Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Biomédica
versão impressa ISSN 0120-4157versão On-line ISSN 2590-7379
Biomédica v.27 n.2 Bogotá abr./jun. 2007
Tuberculosis in patientes treated with tumor necrosis factor-alpha antagonists living in an endemic area. Is the risk worthwhile?
Adriana Rojas-Villarraga 1, 2, *, Carlos Andrés Agudelo 1, 2, *, Ricardo Pineda-Tamayo 1, 2, Alvaro Porras 3, Gustavo Matute 1, Juan Manuel Anaya 2, 4
1 Clínica Universitaria Bolivariana, Universidad Pontificia Bolivariana, Medellín, Colombia
2
Corporación para Investigaciones Biológicas, (CIB), Medellín, Colombia3
Centro Cardiovascular Colombiano-Clínica Santa María, Medellín, Colombia4
Facultad de Medicina, Universidad del Rosario, Medellín, Colombia* Both authors contributed equally to this work.
Recibido: 31/07/06; aceptado: 22/03/07
Tumor necrosis factor alpha antagonists (TNFA) are biological agents to treat chronic inflammatory and autoimmune diseases. However, their use is associated with an increased rate of tuberculosis, endemic mycoses, and intracellular bacterial infections. Since tuberculosis is moderately to highly endemic in Colombia, the risk of these infections in patients treated with TNFAs may be higher than previously reported in Colombia. Recently, four patients have developed tuberculosis during TNFA therapy. Tuberculosis appeared between 3 to 24 months after initiation of TFNA therapy and was independent of previous tuberculin skin test status. A review of the relevant literature and recommendations are presented as guides for surveillance and prophylaxis on a country-wide basis.
Key words: tuberculosis, tumor necrosis factor-alpha/ immunology, endemic diseases, Colombia
Tuberculosis en pacientes tratados con antagonistas del factor de necrosis tumoral alfa en un área endémica, ¿vale la pena el riesgo?
Los antagonistas del factor de necrosis tumoral alfa (infliximab, adalimumab y etanercept) son agentes biológicos utilizados en el tratamiento de enfermedades inflamatorias crónicas y autoinmunes. Sin embargo, su uso está asociado con el incremento de la tasa de tuberculosis, micosis endémicas e infecciones bacterianas intracelulares. Dado que la tuberculosis es moderada/altamente endémica en Colombia, el riesgo de esta infección en los pacientes tratados con estos agentes biológicos puede incrementarse y hacer dicha tasa mayor que la informada previamente (tanto en Colombia como en el mundo). Se presentan cuatro pacientes que desarrollaron tuberculosis durante el tratamiento con antagonistas del factor de necrosis tumoral alfa. La presentación de la tuberculosis ocurrió en promedio 15 meses después del inicio del agente biológico y fue independiente de la prueba de tuberculina. Se hace una revisión del tema y se plantea la necesidad de implementar guías y estrategias gubernamentales orientadas a la detección y profilaxis de tuberculosis en este grupo de pacientes.
Palabras clave: tuberculosis, factor de necrosis tumoral alfa/inmunología, enfermedades endémicas, Colombia
Tumor necrosis factor (TNF)-alpha antagonists (infliximab, adalimumab and etanercept) are biological agents designed to treat inflammatory and autoimmune diseases including rheumatoid arthritis (RA), ankylosing spondylitis (AS), psoriasis and Crohns disease, as TNF-alpha, a cytokine, plays an essential pathogenic role in these diseases (1-3). Patients with these disorders are unresponsive to conventional immuno-modulatory treatment; however, dramatic improvement has been demonstrated with the use of the TNF-alpha antagonist therapy. This use is associated with an increased rate of tuberculosis (TB), endemic mycoses, and intracellular bacterial infections (4). Since TB is moderately to highly endemic in Colombia, the risk of these infections in patients treated with these agents are predicted to be higher than previously reported elsewhere. Herein, four patients are presented who developed TB during TNF-alpha antagonist therapy. The relevant literature is reviewed, and guidelines for surveillance and prophylaxis are proposed. These patients were seen at the Unidad de Inmunología Clínica y Reumatología in the Clinica Universitaria Bolivariana, a third level clinic, in Medellin, Colombia. The total number of patients with RA seen in the Unit was approximately 440 out-patients, of whom 66 (15%) were under TNF-alpha antagonist therapy.
Case description (
table 1)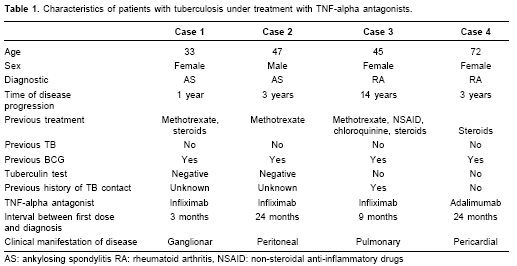
Case 1
A 33-year-old female patient, with a history of ankylosing spondylitis (AS) of one years duration and a recurring chronic anterior uveitis was treated successfully for both with prednisone (60 mg/day) and methotrexate at 15 mg intramuscular (IM) per week. She was then initiated on infliximab at 200 mg intravenous (IV) every 8 weeks and achieved complete recovery of her uveitis and of the Inflammatory back and axial pain.
After three months on infliximab therapy, she was admitted to the Rheumatology Unit of Bolivarian University Clinic (Medellín, Colombia) due to a 15-day condition consisting of a painful, developing cervical mass and of dysphagia that had not responded to oral treatment with antibiotics.
On physical examination, her temperature was 36.8°C, blood pressure 110/70 mm Hg, heart rate 70 beats per minute and respiratory rate 18 breaths per minute. In the right cervical region, a 3 cm diam erythematous adenopathy was found-it was hard, deeply rooted and painful. The examination was otherwise normal. Presumptive evidence for reactive syphilis was found in laboratory examinations, albeit at low titers; this was later confirmed by a FTA-ABS test. Neurosyphilis was ruled out by lumbar puncture results. Test for human immunodeficiency virus (HIV) infection (by Axsym assays, Abbott Laboratories, Chicago, USA) was negative. Blood count, coagulation tests, renal and liver function tests were within the normal range. A significant increase in reactive C protein (9.8 mg/dl, VN: 0-1) was noted. Chest X-ray was normal and cervical tomography showed a conglomerate of cervical adenopathies in the right jugular groove. A fine-needle aspiration biopsy on the palpable adenopathy showed lymphocytic hyperplasia. The biopsy by excision was compatible with chronic caseous granulomatous inflammation (
figure 1). Stains for acid-fast bacilli (Ziehl Nielsen) and for fungus (methenamine silver) were negative, but later Mycobacterium cultures proved positive for M. tuberculosis.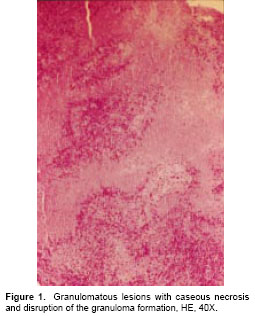
The patient was sent to the public health tuberculosis program for approppiate treatment. The immunosuppressant therapy was not reinitiated, given that her clinical uveitis had gone into complete remission and articular symptoms were absent.
Case 2
A 47-year-old male was given a diagnosis of AS of three years duration. He had no clinical response to methotrexate treatment at 15 mg IM per week and indomethacin at 150 mg/day. Therefore, infliximab was initiated at 200 mg IV every 8 weeks. The patient had a negative tuberculin skin test (TST) done three months before starting treatment with infliximab.
He was admitted to our Rheumatology Unit with a history of excessive bowel movements, and mucus diarrhea, fever, nocturnal diaphoresis, facial swelling, loss of appetite and weight loss of 7 kg in 20 days. Physical examination indicated a temperature of 37.5 °C, blood pressure of 120/80 mm Hg, heart rate of 100 beats per min and respiratory rate of 16 breaths per min. Abdominal palpation produced pain, without peritoneal irritational signs. The remainder of the physical examination was normal.
Laboratory tests showed a significant increase in lactic dehydrogenase (1,398 units/ml. VN: 313-618) and in C reactive protein (11 mg/dl, VN: 0-1). Hematologic laboratory values showed only a slight thrombocytosis (507,000 cells/µl, VN: 150,000-450,000). The results of liver and renal function tests, and the levels of serum electrolytes were within the normal range. Stool tests were normal. He tested negative for HIV.
Chest X-ray showed alveolar infiltrates in the lower left lobe and a small pleural effusion. An abdominal sonography revealed splenomegaly and free liquid in the cavity making possible a needle-guided paracentesis. Ascitic fluid was obtained which showed pH: 7.56, albumin: 2.0 g per dl, white-cell count of 3,040 cells/µl, 95% of which were lymphocytes. The albumin gradient was 1.3 mg/dl. The cultures were negative for aerobic bacteria, fungi, or mycobacteria. An upper gastrointestinal endoscopy and a colonoscopy failed to reveal pathologic changes. Consequently, an abdominal computerized axial tomography was done, in which an increase in fat density and adenomegalies were observed at the root of the mesentery. In order to rule out a neoplastic or granulomatous disease, a guided laparoscopic peritoneal biopsy was done. This procedure revealed a chronic caseous granulomatous inflammation (
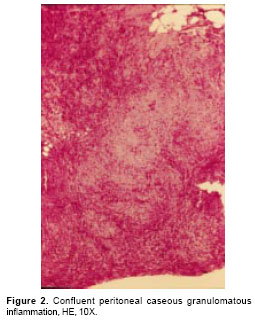
Case 3
A 45-year-old female patient was presented with a 14 years history of RA. She recently had been treated with prednisone at 15 mg/day, naproxen at 500 mg twice a day, methotrexate at 15 mg PO per week, and chloroquinine at 250 mg/day PO. Previously, intolerance to sulfasalazine had been recorded. Despite this regimen of treatment, she persisted with a high articular index (>10). Due to the refractory nature of the disease, treatment with infliximab was started at 200 mg IV every eight weeks. She received this treatment for ten months with an excellent clinical response (ACR70).
During the follow up, she presented a month-long febrile condition with tachycardia, migraine headaches and lumbar pain. In addition, left knee effusion and arthralgia of the wrists and left elbow was detected. She was then admitted to the Rheumatology Unit as an in-patient for studies of prolonged febrile syndrome. On admission, she denied respiratory symptoms. A left knee arthrocentesis was done, ruling out pyogenic arthritis.
Laboratory testing showed lymphopenia (770 cells/µl, VN: 900-5000) and a significant increase in C reactive protein (7.7 mg/dl) and sedimentation rate (70 mm/h). Liver and renal function tests were within the normal ranges. She tested negative for HIV, fungi, and cytomegalovirus infection. Synovial fluid smear, blood cultures for aerobic bacteria, fungi and Mycobacterium were negative.
An abdominal sonography revealed splenomegaly. Chest x-ray showed a range of sizes of poorly defined, bilateral alveolar opacities, situated mainly in the upper lobes, along with liquid in the posterior pleural cavity (figure 3A). A high resolution computed tomography of the thorax (figure 3B) showed poorly defined pulmonary nodules with spiculated lesions, between 2 mm and 1 cm in size, and surrounding pneumonitis mainly located in the upper lobes. In addition, interstitial lesions were seen as well as enlargement of the posterior pleural and multiple small, irregular, peripheral nodules.
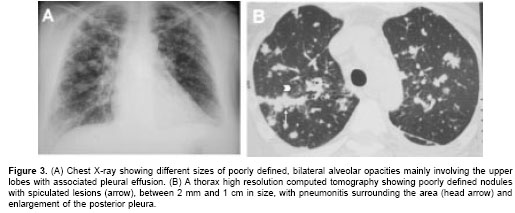
A fiberoptic bronchoscopy was performed to obtain a bronchoalveolar lavage (BAL) and a transbronchial biopsy. The latter was insufficient for an adequate analysis, but BAL was positive for acid-fast bacilli (AFB) and M. tuberculosis grew in the cultures. Anti-TB treatment was initiated. Infliximab was suspended and methotrexate temporarily suspended, given the low autoimmune disease activity, and to avoid the hepatotoxicity of the association with isoniazid. She was released to continue treatment on an outpatient basis.
Case 4
A 72-year-old female patient was presented with a two-month history of dyspnea, cough, and thoracic pain. She was under treatment with digoxin, metoprolol, enalapril, furosemide and spironolactone for arterial hypertension, auricular fibrillation and left ventricular hypertrophy. In addition, she had a three-year history of RA, treated for the last two years with adalimumab (previous use of methotrexate and low dose steroids were considered inadequate).
Chest x-rays showed alveolar infiltrates and central calcified granulomas. A computed tomography scan of the paranasal cavities showed chronic sinusitis. A fungal diagnosis was ruled out by biopsy and appropriate smears. The patient received treatment with levofloxacine.
One month later, she was readmitted due to auricular fibrillation with a rapid ventricular reaction and fever. She was treated with cefepime and amikacin because of a suspicion of partially treated pneumonia. A slight pericardial effusion was found in a transthoracic echocardiogram, and pulmonary thromboembolism was confirmed by a ventilation-perfusion scintigraphy. Anticoagulation was given and she was released with oral antibiotics.
She was readmitted two weeks later because of a sudden episode of dyspnea, asthenia, adynamia, fever and diaphoresis. High sedimentation rate (62 mm/h) and C-reactive protein (13 mg/dl) were found, accompanied by normocytic and normochromic anemia (10.1 g/dl) and negative blood cultures for bacteria. An increased heart size on a chest radiograph was recorded. A new echocardiogram showed an increase of pericardial effusion. Thickening and pericardial effusion, medias-tinal adenopathies and a calcified granuloma in the lower left lobe were observed in a high resolution thorax computed tomography (
figure 4). A pericardial biopsy was taken that disclosed caseous necrosis and AFB in the Ziehl Nielsen stain. Treatment for pericardial TB was started. The patient developed lymphopenia (438 cells/µl, VN: 1,000-5,000). Prednisone treatment at 60 mg/day was initiated, and a few days later the patient was released to continue treatment under the public health TB program. Adalimumab was suspended.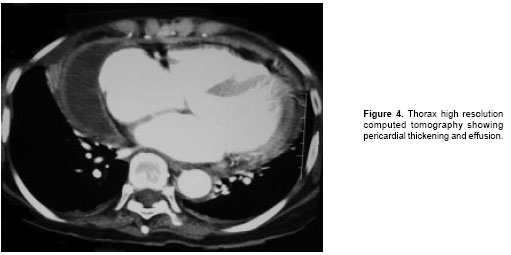
Discussion
Tuberculosis is a serious disease in Colombia. Its rapid growth is encouraged by poor social-economic conditions, the low quality of the public health programs and, recently, by the HIV epidemic. At present, a new population of patients is being threatened. As a result of recent advances in the medical sciences, the new TNF-alpha antagonists are revolutionizing treatment for patients with chronic inflammatory and autoimmune diseases and noticeably improving the state of their health and quality of life. Nevertheless, such treatment has side effects, one of the most troubling is the reactivation of TB. Here, four patients are presented who developed severe TB disease during TNF- alpha antagonist therapy. The bases of the problem are reviewed and the status of the current situation is summarized.
TNF alpha and inflammation
TNF-alpha is a cytokine that plays a critical role in the regulation of inflammatory processes and takes a part in apoptosis, cell activation, cell recruitment and differentiation. It is produced primarily by macrophages in response to stimuli activating toll-like receptors, but can also be expressed by activated T cells, B cells, and NK cells (5). This cytokine has a role in septic shock and acute respiratory distress syndrome (ARDS); in addition, it has been implicated in the pathogenesis of chronic processes such as autoimmunity, graft-versus-host disease, Crohns disease, and cachexia accompanying cancer, as well as the acquired immunodeficiency syndrome. TNF has also been implicated in insulin resistance, endothelial activation, congestive heart failure, atherosclerosis, pancreatitis, and alcohol-induced liver injury, among others (6,7).
TNF alpha is present at biologically significant levels in RA synovial tissue and fluid. Furthermore, the level of TNF alpha in the synovium seems to parallel the extent of both inflammation and bone erosion. TNF triggers several of the most central and critical events in the acute and chronic synovial inflammation, as well as the ultimate tissue destruction characteristic of RA, including induction of multiple additional cytokines and chemokines, expression of adhesion molecules and synovial neoangiogenesis (8). TNF alpha is highly expressed at the site of inflammation in AS-although it does not seem to be elevated systemically, but rather, highly expressed locally at the primary site of inflammation, such as the sacroiliac joint (9).
TNF-alpha and TB
TNF-alpha is an essential component in the processes leading to an effective host response against TB. It is necessary for optimal coordination of the two aspects of TB immunity: (1) the adequate differentiation of specific T cells to secrete an appropriate Th1 cytokine profile and (2) the development of granulomas in which activated epithelioid macrophages restrict mycobacterial growth and dissemination (5). A synergistic activity occurs between interferon (IFN) gamma, one of the central mediator of Th1 type response, and TNF-alpha in the activation of macrophages. The activation induces macrophage differentiation into epithelioid cells and increases their phagocytic capacity (10,11).
The formation of granulomas at the site of mycobacterial infection is an essential component of host immunity designed to control infection. This process is dependent on the activation of mycobacteria-reactive T lymphocytes, particularly IFN -secreting CD4 and CD8 T cells. Granuloma formation, however, is a complex process that requires not only the activation of the lymphocytes, but also their recruitment with monocytes to the site of the infection, migration into the tissues, and juxtaposition around mycobacteria-infected macrophages (12). TNF alpha is required for the early induction of chemokines that initiates timely cell recruitment as well as the establishment and maintenance of the microenvironment in the protective granulomas (12).
In the absence of TNF-alpha, the granuloma becomes disorganized and diffuse, with the development of caseous lesions and an incapacity to contain the bacteria (a sign of the disruption of the protecting mechanism) and concomitant dissemination of the infection (5,10). The importance of this cytokine in the expression of the HIV mediated by nuclear factor kappa beta during co-infection with TB is also known (13,14).
TNF-alpha antagonists
Three TNF-alpha antagonists for the treatment of RA (infliximab, adalimumab and etanercept) have been approved in the last several years. Infliximab has been approved also for the treatment of psoriatic arthritis (PsA) and Crohns disease, and etanercept for the treatment of PsA.
Etanercept is a soluble TNF-receptor fusion protein and is composed of two dimers. Each has an extracellular, ligand-binding portion of the higher-affinity type 2 TNF receptor (p75) linked to the Fc portion of human IgG1 (15). Infliximab is a chimeric monoclonal antibody (murine-binding VK and VH domains, with human constant Fc domains) that combines with TNF-alpha with great affinity and thereby impairs the binding of TNF alpha to its receptors. It also kills cells that express TNF alpha through antibody- dependent and complement-dependent cytotoxicity (16). Adalimumab, the latest TNF-alpha antagonist, is a recombinant monoclonal antibody that binds TNF-alpha and also causes lymphocyte apoptosis and rupture of the cells that express TNF-alpha on their surface (17).
The value of TNF-alpha antagonist therapy in RA is its ability to (a) decrease the acute phase reactants in serum, (b) reduce lymphocyte infiltration in the synovium, (c) inhibit the cascade of proinflamatory cytokines, (d) deactivate the endothelium, modulate chemotaxis at the sinovium level (18) and (e) decrease levels of soluble leukocyte-endothelial adhesion molecules (IL-1 and IL-6) at the systemic level. Multiple studies have shown that these medications given either as single-therapy or as combined-therapy allow a rapid, significant, and sustained clinical improve-ment, as well as a decrease in the radiological progression of RA.
The effectiveness of TNF-alpha antagonist therapy has also been demonstrated for Crohns disease (19), PsA (20), AS (9), some types of vasculitis (21) and uveitis (22). Variable success has been reported for sarcoidosis (23) and it has proven an unsuccessful treatment in Sjogrens syndrome (24,25), as well as an association with moderate to severe chronic heart failure (26).
TNF-alpha antagonists and TB
Since the introduction of TNF-alpha antagonists, an increase in the cases of TB within the treated population has been observed. In the United States. TB rates have increased from 6.2 per 100,000 in the general population to 144 per 100,000 in patients who are receiving treatment with infliximab and 35 per 100,000 in those who receive etanercept (27). In Spain, the estimated incidence of TB associated with infliximab in RA patients was 1,893 per 100,000 in the year 2000, compared with a previous incidence of 21 per 100,000 in the general population and 95 per 100,000 among RA patients not exposed to TNF-alpha antagonist therapy (28). A world rate of 47.6 per 100,000 for infliximab and 20.7 per 100,000 for etanercept has been calculated (29). Soluble TNF-alpha mainly interacts with TNFR1 (TNF-alpha receptor), while membrane-bound TNF-alpha exerts its actions via TNFR2. TNFR1 is essential for both granuloma formation and containment of intracellular pathogens, whereas TNFR2 plays a less significant role (4). This difference partially explains the varying infectious risk between infliximab and etanercept, and is in accord with the observation that infliximab is more useful than etanercept in treating granulomatous diseases such as Crohns disease and sarcoidosis (4).
Quite probably, the majority of the TB cases induced by the use of TNF-alpha antagonist represent reactivation of a latent condition. Usually, reactivation occurs within the first two to five months, corresponding to three doses of treatment (30), although this complication has been reported to occur up to 20 months after treatment with etanercept. In our series, we observed three TB cases under infliximab treatment; they appeared within a time interval between the first dose and the TB diagnosis of three to ten months. With adalimumab, this occurred after 24 months of treatment. In some studies, the majority of the patients came from countries with a low incidence of TB, such as the United States and Europe (28,30). Nevertheless, eight to twelve of the patients reported in the state of California (United States) between 2002 and 2003 were originally from countries where TB is prevalent (31).
The disease manifestations in this group of patients has been shown to be similar to that of other immunosuppressed patients, with the majority being extrapulmonary forms (56%-65%) and several disseminated forms (24%); mortality rates are between 5%-11% (28,30). The unusual manifestations induced by the TNF-alpha antagonist possibly accounts for the delay in diagnosis of TB, since these delays occur in cases of pulmonary TB in patients with RA and ileocaecal TB in patients with Crohns disease. For these cases, accurate diagnosis may be difficult because of the inability to differentiate degree of pulmonary involvement in the RA cases and TB from Chrohns relapse in the latter cases (32,33). The main clinical manifestation of disease in our series was extrapulmonary, and consisted of two cases with infliximab (cervical and abdominal lymphadenopathy) and one case with adalimumab (pericardial). The other patient, who was treated with infliximab, had a pulmonary manifestation of TB disease (table 1).
A series of recommendations for TB screening and treatment of latent infection and active TB in those patients have been generated. The Centers for Disease Control in Atlanta (United States) has recommended the following, complete checklist of risk factors for M. tuberculosis infection in all patients before initiating TNF-alpha antagonists: (a) birth in a country where TB is prevalent or (b) history residence in a congregate setting (e.g., jail or prison, homeless shelter, or chronic-care facility), (c) a positive tuberculin skin test (TST) result, (d) substance abuse (injection or otherwise), (e) health-care employment in settings with TB patients, and (f) chest radiographic findings consistent with previous TB (31).
Application of the Purified protein derivative (PPD) TST is the only widely used method to detect latent tuberculosis infection (LTBI) and is dependent on a normal T cell function. In RA, the T cell function is altered and may result in an inability to develop an adequate PPD reaction. Several authors have demonstrated that a PPD skin test is not appropriate for recognizing LTBI in patients with RA in a specific population (34).
Although false-positive results can occur in countries where BCG vaccine is given after infancy, Gardam et al (29) have recommended a conservative approach in the setting of TNF alpha antagonists, whether or not BCG vaccination was the cause of a positive skin test. They also recommend that patients who are to start an immunosuppressive therapy or who have impaired immunity due to illness should undergo TST before therapy or as soon as possible after the diagnosis of the immunosuppressive disease. The will keep to a minimum the risk of false-negative reactions due to anergy (29).
Treatment for LTBI should be initiated for immunosuppressed patients with a TST result of >5 mm and/or chest radiograph findings suggestive of past TB. This treatment is also recommended for patients with a negative TST in whom the clinical and epidemiological circumstances suggest M. tuberculosis infection. The treatment suggested by the Centers for Disease Control and the Spanish Rheumatology Society (35) is isoniazid at 300 mg per day for nine months. In some highly endemic countries, a six month treatment with LTBI is recommended (36). The time for initiating biological therapy has not been defined but it is preferable to start it once the treatment with INH has been completed. In any case, TNF-alpha antagonist must not be the first step in treatment. Preferably TNF-alpha antagonist therapy should is not to be initiated during INH prophylaxis, since patients can develop TB under this treatment (37). Recommendations based on the treatment of LTBI in patients with HIV indicate that it is not necessary to continue treatment with isoniazid even if the immunosuppressant treatment has to continue for a long time (38). Liver toxicity caused by isoniazid may be enhanced in these patients, probably due to concomitant treatment with other drugs such as methotrexate and NSAIDs (non steroidal anti-Inflammatory drugs).The combination use of pyrazinamide and rifampicin has not been recommended due to the excessive risk of hepatotoxicicity.
It has been demonstrated that strategies to treat LTBI that are tailored to the at-risk population can effectively and safely lessen the likelihood of active TB in patients treated with TNF-alpha antagonist therapy, decreasing the TB rate by 78% (35). Noteworthy, TB disease can occur despite the above recommendations for the treatment of LTBI. This is not very surprising, since the evidence indicates that 9 months of treatment with isoniazid does not fully protect against the development of active TB. Although the decrease in the rate is ~70%, this figure rises to approximately 90% in patients who are compliant with the medication (39).
Active TB must always be considered a potential cause of febrile or respiratory disease in patients who are receiving TNF-alpha antagonist therapy. Because it blocks of the cytokine activity, manifestations of TB disease in this group of patients may be more subtle. Chest X-ray and sputum cultures are recommended in all cases (32).
The British Thoracic Society guidelines recommend that a full course of standard anti-TB therapy be completed prior to the initiation of TNF-alpha antagonist therapy if the patient is found to have active TB. However, under some circumstances, it can be initiated in consultation with other specialists caring for TB patients (40). Treatment for TB is to be given for at least 2 months (with full compliance), or until the drug susceptibility profile of the organism is known, prior to commencing TNF-alpha antagonist therapy (40). If active TB is found during TNF-alpha antagonist therapy, the treatment must be adjusted to that defined by the TB program; furthermore the TNF-alpha antagonist therapy must be suspended at least until anti-TB treatment has been initiated and the patients condition has improved (31). However, some professionals recommend the resumption of TNF-alpha antagonist therapy be avoided in patients who had developed TB under its use (41). Table 2 summarizes expert panel committees (evidence based recommendation Class IV), including the British Thoracic Society (40), California County Health Department and American Thoracic Society/CDC, United States (31), as well as other important reviews (29,32). The main recommendations are listed for the prevention and treatment of those TB cases associated with the use of TNF-alpha antagonist therapy.

A rate of 11.8% and 2.4% of global and multidrug resistant TB has been described recently in Colombia, respectively (42). Therefore, health care policies must ensure the best possible strategy for the management and prevention of multidrug-resistant TB in association with control campaigns in low and middle income countries.
Concluding remarks
The introduction of TNF-alpha antagonist therapy in Colombia with the concomitant appearance of associated TB cases has set off the alarms about what may be an increasing problem. One previous case report has been made in Colombia, which described a TB pulmonary reactivation case related to infliximab therapy (43). Positive results in TST greater than 40% (44) and the TB profile denoted a situation consisting of a highly endemic country with an incidence of 50/100,000, a prevalence of 75/100,000 and mortality of 8.0 deaths/100,000/yr (45). This is associated with the growing use of TNF-alpha antagonists therapy. This therapy can increase the reactivation of a latent condition and also a presentation of newly active TB.
In high endemic areas of M tuberculosis infection such as Peru (34), TST is not recommended for the recognition of LTBI in patients with RA. This is a policy that is recommended for application in all highly endemic areas, including Colombia. As shown in table 1, the usefulness of TST prior to initiation of the TNF-alpha antagonist does not have unanimous agreement among rheumatologists. Other more sensitive diagnostic tests exist for the identification of LTBI (QuantiFERON-TB Gold test, T SPOT-TB). These are based upon detection of interferon-gamma released by T-cells in response to M. tuberculosis-specific antigens early secretory antigenic target 6 (ESAT-6) and culture filtrate protein-10 (CFP-10). However, they are not yet routinely available and their validation awaits confirmation in larger studies (46-48).
Clear guidelines are necessary as shown by case reports from highly endemic areas. Formal screening for latent infection is recommended for patients who are candidates for TNF-alpha antagonist therapy. This approach will detect the clinical cases amongst the different presentation forms, and will allow the selection of an appropriate treatment of active and latent cases. The four cases presented herein permitted the illustration of the special characteristics and progression of the agonist treatment problem. These will be useful for encouraging health authorities and rheumatology expert committees to delineate policies for detecting and prevention of this serious complications in cases treated with TNF-alpha antagonists.
The Asociación Colombiana de Reumatología has published recommendations to treat rheumatoid arthritis (49,50) and spondiloartropathies (51) with a short section focusing on the use of anti TNF therapy in these pathologies. These recommendations indicate that prior to the initiation of anti TNF therapy, the TST and chest X Ray must be done, and recommend the avoidance of anti TNF treatment in the case of latent TB or active TB infection. However, neither a clear discussion nor mandate is implicit in these recommendations.
Concerning the rate of anti TNF therapy used in Colombia, one report (52) indicates that rheumatologists initiate anti-TNF therapy as second line drug treatment in 16% of patients with rheumatoid arthritis.
Subsequent to the submission of this manuscript, two additional cases were observed (Rojas-Villarraga A, Agudelo CA, Pineda-Tamayo R, Porras A, Matute G, Anaya JM. Tuberculosis en pacientes con enfermedades autoinmunes en tratamiento con antagonistas del factor de necrosis tumoral alfa. Resumen. Acta Med Colomb 2006;31(Suppl.):313), one under adalimubab treatment for AS and another under etanercept treatment for RA. However, because studies on RA prevalence in Colombia or obligatory report of TB cases in these patients have not been done, an objective calculation of ratio or likelihood of being helped vs. harmed is precluded.
Finally, new developments are continuously occurring in the field of biological therapy. The increasing numbers of therapies for the modulation of inflammatory response can bring the patients enormous benefits; however, they can also generate problems that remain to be discovered.
Acknowledgments
We thank the staff members of the Clínica Universitaria Bolivariana for their help, the colleagues who allowed us access to the care of their patients, and Ángela Restrepo for a critique of our views. The authors declare that they have no potential conflict of interest and any source of financing to this work.
Correspondencia:
Juan-Manuel Anaya, Corporación para Investigaciones Biológicas, Cra 72ª No. 78 B-141, Medellín, Colombia.
References
1. Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ. 2003;10:45-65. [ Links ]
2. Camargo JF, Delgado A, Tobón GJ, Anaya JM. Citoquinas, quimioquinas y osteoinmunología. En: Artritis reumatoide. Bases moleculares, clínicas y terapéuticas. En: Anaya JM, Pineda-Tamayo R, Gómez LM, Galarza-Maldonado C, Rojas-Villarraga A, Martín J, editores. Primera edición. Medellín: Corporación para Investigaciones Biológicas; 2006.p. 95-116. [ Links ]
3. Monteleone I, Vavassori P, Biancone L, Monteleone G, Pallone F. Immunoregulation in the gut: success and failures in human disease. Gut. 2002; 50 (Suppl.3):iii60-4. [ Links ]
4. Crum NF, Lederman ER, Wallace MR. Infections associated with tumor necrosis factor- antagonists. Medicine (Baltimore). 2005;84:291-302. [ Links ]
5. Ehlers S. Role of tumor necrosis factor (TNF) in host defense against tuberculosis: implications for immunotherapies targeting TNF. Ann Rheum Dis. 2003; 62 (Suppl.2):ii37-42. [ Links ]
6. Tracey KJ, Cerami A. Tumor necrosis factor: pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491-503. [ Links ]
7. Papadakis KA, Targan SR. Tumor necrosis factor: biology and therapeutic inhibitors. Gastroenterology. 2000;119:1148-57. [ Links ]
8. Fox DA. Cytokine blockade as a new strategy to treat rheumatoid arthritis. Arch Intern Med. 2000;160:437-44. [ Links ]
9. Gorman JD, Sack KE, Davis JC Jr. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med. 2002;346:1349-56. [ Links ]
10. Kaufmann SH. Protection against tuberculosis: cytokines, T cells, and macrophages. Ann Rheum Dis. 2002;61(Suppl.2):ii54-8. [ Links ]
11. van Crevel R, Ottenhoff TH, van der Meer JW. Innate immunity to Mycobaterium tuberculosis. Clin Microbiol Rev. 2002;15:294-309. [ Links ]
12. Roach DR, Bean AG, Demangel C, France MP, Briscoe H, Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168:4620-7. [ Links ]
13. Osborn L, Kunkel S, Nabel GJ. Tumor necrosis factor a and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa b. Proc Natl Acad Sci USA. 1989;86:2336-40. [ Links ]
14. Toossi Z, Mayanja-Kizza H, Hirsch CS, Edmonds KL, Spahlinger T, Hom DL, et al. Impact of tuberculosis (TB) on HIV-1 activity in dually infected patients. Clin Exp Immunol. 2001;123:233-8. [ Links ]
15. Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, et al. A trial of etanercept, a recombinant tumor necrosis factor receptor: Fc protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999; 340:253-9. [ Links ]
16. Scallon BJ, Moore MA, Trinh H, Knight DM, Ghrayeb J. Chimeric anti-TNFa monoclonal antibody cA2 binds recombinant transmembrane TNF-a and activates immune effector functions. Cytokine. 1995;7:251-9. [ Links ]
17. Salfeld J, Kaymakcalan Z, Tracey D, Roberts A, Kamen R. Generation of fully human anti-TNF antibody D2E7. Arthritis Rheum. 1998;41(Suppl.):S57. [ Links ]
18. Criscione LC, St Clair EW. Tumor necrosis factor–a antagonists for the treatment of rheumatic diseases. Curr Opin Rheum. 2002;14:204-11. [ Links ]
19. Sands BE, Anderson FH, Bernstein CN, Chey WY, Feagan BG, Fedorak RN, et al. Infliximab maintenance therapy for fistulizing Crohn´s disease. N Engl J Med. 2004;350:876-85. [ Links ]
20. Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy and effect on disease progression. Arthritis Rheum. 2004;50:2264-72. [ Links ]
21. Hoffman GS, Merkel PA, Brasington RD, Lenschow DJ, Liang P. Anti-tumor necrosis factor therapy in patients with difficult to treat Takayasu arteritis. Arthritis Rheum. 2004;50:2296-304. [ Links ]
22. Murphy CC, Ayliffe WH, Booth A, Makanjuola D, Andrews PA, Jayne D. Tumor necrosis factor alpha blockade with infliximab for refractory uveitis and scleritis. Ophthalmology. 2004;111:352-6. [ Links ]
23. Baughman RP, Iannuzzi M. Tumour necrosis factor in sarcoidosis and its potential for targeted therapy. BioDrugs. 2003;17:425-31. [ Links ]
24. Sankar V, Brennan MT, Kok MR, Leakan RA, Smith JA, Manny J, et al. Etanercept in Sjogren syndrome: a twelve-week, randomized, double blind, placebo-controlled pilot clinical trial. Arthritis Rheum. 2004; 50:2240-5. [ Links ]
25. Pessler F, Monash B, Rettig P, Forbes B, Kreiger PA, Cron RQ. Sjogren syndrome in a child: favorable response of the arthritis to TNFalpha blockade. Clin Rheumatol 2006;25:746-8. [ Links ]
26. Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL). Circulation. 2004;109:1594-602. [ Links ]
27. Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infections diseases associated with tumor necrosis factor antagonists. Clin Infect Dis. 2004;38:1261-5. [ Links ]
28. Gómez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD, BIOBADASER Group. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum. 2003;48:2122-2. [ Links ]
29. Gardam MA, Keystone EC, Menzies R, Manners S, Skamene E, Long R, et al. Anti tumor necrosis factor agents and tuberculosis risk: mechanism of action and clinical management. Lancet Infect Dis. 2003;3:148-55. [ Links ]
30. Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associate with infliximab, a tumor necrosis factora-neutralizing agent. N Engl J Med. 2001;345;1098-104. [ Links ]
31. Centers for Disease Control and Prevention (CDC). Tuberculosis associated with blocking agents against necrosis factor-alpha. California 2002-2003. MMWR Morb Mortal Wkly Rep. 2004;53:683-6. [ Links ]
32. Long R, Gardam M. Tumor necrosis factor-a inhibitors and the reactivation of latent tuberculosis. CMAJ. 2003;168:1153-6. [ Links ]
33. Wagner TE, Huseby ES, Huseby JS. Exacerbation of Mycobacterium tuberculosis enteritis masquerading as Crohn´s disease after treatment with a tumor necrosis factor-a inhibitor. Am J Med. 2002;112:67-9. [ Links ]
34. Ponce de Leon D, Acevedo-Vasquez E, Sanchez-Torres A, Cucho M, Alfaro J, Perich R, et al. Attenuated response to purified protein derivative in patients with rheumatoid arthritis: study in a population with a high prevalence of tuberculosis. Ann Rheum Dis. 2005;64:1360-1. [ Links ]
35. Carmona L, Gómez-Reino JJ, Rodríguez-Valverde V, Montero D, Pascual-Gomez E, Mola EM, et al. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum. 2005;52:1766-72. [ Links ]
36. Da Rocha G. Rheumatoid arthritis and tuberculosis in the tumor necrosis factor inhibitors era: observations from Brazil. J Clin Rheumatol. 2005;11:344-6. [ Links ]
37. Den Broeder A, Vonkeman H, Creemers M, De Jong E, Van de Laar M. Characteristics of Tuberculosis during Anti-tnf Treatment in RA Patients in The Netherlands and the Influence of Pre-Treatment Screening and Treatment. Arthritis and rheumatism. Ann Rheum Dis. 2005;52:S342-3. [ Links ]
38. Hamilton CD. Tuberculosis in the cytokine era: What rheumatologists need to know. Arthritis Rheum. 2003;48:2085-91. [ Links ]
39. Cohn DL, OBrien RJ. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49:1-54. [ Links ]
40. Ledingham J, Wilkinson C, Deighton C. British Thoracic Society (BTS) recommendations for assessing risk and managing tuberculosis in patients due to start anti-TNF-alpha treatments. Rheumatology. (Oxford) 2005;44:1205-6. [ Links ]
41. Mariette X, Salmon D. French guidelines for diagnosis and treating latent and active tuberculosis in patients with RA treated with TNF blockers. Ann Rheum Dis. 2003;62:791. [ Links ]
42. Ministerio de la Protección Social. Dirección General de Salud Pública. Actualización análisis de la situación de la tuberculosis en Colombia. Noviembre de 2006. Consultado: 5 de diciembre de 2006. Disponible en: http://www.minproteccionsocial.gov.co/VBeContent/library/documents/DocNewsNo15417DocumentNo2660.PDF [ Links ]
43. Martínez JV, Medina Y, Parga R, Restrepo JF, Iglesias-Gamarra A, Rondón F. Reactivación de tuberculosis pulmonar (TBC) con el uso de antagonistas del factor de necrosis tumoral alfa (FNTa) en artritis reumatoide. A propósito de un caso. Rev Colomb Reumatol. 2005;12:54-61 [ Links ] 44. Gomez LM, Anaya JM, Martin J. Genetic influence of PTPN22 R620W polymorphism in tuberculosis. Hum Immunol. 2005;66:1242-7. [ Links ] 45. World Health Organization. Global tuberculosis database. Brief country profile for Colombia for 2004. [Consultado: 5 de mayo de 2006]. Disponible en:
46. Taggart EW, Hill HR, Ruegner RG, Litwin CM. Evaluation of an in vitro assay for interferon gamma production in response to the Mycobacterium tuberculosis-synthesized peptide antigens ESAT-6 and CFP-10 and the PPD skin test. Am J Clin Pathol. 2006; 125:467-73. [ Links ]
47. Meier T, Eulenbruch HP, Wrighton-Smith P, Enders G, Regnath T. Sensitivity of a new commercial enzyme-linked immunospot assay (T SPOT-TB) for diagnosis of tuberculosis in clinical practice. Eur J Clin Microbiol Infect Dis. 2005;24:529-36. [ Links ]
48. Mazurek GH, Jereb J, Lobue P, Iademarco MF, Metchock B, Vernon A et al. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection. MMWR Recomm Rep. 2005;54:49-55. [ Links ]
49. Londoño JD, Rueda J, Díaz M, Uribe O, González H, et al. Asociación Colombiana de Reumatología. Recomendaciones del comité de expertos de la Asociación Colombiana de Reumatología para el empleo de terapia bloqueadora del factor de necrosis tumoral en artritis reumatoide. Rev Colomb Reumatol. 2001;8:296-302 [ Links ]
50. Londoño JD, Saibi DL, Anaya JM, Caballero CV, Moline JF, Díaz M, et al. Asociación Colombiana de Reumatología. Normatización para la administración y el seguimiento de los agentes biológicos y de quimioterapia usados en reumatología. Rev Colomb Reumatol. 2004;11:141-9. [ Links ]
51. Valle-Oñate R, Jáuregui E, Otero W, Vélez P, Salas J, Uribe O, et al. Asociación Colombiana de Reumatología. Recomendaciones del Comité de Expertos de la Asociación Colombiana de Reumatología para el empleo de terapia biológica en las espondiloartropatías, Guía Académica. Rev Colomb Reumatol. 2005;12:95-106. [ Links ]
52. Caballero C, Londoño JD, Chalem P. Tratamiento de la artritis reumatoide en Colombia: aplicación práctica de los conceptos teóricos por parte de los reumatólogos colombianos. Rev Colomb Reumatol. 2002;9:242-50. [ Links ]














