants in the two study groups showed skin test conversion, but a larger proportion in the control group developed American cutaneous leishmaniasis.
Similar results were observed in Sudan (1995- 1996), where a trial was conducted to assess the efficacy of pyrethroid-impregnated bednets on the transmission of visceral leishmaniasis, and a significant reduction in the number of clinical cases in the intervention village observed. Also, the high disease/infection ratio in the control village was reversed into a high infection/disease ratio in the intervention village (29). According to the WHO these changes in the pathogenesis of the disease may be due to a reduced number of infective bites or to reduced parasite doses received by people using the bednets. Similarly, in our study the disease/infection ratio was higher in the control (0.16) than in the intervention group (0.07).
Previous studies using pyrethroid-impregnated bednets have been effective in reducing cutaneous leishmaniasis due to L. tropica in Syria (30), and visceral leishmaniasis in Sudan (29). We report for the first time the use of a multifaceted intervention, which includes impregnated bednets, for the prevention of cutaneous leishmaniasis in the New World. Current control strategies are limited to detection and treatment of cases; however, since humans have not been proven to be reservoirs for American cutaneous leishmaniasis, this strategy does not have any effect on the transmission and appearance of new cases.
Although pyrethroid-impregnated bednets have been shown to reduce malaria transmission significantly (31) and would be expected to have a similar effect on leishmaniasis, there are several drawbacks attached to their widespread use. These include (a) problems with ensuring adequate coverage of the mesh during impregnation, (b) risk of intoxication if users miscalculate the correct concentration of insecticide or substitute pyrethroids for other, more toxic chemicals and (c) environmental contamination associated with washing nets or disposal of surplus insecticides. Fish, an important economic resource in the study area, are particularly susceptible to pyrethroid intoxication. These problems could be reduced (although not eliminated) by supplying people with pre-impregnated bednets.
A drawback of the repellent used was that it was formulated as a soap whose protective effect was not retained after rinsing. This might have discouraged some participants from using it when necessary.
Although not evaluated adequately during the present study, regular whitewashing of the lower trunks of trees used by sand flies as diurnal resting sites is a low-cost, sustainable measure that can be carried out by untrained individuals without safety equipment. This measure would not kill sand flies but might have the effect of distancing them from the communities further into the forest where other blood meal sources are available. Sand flies have relatively short flight ranges (32) so the width of such barrier zones (and therefore the number of trees that would have to be treated) is not large. Many of the large trees favored by sand flies as diurnal resting sites were also used by the communities as latrines, particularly examples of breadfruit (Artocarpus artilis) with large buttress roots. This would be a further incentive to maintain these trees free from sand flies.
The policy implications for the control of American cutaneous leishmaniasis with a multifaceted intervention need to be considered carefully. A cost effectiveness analysis conducted during the first year of the intervention yielded a big incremental cost effectiveness ratio (ICER) (33). This represents the additional cost that society would have to pay for the intervention in order to prevent an additional case of American cutaneous leishmaniasis, compared to the cost effectiveness of current case management activities (34). Sensitivity analysis showed that the ICER decreases significantly with an increment in the incidence of American cutaneous leishmaniasis (33). Also, a multifaceted intervention could be more cost effective if the beneficial effect on the transmission of other vector-borne diseases endemic in the study area, such as malaria and dengue, is taken into account. Sustainability of this intervention also has constraints, which have been discussed in detail elsewhere (35).
One limitation of our study is that we could not evaluate the effect of the different intervention measures separately, although the effect modification pattern observed implies that the bednets were more effective than the repellent in the present study. The lack of an intervention effect in farmers may indicate that the repellent had little effect. To target interventions to high-risk populations such as children and villages with a high prevalence of infection could be a more cost effective alternative. Our data shows how the effect was higher in these groups.
Acknowledgements
The authors thank the 20 communities that were part of the study, especially the volunteers who participated in project activities. We are grateful to Wilson Cortés Montaño, Aura García, Edgar Muñoz, Nancy Gore Saravia and the clinical staff from CIDEIM; Ricardo Rodríguez, Adalberto Ruiz and Esmeralda Burbano from CIMDER. We appreciate the collaboration of the Hospital San Andrés and the Malaria Control Program of Tumaco, especially Dr. Jesús Quiñones, Wilson Casierra, Rigoberto Erazo, Andrés Dajome, Guido Pantoja, Beatriz Caicedo, Margarita Cortés, Alberto Reynel, Rosa Aída Ramirez, and Alirio Castillo.
Financial support
A first draft of this paper was written while the principal author was in receipt of a WHO Research Training Grant. The study was supported by the International Development Research Center of Canada, IDRC file 92-0223-01.
Conflicts of interest
The authors have no conflicts of interest concerning the work reported in this paper.
Correspondencia:
Carlos A. Rojas, Grupo de Epidemiología, Facultad Nacional de Salud Pública, Universidad de Antioquia, Calle 62 No. 52-68, Medellín, Colombia.
Phone (574) 210 6433, fax (574) 511 2506
crojas@guajiros.udea.edu.co
References
1. Weigle KA, Saravia NG. Natural history, clinical evolution, and the host-parasite interaction in New World cutaneous leishmaniasis. Clin Dermatol 1996;14:433-50. [ Links ]
2. Ashford RW. Leishmaniasis reservoirs and their significance in control. Clin Dermatol 1996;14:523-32. [ Links ]
3. Desjeux P. Leishmaniasis. Public health aspects and control. Clin Dermatol 1996;14:417-23. [ Links ]
4. Ashford RW, Desjeux P, Deraadt P. Estimation of population at risk of infection and number of cases of leishmaniasis. Parasitol Today 1992;8:104-5. [ Links ]
5. WHO. Technical Report Series, No 793. Control of the leishmaniases: report of a WHO Expert Committee. Geneva: WHO; 1990. [ Links ]
6. Escobar MA, Martínez F, Scott Smith D, Palma GI. American cutaneous and mucocutaneous leishmaniasis (tegumentary): a diagnostic challenge. Trop Doct 1992;22(Suppl.1):69-78. [ Links ]
7. Herwaldt, BL, Berman JB. Recommendations for treating leishmaniasis with sodium stibogluconate (pentostam) and review of pertinent clinical studies. Am J Trop Med Hyg 1992;46:296-306. [ Links ]
8. Alexander JB, Maroli M. Control of phlebotomine sandflies. Med Vet Entomol 2003;17:1-18. [ Links ]
9. Davies CR, Kaye P, Croft SL, Sundar S. Leishmaniasis: new approaches to disease control. BMJ 2003;326:377-82. [ Links ]
10. Ready PD, Arias JR, Freitas RA. A pilot study to control Lutzomyia umbratilis (Diptera: Psychodidae), the major vector of Leishmania braziliensis guyanensis, in a peri-urban rainforest of Manaus, Amazonas State, Brazil. Mem Inst Oswaldo Cruz 1985;80:27-36. [ Links ]
11. Davies CR, Llanos-Cuentas A, Canales J, León E, Álvarez E, Monje J et al. The fall and rise of Andean cutaneous leishmaniasis: Transient impact of the DDT campaign in Peru. Trans R Soc Trop Med Hyg 1994;88:389-93. [ Links ]
12. Davies CR, Llanos-Cuentas A, Campos P, Monge J, León E, Canales J. Spraying houses in the Peruvian Andes with lambda-cyhalothrin protects residents against cutaneous leishmaniasis. Trans R Soc Trop Med Hyg 2000;94:631-6. [ Links ]
13. Reithinger R, Davies CR. Is the domestic dog ( Canis familiaris) a reservoir host of American cutaneous leishmaniasis? A critical review of the current evidence. Am J Trop Med Hyg 1999;61:530-41. [ Links ]
14. Yadon ZE, Rodríguez LC, Davies CR, Quigley MA. Indoor and peridomestic transmission of American cutaneous leishmaniasis in northwestern Argentina: a retrospective case-control study. Am J Trop Med Hyg 2003;68:519-26. [ Links ]
15. Kroeger A, Mancheno M, Alarcon J, Pesse K. Insecticide- impregnated bed nets for malaria control: varying experiences from Ecuador, Colombia and Peru concerning acceptability and effectiveness. Am J Trop Med Hyg 1995;53:313-23. [ Links ]
16. Alexander JB, Usma MC, Cadena H, Quesada BL, Solarte Y, Roa W et al. Evaluation of deltamethrinimpregnated bednets and curtains against phlebotomine sandflies in Valle del Cauca, Colombia. Med Vet Entomol 1995;9:279-83. [ Links ]
17. Alexander JB, Cadena H, Usma MC, Rojas CA. Laboratory and field evaluations of a repellent soap containing DEET and Permethrin against phlebotomine sand flies (Diptera: psychodidae) in Valle del Cauca, Colombia. Am J Trop Med Hyg 1995;52:169-73. [ Links ]
18. Weigle KA, Saravia NG, de Davalos M, Moreno LH, DAlessandro A. Leishmania braziliensis from the Pacific coast region of Colombia: foci of transmission, clinical spectrum and isoenzyme phenotypes. Am J Trop Med Hyg 1986;35:722-31. [ Links ]
19. Saravia NG, Weigle KA, Segura I, Giannini SH, Pacheco R, Labrada LA et al. Recurrent lesions in human L. braziliensis infection: reactivation or re-infection? Lancet 1990;336:398-402. [ Links ]
20. Saravia NG, Segura I, Holguin AF, Santrich C, Valderrama L, Ocampo C. Epidemiologic, genetic and clinical associations among phenotypically distinct populations of Leishmania (Viannia) in Colombia. Am J Trop Med Hyg 1998;59:86-94. [ Links ]
21. Travi BL, Montoya J, Solarte Y, Lozano L, Jaramillo C. Leishmaniasis in Colombia I: Studies on the phlebotomine fauna associated with endemic foci in the Pacific Coast region. Am J Trop Med Hyg 1988;39:261-6. [ Links ]
22. Weigle KA, Santrich C, Martinez F, Valderrama L, Saravia N. Epidemiology of cutaneous leishmaniasis in Colombia: A longitudinal study of the natural history, prevalence and incidence of infection and clinical manifestations. J Infect Dis 1993;168:699-708. [ Links ]
23. Weigle KA, Valderrama L, Arias AL, Santrich C, Saravia N. Leishmanin skin test standardization and evaluation of safety, dose, storage, longevity of reaction and sensitization. Am J Trop Med Hyg 1991;44:260-71. [ Links ]
24. Weigle KA, Santrich C, Martinez F, Valderrama L, Saravia N. Epidemiology of cutaneous leishmaniasis in Colombia: environmental and behavioral risk factors for infection, clinical manifestations, and pathogenicity. J Infect Dis 1993;168:709-14. [ Links ]
25. Rothman KJ, Greenland S. Modern epidemiology. Second edition. Philadelphia, Pennsylvania: Lippincott-Raven; 1998. [ Links ]
26. Kleinbaum DG, Kupper LL, Muller KE. Applied regression analysis and other multivariable methods. Second edition. Belmont, CA: Duxbury Press; 1988. [ Links ]
27. Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika 1986;73:13-22. [ Links ]
28. Menon, JN, Bretscher PA. Parasite dose determines the Th1/Th2 nature of the response to Leishmania major independently of infection route and strain of host or parasite. Eur J Immunol 1998;28:4020-8. [ Links ]
29. WHO. Thirteenth Programme Report. Progress 1995-96. (WHO/TDR). Geneva: WHO; 1996. [ Links ]
30. Tayeh A, Jalouk L, Al-Khiami AM. A cutaneous leishmaniasis control trial using pyrethroid-impregnated bednets in villages near Aleppo, Syria. World Health Organization 1997. WHO/LEISH/97.41, Geneva: WHO: 1997. [ Links ]
31. Choi HW, Breman JG, Teutsch SM, Liu S, Hightower AW, Sexton JD. The effectiveness of insecticide-impregnated bed nets in reducing cases of malaria infection: a meta-analysis of published studies. Am J Trop Med Hyg 1995;52:377-82. [ Links ]
32. Alexander JB. Dispersal of phlebotomine sand flies (Diptera: Psychodidae) in Colombian coffee plantations. J Med Entomol 1987;24:552-8. [ Links ]
33. Rojas CA. Evaluation of a multifaceted intervention to prevent the transmission of American cutaneous leishmaniasis in Colombia (thesis). Chapel Hill, NC: University of North Carolina; 1999. [ Links ]
34. Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-effectiveness in health and medicine. New York: Oxford University Press; 1996. [ Links ]
35. Rojas CA. An ecosystem approach to human health and the prevention of cutaneous leishmaniasis in Tumaco, Colombia. Cad Saude Publica 2001;17(Suppl.1):193-200. [ Links ]














