Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biomédica
Print version ISSN 0120-4157On-line version ISSN 2590-7379
Biomédica vol.27 no.3 Bogotá July/Sept. 2007
Rickettsial infection in capybaras (Hydrochoerus hydrochaeris) from São Paulo, Brazil: serological evidence for infection by Rickettsia bellii and Rickettsia parkeri
Richard C. Pacheco 1, Mauricio C. Horta 1, Jonas Moraes-Filho 1, Alexandre C. Ataliba 1, Adriano Pinter 1, 2, Marcelo B. Labruna 1
1 Departamento de Medicina Veterinária Preventiva e Saúde Animal, Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo, São Paulo, Brasil
2
Superintendência de Controle de Endemias (Sucen), São Paulo, BrasilThis study was carried out in the Faculty of Veterinary Medicine of the University of São Paulo, Brazil
Recibido: 01/03/07; aceptado: 11/05/07
Introduction.
In Brazil, capybaras (Hydrochoerus hydrochaeris) are important hosts for Amblyomma ticks, which in turn can transmit rickettsiae to humans and animals. Therefore, capybaras are potential sentinels for rickettsial infection.Objective. The present study evaluated rickettsial infection in capybaras in different areas of the state of São Paulo, where rickettsiosis has never been reported.
Materials and methods. Blood sera from 73 capybaras from six localities in São Paulo were tested by indirect immunofluorescence assay using Rickettsia rickettsii, Rickettsia parkeri, and Rickettsia bellii antigens. Capybara spleens were tested by PCR, targeting a fragment of the rickettsial gltA gene. Ticks were collected from each capybara sample and taxonomically identified to species.
Results. A total of 94 positively reacting capybara samples, 19 (26.0%), 25 (34.2%), and 50 (68.5%) capybara sera reacted to R. rickettsii, R. parkeri, and R. bellii, respectively. Twenty-five capybara sera showed titers to R. bellii at least four-fold higher than to any of the other two antigens. These sera were considered homologous to R. bellii. Using the same criteria, 3 capybara sera were considered homologous to R. parkeri. No sera were be considered homologous to R. rickettsii. No rickettsial DNA was detected in capybara spleen samples. Ticks collected on capybaras were Amblyomma dubitatum and Amblyomma cajennense.
Conclusions. The first evidence is reported of R. bellii natural infection in vertebrate hosts, and the first evidence of R. parkeri infection in capybaras. While R. parkeri is known to infect and cause disease in humans, no similar evidence for human infection has been indicated by R. bellii.
Key words: Rickettsia, rickettsia infections/diagnosis, rodentia, serology, zoonoses, Brazil
Infección por rickettsia en capibaras (Hydrochoerus hydrochaeris) de São Paulo, Brasil: evidencia serológica de infección por Rickettsia bellii y Rickettsia parkeri
Introducción. En Brasil, los capibaras (Hydrochoerus hydrochaeris) son importantes huéspedes para garrapatas del género Amblyomma, las cuales transmiten rickettsiosis a humanos y animales. Por lo tanto, estos roedores pueden ser potenciales centinelas para detectar infección por rickettsia.
Objetivos. Este trabajo evaluó la infección por rickettsia en capibaras de diferentes regiones del estado de São Paulo, donde las rickettsiosis nunca han sido reportadas.
Materiales y métodos. Se examinarion los sueros de 73 capibaras de seis localidades en São Paulo con la prueba de immunofluorescencia indirecta con antígenos de Rickettsia rickettsii, Rickettsia parkeri y Rickettsia bellii. Los bazos de los capibaras se extrajeron y se analizaron por reacción en cadena de la polimerasa para un fragmento del gene gltA de rickettsia. Las garrapatas se recolectaron de los capibaras y se identificaron hasta especie.
Resultados. Diecinueve (26,0%), 25 (34,2%) y 50 (68,5%) sueros de los capibaras reaccionaron con R. rickettsii, R. parkeri y R. bellii, respectivamente. De los 50 sueros que reaccionaron con antígenos de R. bellii, 25 presentaron títulos, por lo menos, cuatro veces mayores que los otros dos antígenos. Estos sueros fueron considerados homólogos de R. bellii. Usando el mismo criterio, tres sueros de los capibaras se consideraron homólogos de R. parkeri. Ningún suero se consideró homólogo de R. rickettsii. No se detectó ADN de rickettsia en bazo. Las garrapatas recolectadas de los capibaras fueron identificadas como Amblyomma dubitatum y Amblyomma cajennense.
Conclusiones. Este trabajo reporta la primera evidencia de infección natural por R. bellii en vertebrados y, también, la primera evidencia de infección por R. parkeri en capibaras. Se sabe que R. parkeri infecta y produce enfermedad en humanos; sin embargo, no hay evidencia de infección humana por R. bellii.
Palabras clave: Rickettsia, rickettsiosis/diagnóstico, roedores, serología, zoonosis, Brasil
Rickettsia are obligate intracellular bacteria, many of which cause zoonotic diseases in different parts of the world. Rickettsia species not associated with specific diseases are considered as having an unknown pathogenicity, or are symbionts of invertebrate organisms (1). Pathogenic rickettsiae have been divided into two groups: (i) the typhus group (TG), composed by Rickettsia prowazekii and Rickettsia typhi, transmitted primarily by lice and fleas, respectively; (ii) and the spotted fever group (SFG), including Rickettsia rickettsii and Rickettsia parkeri (2) transmitted mostly by ticks,
Rickettsia rickettsii is the etiological agent of the rickettsial disease with the greatest mortality and is known in different parts of its range as Brazilian spotted fever (BSF, Brazil), Rocky Mountain spotted fever (United States), and Tobia fever (Colombia). In the pre-antibiotic era, mortality rates of BSF were nearly 80% (3). Currently, in the Brazilian state of São Paulo, mortality rates can reach 40% (4). In addition to R. rickettsii, another pathogenic tick-borne rickettsia occurring in Brazil is R. parkeri. It is the etiological agent of a recently recognized spotted fever rickettsiosis in the United States and Uruguay, where it is transmitted by the ticks Amblyomma maculatum and A. triste, respectively (5,6). Spotted fever caused by R. parkeri seems to have milder clinical manifestations than the classical BSF, with no fatal cases yet reported (5,6). In Brazil, human cases due to R. parkeri have not been reported; however the infection by R. parkeri and a close-related genotype (strain COOPERI) have been reported in the ticks A. triste and Amblyomma dubitatum (=Amblyomma cooperi), respectively, in the state of São Paulo (7,8). In addition, serological evidence of R. parkeri infection has been reported in dogs, horses, and opossums in the state of São Paulo (9, Horta MC, Labruna MB, Pinter A, Linardi PM, Schumaker TT. Rickettsia infection in five areas of the State of Sao Paulo, Brazil. Mem Inst Oswaldo Cruz. Submitted).
Other tick-associated rickettsiae of unrecognized pathogenicity for humans have been reported recently in Brazil. These include the SFG species R. amblyommii in the state of Rondônia (10) and R. rhipicephali in Rondônia and São Paulo (11,12). R. bellii, a species classified neither in the TG or SFG, has also been reported in Rondônia and São Paulo (7,10,12). While serological evidence of canine infection due to R. amblyommii and R. rhipicephali has been reported in the state of Rondônia (13) there has been no evidence for R. bellii natural infection in vertebrate hosts.
The indirect immunofluorescence assay (IFA) is currently the test of choice for serologic diagnosis of rickettsial infection in humans and animals (14,15). However, cross-reactive antibodies between Rickettsia species are often observed, rendering difficult the serologic identification of the Rickettsia species involved in an infection. The geographic origin of the infection has been one of the best indicators of species identity. Testing a clinical serum against the possible Rickettsia species known to occur in a given area is ideal, because often homologous antibody titers are higher than heterologous antibody titers. In some cases, the differences in titers may be great enough to differentiate among the rickettsial species potentially stimulating the immune response (14). Using this criteria, serological evidence for human and animal infection due to R. rickettsii, and animal infection due to R. parkeri, R. amblyommii, and R. rhipicephali have been reported in the states of São Paulo and Rondônia (9,13, Horta MC, Labruna MB, Pinter A, Linardi PM, Schumaker TT. Rickettsia infection in five areas of the State of Sao Paulo, Brazil. Mem Inst Oswaldo Cruz. Submitted).
In the state of São Paulo, capybaras (Hydrochoerus hydrochaeris) are incriminated as primary hosts for the ticks Amblyomma cajennense and A. dubitatum (16). Since A. cajennense is incriminated as a vector of R. rickettsii (17), and A. dubitatum as a potential vector of R. parkeri (7), capybaras are potential sentinel hosts for rickettsial infection. The current study evaluated rickettsial infections in capybaras in several areas of the state of São Paulo, where BSF has not been reported previously.
Materials and methods
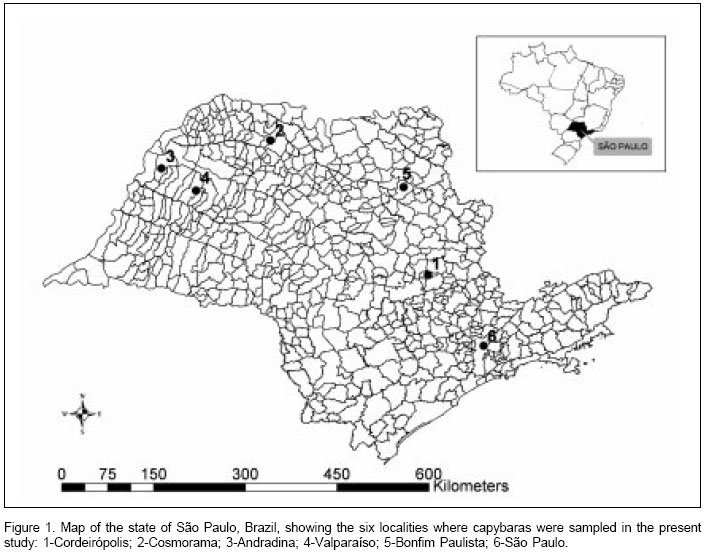
Capybaras were sampled from six different localities in the state of São Paulo (Figure 1): São Paulo city on the banks of Tietê River (23o32S, 46o37W), Cordeirópolis (22o28S, 47o27W), Bonfim Paulista (21o09S, 47o49W), Andradina (20o53S, 51o22W), Valparaíso (21o13S, 50o51W), and Cosmorama (20o28S, 49o46W). Capture of capybara samples have been authorized by the state office of the "Instituto Brasileiro do Meio Ambiente e Recursos Naturais Renováveis" (IBAMA). Samples were collected during slaughtering in an official wildlife abattoir in Iguape Municipality. Serum, spleen, and ticks were collected from each capybara.
A 3-min time regime was adopted for tick evaluation on each capybara; attached ticks were collected as they were encountered and stored within plastic tubes. Tick samples were available for 3 localities: São Paulo city, Cordeirópolis, and Bonfim Paulista. Ticks were brought to the laboratory, where they were taxonomically identified according to Barros-Battesti et al. (18).
The spleens were stored in individual sealed plastic bags, placed immediately on dry ice, brought to the laboratory, and refrigerated at -80oC freezer until use. Spleens were not available from the Valparaiso capybaras from Valparaíso and half of those from São Paulo. Each spleen was subjected to DNA extraction using the DNeasy Tissue Kit (Qiagen, Chatsworth, CA) following the manufacturers protocol, and tested by PCR using the primers CS-5 (forward) and CS-6 (reverse) that amplify a 147 bp fragment of the citrate synthase gene (gltA). These primers have a sensitivity for detection of a single copy of R. rickettsii DNA and 10 copies of R. bellii (7). PCR conditions were as described previously (17). For each reaction, three negative controls (water) and a positive control 300 ng of DNA of R. parkeri-infected A. cajennense ticks (19) were included.
Blood samples were transported to the laboratory at room temperature where samples were centrifuged (1,500xg for 10 minutes), aliquoted into labeled microtubes and stored at -20°C. The indirect immunofluorescence assay (IFA) used crude antigens derived from three Rickettsia isolates from Brazil: R. rickettsii strain Taiaçu, R. parkeri strain At24, and R. bellii strain Mogi. These three rickettsiae comprise the Rickettsia species known to infect Amblyomma ticks that infest capybaras in Brazil (7,17).
For antigen preparation, each Rickettsia was cultivated in Vero cells and harvested when the 100% infection level was approached. The infected cells were centrifuged at 12,000xg for 10 min and the pellet was washed in 0.1 M phosphate buffered saline (PBS, pH 7.4), centrifuged again, and resuspended in PBS containing 1% bovine calf serum and 0.1% sodium azide. Ten microliters of rickettsiae-infected cells were applied onto each well of 12-antigen slides. The antigens on the slides were air-dried and then fixed in acetone for 10 min. Slides were held at –80oC until inspection.
Capybara sera were diluted in two-fold increments with PBS starting from a 1:64 dilution. Ten microliters of diluted sera were added to each well of the antigen slides. The slides were incubated at 37oC for 30 min in a humidity box, rinsed once, and washed twice for 15 min per wash in PBS. The slides were incubated with fluorescein isothiocyanate-labeled sheep anti-capybara IgG (produced by the Centro de Controle de Zoonoses, São Paulo city) and washed as described above. The slides were prepared for inspection with buffered glycerin under cover slips. The slides were read using an ultraviolet microscope (Olympus BX60, Japan) at 400X magnification. For each sample, the endpoint titer reacting with each of the three Rickettsia antigens was determined. Sera showing at least four-fold higher titer for a given Rickettsia species than the other Ricketttsia species was considered homologous to the first Rickettsia species or to a very closely related genotype (9,13).
All reactions were performed with the same preparation lot of reagents; this including the secondary antibody, which was always used at the 1:400 dilution, as previously titrated in our laboratory. All slides were read by each of the three authors. Reactive sera were tested in two or three replications before determining the endpoint titer. In each slide, a serum previously shown to be non-reactive (negative control) and a known reactive serum (positive control) were tested at the 1:64 dilution. This positive control serum was previously shown to react with R. rickettsii, R. parkeri, and R. bellii at the endpoint titers 1:1024, 1:512, and 1:128, respectively.
Results
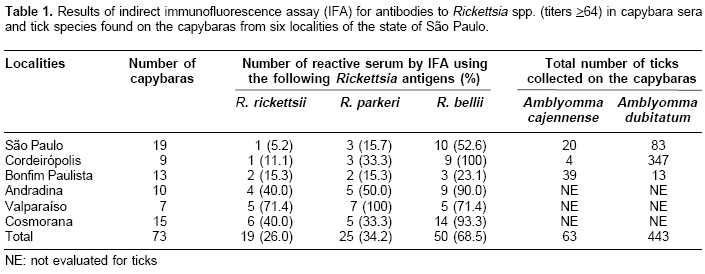
Of the 73 capybaras tested by IFA, 54 (74.0%) reacted positively to at least one Rickettsia species. A total of 19 (26.0%), 25 (34.2%), and 50 (68.5%) capybara sera reacted to R. rickettsii, R. parkeri, and R. bellii, respectively. In five localities (São Paulo, Cordeirópolis, Bonfim Paulista, Andradina, and Cosmorama), R. bellii was the antigen that elicited the highest frequency of seropositive capybaras, with values ranging from 23.1 to 100% (
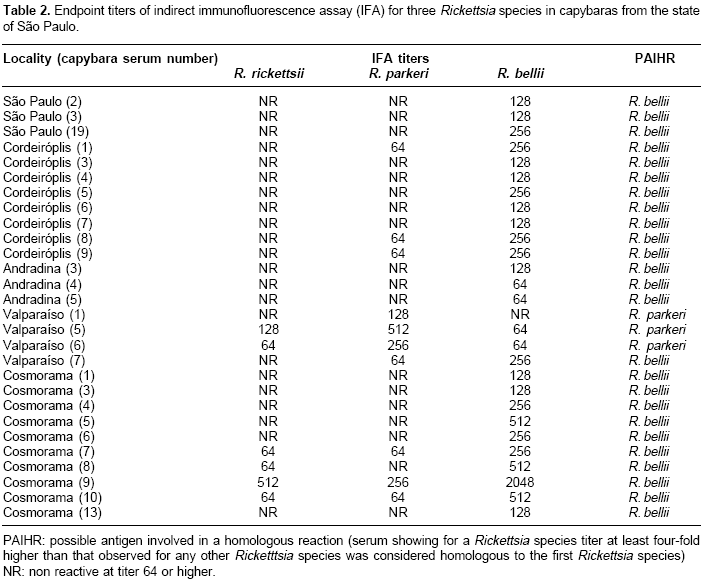
Table 1). In contrast, capybaras from Valparaíso showed highest seropositive frequency for R. parkeri (100%), followed by R. bellii and R. rickettsii at equal frequencies (71.4%).Three, 8, 3, 10, and 1 capybara sera from São Paulo, Cordeirópolis, Andradina, Cosmorama, and Valparaíso, respectively, showed titers to R. bellii at least four-fold higher than to any of the other two antigens (
Table 2). These sera were considered homologous to R. bellii or a very closely related genotype. Using the same criteria, 3 sera from Valparaíso were considered homologous to R. parkeri (Table 2). No sera were found homologous to R. rickettsii.Adult ticks from the three localities were identified as A. cajennense (63 specimens) and A. dubitatum (443 specimens). In São Paulo and Cordeirópolis, most of the ticks (80.6 and 98.9%, respectively) were A. dubitatum, whereas A. cajennense comprised 75% of the ticks collected on capybaras from Bonfim Paulista. A total of 57 capybara spleens were tested by PCR; none showed to contain rickettsial DNA.
Discussion
The current study tested capybara sera against all known Rickettsia species found in ticks that infest capybaras in São Paulo state. In the six localities tested, serological evidence for rickettsial infection one or more of the capybaras sampled. In five localities (São Paulo, Cordeirópolis, Bonfim Paulista, Andradina, and Cosmorama), more animals were seroreactive to R. bellii than to R. rickettsii or R. parkeri. In addition, we present serological evidence for R. bellii infection in at least 25 capybaras (
Table 2). R. bellii has been reported infecting A. dubitatum ticks from several parts of the state of São Paulo (7, Horta MC, Pinter A, Souza CE, Neto EJ, Souza SS, Soares RM et al. Ocorrência de Rickettsia bellii em Carrapatos colhidos nos Municípios de Piracicaba, Pedreira, Campinas, Itu e Cordeirópolis, Estado de São Paulo,. In: XIII Congresso Brasileiro de Parasitologia Veterinária e I Simpósio Latino-Americano de Rickettsioses, 2004, Ouro Preto-MG). Since R. bellii is found in the A. dubitatum hemolymph (7), this agent possibly can infect tick salivary glands and subsequently be transmitted via tick feeding. Therefore, A. dubitatum may have transmitted R. bellii to the seropositive capybaras in the current study, since capybaras are primary hosts for all parasitic stages of A. dubitatum (20. This hypothesis is corroborated by the tick infestation data (Table 1). In São Paulo and Cordeirópolis, where >80% of the ticks collected on capybaras were A. dubitatum, 52.6 to 100% of the capybaras were seropositive to R. bellii. On the other hand, in Bonfim Paulista, where only 25% of the ticks were A. dubitatum, only 23.1% of the capybaras were seropositive for R. bellii.In Valparaíso, substantial evidence for R. parkeri infection were observed in at least three capybaras (
Table 2), although ticks were not collected from these capybaras. Nonetheless, capybaras are frequently found to be parasitized by A. dubitatum ticks in the state of São Paulo (20, Pacheco RC, Pinter A, Ferreira PM, Ferreira F, Labruna MB. Carrapatos infestando capivaras em cinco áreas do estado de São Paulo. In: XIII Congresso Brasileiro de Parasitologia Veterinária e I Simpósio Latino-Americano de Rickettsioses, 2004, Ouro Preto, MG. Revista Brasileira de Parasitologia Veterinária 2004;13:315). Since R. parkeri (strain COOPERI) has been reported infecting A. dubitatum in the state of São Paulo (7), the A. dubitatum population from Valparaíso were probably infected with R. parkeri. This finding indicates the possibility of clinical cases of human infection by R. parkeri, since it is a recognized agent of spotted fever.No serological evidence for the presence of R. rickettsii infection was recovered from the capybaras sampled. This result was not surprising since the samples were from areas where BSF has never been reported. Capybaras are also the primary hosts of A. cajennense, one of the vectors of BSF in Brazil (16). Consequently, the serological results indicated that R. rickettsii was not circulating in A. cajennense populations currently sampled.
Rickettsial DNA were not found in any of the capybara spleen samples. Spleen is one of the organs of choice for rickettsial infection surveillance (14). However, detection of rickettsia in vertebrate animals is usually a rare event. Once infected, vertebrates display a rickettsemia for only a few days or weeks, and thereafter no rickettsia is found in the organism (21).
The current study reports the first evidence of R. bellii natural infection in vertebrate hosts, and also the first evidence of R. parkeri infection in capybaras. While R. parkeri is known to infect and cause disease in humans (5), there has been no evidence of human infection by R. bellii. In fact, previous studies in areas of occurrence of R. bellii-infected ticks failed to demonstrate serological evidence of R. bellii infection among dogs, horses, cats, opossums, or humans (9,13, Horta MC, Labruna MB, Pinter A, Linardi PM, Schumaker TT. Rickettsia infection in five areas of the State of Sao Paulo, Brazil. Mem Inst Oswaldo Cruz. Submitted). If capybaras are more susceptible to R. bellii infection than other vertebrates, this has yet to be demonstrated. Nevertheless, the possible role of R. bellii causing infection in vertebrate hosts, including humans, is worthy of further investigation.
Acknowledgments
We thank Fernando Ferreira (University of São Paulo) for drawing the
figure 1 of this manuscript, the "Instituto Brasileiro do Meio Ambiente e Recursos Naturais Renováveis" (IBAMA) for authorizing our work with capybaras, and the Centro de Controle de Zoonoses (CCZ) of São Paulo city and Celso E. Souza (Sucen) for producing and providing the capybara conjugate.Conflict of interests
We declare that there is no conflict of interests on the results presented in this article.
Financial support
This work was supported by "Fundação de Amparo a Pesquisa do Estado de São Paulo" (FAPESP) and "Conselho Nacional de Desenvolvimento Científico e Tecnológico" (CNPq).
Correspondencia:
Marcelo B. Labruna, Departamento de Medicina Veterinária Preventiva e Saúde Animal, Faculdade de Medicina Veterinária e Zootecnia, Universidade de São Paulo, Av. Prof. Orlando Marques de Paiva 87, 05508-270 São Paulo, SP, Brasil Phone: 55-11-3091 1394; Fax: 55-11-3091 7928.
labruna@usp.References
1. Perlman SJ, Hunter MS, Zchori-Fein E. The emerging diversity of Rickettsia. Proc Biol Sci. 2006;273:2097-106. [ Links ]
2.Roux V, Rydkina E, Eremeeva M, Raoult D. Citrate synthase gene comparison, a new tool for phylogenetic analysis and its application for the Rickettsiae. Int J Syst Bacteriol. 1997;47:252-61. [ Links ]
3. Monteiro JL. Estudos sobre o typho exantemático de S. Paulo. Mem Inst Butantan. 1931;6:1-136. [ Links ]
4. Angerami RN, Resende MR, Feltrin AF, Katz G, Nascimento EM, Stucchi RS et al. Brazilian spotted fever: a case series from an endemic area in southeastern Brazil: clinical aspects. Ann N Y Acad Sci. 2006;1078:252-4. [ Links ]
5. Paddock CD, Sumner JW, Comer JA, Zaki SR. Rickettsia parkeri: A newly recognized cause of spotted fever rickettsiosis in the United States. Clin Infect Dis. 2004;38:15-21. [ Links ]
6. Venzal JM, Portillo A, Estrada-Peña A, Castro O, Cabrera PA, Oteo JA. Rickettsia parkeri in Amblyomma triste from Uruguay. Emerg Infect Dis. 2004;10:1493-5. [ Links ]
7. Labruna MB, Whitworth T, Horta MC, Bouyer DH, McBride J, Pinter A et al. Rickettsia species infecting Amblyomma cooperi ticks from an area in the State of São Paulo, Brazil, where Brazilian spotted fever is endemic. J Clin Microbiol. 2004;42:90-8. [ Links ]
8. Silveira I, Pacheco RC, Szabo MP, Ramos HG, Labruna MB. Rickettsia parkeri in Brazil. Emerg Infect Dis. 2007;13:1111-3. [ Links ]
9. Horta MC, Labruna MB, Sangioni LA, Vianna MC, Gennari SM, Galvão MA et al. Prevalence of antibodies to Spotted Fever Group Rickettsiae in humans and domestic animals in a Brazilian Spotted Fever-Endemic area in the State of São Paulo, Brazil: Serologic evidence for infection by Rickettsia rickettsii and another spotted fever group Rickettsia. Am J Trop Med Hyg. 2004;71:93-7. [ Links ]
10. Labruna MB, Whitworth T, Bouyer DH, McBride J, Camargo LM, Camargo EP et al. Rickettsia bellii and Rickettsia amblyommii in Amblyomma ticks from the state of Rondônia, Western Amazon, Brazil. J Med Entomol. 2004;41:1073-81. [ Links ]
11. Labruna MB, Camargo LM, Camargo EP, Walker DH. Detection of a spotted fever group Rickettsia in the tick Haemaphysalis juxtakochi in Rondonia, Brazil. Vet Parasitol. 2005;127:169-74. [ Links ]
12. Labruna MB, Pacheco RC, Richtzenhain LJ, Szabo MP. Isolation of Rickettsia rhipicephali and Rickettsia bellii from ticks Haemaphysalis juxtakochi in the state of Sao Paulo, Brazil. Appl Environ Microbiol. 2007;73:869-73. [ Links ]
13. Labruna MB, Horta MC, Aguiar DM, Calvacante GT, Pinter A, Gennari SM et al. Prevalence of Rickettsia infection in dogs from the urban and rural areas of Monte Negro municipality, Western Amazon, Brazil. Vector Borne Zoonotic Dis. 2007;7:249-55. [ Links ]
14. La Scola B, Raoult D. Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J Clin Microbiol. 1997;35:2715-27. [ Links ]
14. Parola P, Paddock CD, Raoult D. Tick-borne rickettsioses around the world: emerging diseases challenging old concepts. Clin Microbiol Rev. 2005;18:719-56. [ Links ]
15. Vieira AM, Souza CE, Labruna MB, Mayo RC, Souza SS, Camargo-Neves VL. Manual de Vigilância Acarológica, Estado de São Paulo. São Paulo: Secretaria de Estado da Saúde; 2004. [ Links ]
16. Guedes E, Leite RC, Prata MC, Pacheco RC, Walker DH, Labruna MB. Detection of Rickettsia rickettsii in the tick Amblyomma cajennense in a new Brazilian spotted fever-endemic area in the state of Minas Gerais. Mem Inst Oswaldo Cruz. 2005;100:841-5. [ Links ]
17. Barros-Battesti DM, Arzua M, Bechara GH. Carrapatos de importância medico-veterinária da região Neotropical. Um guia ilustrado para identificação de espécies. São Paulo: Vox/ICTTD-3/Butantan; 2006. [ Links ]
18. Sangioni LA, Horta MC, Vianna MC, Gennari SM, Soares RS, Galvão MA et al. Rickettsial infection in animals and Brazilian spotted fever endemicity. Emerg Infect Dis. 2005;11:265-70. [ Links ]
19. Labruna MB, Pinter A, Teixeira RH. Life cycle of Amblyomma cooperi (Acari: Ixodidae) using capybaras (Hydrochaeris hydrochaeris) as hosts. Exp Appl Acarol. 2004;32:79-88. [ Links ]
20 .Burgdorfer W. Ecological and epidemiological considerations of Rock Mountain spotted fever and scrub typhus. EN: DH Walker, editor. Biology of rickettsial diseases. Vol.1. Boca Raton: CRC Inc; 1988. [ Links ]