Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biomédica
Print version ISSN 0120-4157On-line version ISSN 2590-7379
Biomédica vol.30 no.3 Bogotá July/Sept. 2010
PRESENTACIÓN DE CASO
1Grupo de Micobacterias, Instituto Nacional de Salud, Bogotá D.C., Colombia
2Grupo de Microbiología Molecular, Facultad de Medicina, Universidad de La Sabana, Chía, Colombia
3Universidad Industrial de Santander, Bucaramanga, Colombia
Recibido: 29/12/09; aceptado:13/04/10
Introduction. Cutaneous tuberculosis as a result of a needle injection is a rare event; it generally occurs among medical and laboratory personnel and among patients receiving percutaneous treatment.
Objective. Six patients are presented who developed cutaneous tuberculosis after mesotherapy cosmetic treatment.
Material and methods. One to four months after injection of an unknown product as treatment for obesity and cellulites, five women and a man developed papules, nodules and drainage of wax like material at the inoculated sites; this was interpreted clinically as a non tuberculous mycobacterium infection. Skin biopsies were taken for a histopathologic study; the biopsy and exudates were cultured to make a phenotypic identification. Polymerase chain reaction and restriction enzyme pattern analyses (PCR-restriction pattern analysis)) procedures were applied to the skin biopsies.
Results. Mycobacterium tuberculosis was confirmed in the culture and by PRA analysis in the paraffin-embedded biopsies. The patients had never had tuberculosis. Their thoracic X rays were normal and the size of the tuberculin reaction was 17 to 20 mm. Five patients recovered with antituberculosis treatment and the sixth spontaneously healed after the removal of the largest cutaneous module. No satellite adenopathy or recurrences were observed.
Conclusions. A previously undescribed mode of acquisition cutaneous tuberculosis was described. This was the second incident of a demonstrated cutaneous tuberculosis following mesotherapy in Colombia. Skin lesions induced by injections must be tested to detect mycobacterias to include M. tuberculosis.
Keywords: tuberculosis, cutaneous; Mycobacterium tuberculosis, case studies.
Tuberculosis cutánea por mesoterapia, estudio de seis casos
Introducción. La tuberculosis cutánea secundaria a la inyección con agujas es rara; se presenta en personal médico y de laboratorio, y en pacientes que reciben tratamientos percutáneos.
Objetivo. Presentar seis pacientes con tuberculosis cutánea secundaria a tratamiento por mesoterapia.
Materiales y métodos. Entre 1 y 4 meses después de la inyección en la piel glútea y abdominal de material no precisado, como tratamiento para la obesidad y la celulitis, cinco mujeres y un hombre desarrollaron pápulas, nódulos y senos de drenaje de material seroso en los sitios de inoculación, interpretados clínicamente como infección por micobacterias no tuberculosas.
Se practicaron cultivos de las secreciones y de las biopsias de piel para la identificación fenotípica y estudio de histopatología. Con los resultados iniciales se realizaron pruebas moleculares de PRA (PCR-restriction pattern analysis) en las biopsias de piel y estudio ampliado de los pacientes.
Resultados. Se demostró Mycobacterium tuberculosis en los cultivos, hallazgo confirmado por la técnica de PRA en las biopsias incluidas en parafina.Los pacientes no habían padecido tuberculosis. Las placas de tórax fueron normales y la tuberculina midió entre 17 y 20 mm. Cinco curaron con terapia antituberculosa y otro curó espontáneamente luego de la resección-biopsia de la lesión más grande. No se encontraron adenopatías satélites ni recurrencias.
Conclusiones. Se demostró una nueva forma de adquirir la tuberculosis cutánea. Esta es la segunda demostración de tuberculosis cutánea por mesoterapia en Colombia. El estudio de las lesiones de la piel en el sitio de la inyección cutánea debe incluir pruebas para detectar micobacterias, entre ellas M. tuberculosis.Las autoridades sanitarias deben prestar atención y prevenir esta modalidad de adquirir la tuberculosis.
Palabras clave: tuberculosis cutánea, Mycobacterium tuberculosis, estudios de casos.
Mesotherapy or intradermotherapy is a mode of treatment established since the 1950s. The treatment consists of injecting several medications in the middle dermis and are mixed with local anesthetics such as procaine mesocaine or lydocaine.This blend reaches a high and persistent concentration to permit a prolonged activity of up to five days duration (1). This method has proved useful in areas such as pain reduction, traumatology, orthopedics, physiatrist and rheumatology (1). However, cutaneous injections, applied by medical doctors or lay people, can cause important complications such as atypical mycobacteria or nontuberculosis mycobacteria (NTM) infections. The infections are recognizeable because of lesion frequency, profusion and size (2-7).
The current report presents six patients who were subjected to cosmetic mesotherapy treatment and subsequently developed papules and cutaneous nodules, at first diagnosed as caused by NTM. Further culture and molecular analysis showed the causal agent to be Mycobacterium tuberculosis.
Patients
Case 1. Seventy year old woman. Four months after receiving injections in abdominal skin as a mesotherapy treatment for grooves, large nodules appeared on her lower abdomen; ulcerated erythematous plaques with a slight secretion were present at the injection sites. The patient was unsuccessfully treated with multiple antibiotics. Biopsies were performed in the ulcerated plaques to provide tissue for histopathologic examination and to culture for the presence of NTM.On the fourth week of culture incubation, bacterial growth was observed and identified as M. tuberculosis.
Case 2. Forty-three year old woman. Four months after mesotherapy treatment for grooves and abdominal and thigh cellulite depositions, the patient developed nodules and abscesses at the injected sites; the condition was diagnosed as caused by NTM.A biopsy for culture and histopathologic analysis was performed. On the eight week of culture incubation, bacterial growth occurred that was identified as M. tuberculosis through phenotypical and genotypical tests.
Case 3. A fifty-five year old male developed edematous cutaneous papules and nodules on the abdominal skin three months after receiving mesotherapy treatment for abdominal adipose. His wife also developed similar lesions after mesotherapy treatment (Case 6). A biopsy for cultural and histopathologic identification of the causative agent was performed; a culture of the nodule secretions was also taken. Bacterial growth occurred at the ninth week of incubation and was later identified as M. tuberculosis through phenotypical and genotypical tests.
Case 4. Fifty year old woman. The patient was subjected to local injections on abdominal, buttocks and thighs to eliminate grooves and cellulite. A month later, she developed erythematous papules and nodules of slow growth on thighs and abdomen--some of them ulcerated with serous bloodshot secretions. A lesion was excised and diagnosed as non specific inflammation. Because the ulceration and secretion persisted in the remaining lesions, the patient consulted a dermatologist, who ordered cultures for NTM. On the fourth week, the cutaneous lesion culture was positive for M. tuberculosis through phenotypical and genotypical tests. The patient refused antibiotic treatment for tuberculosis but healed spontaneously of the papules six months later. Depressed and hyperpigmented scars remained at the sites of the papules.
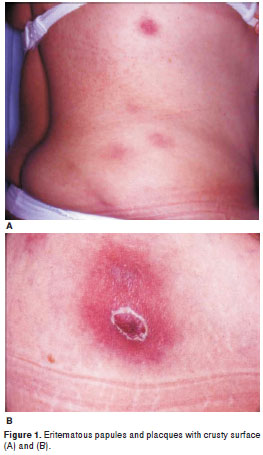
Case 5. Forty seven year old woman. During the previous six months, the patient presented hard, painful nodules and placques on abdomen, back and thighs that spontaneously drained pus (Figure 1). They began to develop four months after receiving local injections to lose weight. She was treated with local triamcinolone infiltrations without success. A biopsy was performed on one of the lesions for culture and histopathological analysis. On the sixth week of incubation, the culture was positive for M. tuberculosis through phenotypical and genotypical tests.
Case 6. Forty three year old woman, wife of case 3. Three months after receiving injection in abdomen skin to reduce obesity, the patient developed several erythematous nodules and papules;several were ulcerated and draining. Skin biopsy was performed in one lesion for culture, and on another for histopathological analysis to confirm NTM abscesses. The culture did not have bacterial growth after 16 weeks of incubation. Molecular tests demonstrated the presence of M. tuberculosis DNA in the skin biopsy.
Materials and methods
Samples. Secretion samples from lesions and skin biopsies were taken from the six patients who were remitted to the laboratory between October 2004 and December 2005. Each sample was subjected to direct tests and culture. Five patients had biopsies for histopathological analysis, consisting in 4x5 mm skin cylinders fixed in neutral formaldehyde, paraffin-embedded, and stained with hematoxylin-eosin and Ziehl-Neelsen (ZN).
Phenotypical identification and susceptibility tests to drugs. After decontamination, samples were placed in culture media (Ogawa Kudoh and Stonebrink), at 32°C and 37°C. Recovered isolates were colored with ZN stain and subjected to biochemical and enzymatic tests for mycobacteria identification (8). Susceptibility to isoniacide, rifampicine, ethambutol and streptomycin was determined by standard methods (9).
Genotypic identification
Mycobacterium genomic DNA extraction. Samples were initially treated with 5% Chelex-100 (Sigma) and then subjected to a standard analysis protocolwas followed (10,11).
Molecular identification. The identifications were performed on skin biopsies embedded in paraffin from five patients at the Laboratory of Mycobacteria on request of the Laboratory of Pathology, both laboratories at the Colombian National Institute of Health in Bogotá. In the Laboratory of Pathology, the case biopsies had been processed for histopathological study over a three-year period before submission to the Laboratory of Mycobacteria. At the Laboratory of Mycobacteria, techniques of restriction pattern analysis (PRA) were used to identify mycobacteria following standard protocols (12). Mycobacterium tuberculosis H37Rv was used as positive control and sterile water as negative control. The restriction patterns were analyzed using this net site (http://app.chuv.ch/prasite/index.html).
Additional study of the patients
Once M. tuberculosis was identified, the attending physicians were informed. The physicians then acquired additional information from their patients that included past or present family history of tuberculosis, thoracic X-rays and tuberculin reaction. Five of the patients received standard treatment established for tuberculosis.
Results
Direct ZN staining. It was negative in all samples tested.
Phenotypical Identification. In five of the six cases, positive cultures at 37°C were obtained for M. tuberculosis within a period ranging from 4 to 9 weeks (table 1). Colonies had a wrinkled, dry aspect, without pigment, and corresponded to acid alcohol-resistant bacilli as indicated by the ZN staining. Biochemical and enzymatic tests of culture were niacin- and catalase-positive at room temperature, catalase-negative at 68°C and positive for nitrate reduction. These tests showed that the isolated bacterial species were M. tuberculosis.
Susceptibility tests. The case 5 isolate showed resistance to isoniacide. The other isolates were sensitive to the antimicrobial drugs studied.
Molecular identification through PRA. The same PRA estriction pattern was present in the five samples and characteristic of Mycobacterium species of the M. tuberculosis complex.
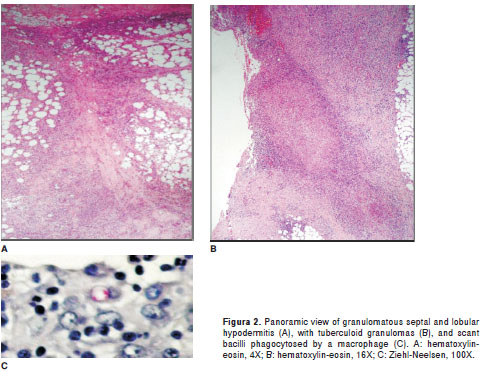
Histopathology. Skin biopsies showed similar lesion structure, located predominantly within the deep dermis and on the hypodermis (figure 2). Granulomatous inflammation with abundant epithelioid cells was present, along with a number of Langhans giant cells, numerous lymphocytes, a few plasma cells, and vacuolated macrophages. Many polymorphonuclear leucocytes were seen, either dispersed or forming abscesses, at the center of some granulomas or surrounding small vacuoles.Small areas of fibrinoid necrosis was observed at the center of some granulomas. The lesions were typically deep into the adipose lobules. Only in Case five were acid fast bacilli found to be phagocytized in few macrophages (figure 2C). The image did not suggest tuberculosis but inflammation by NTM, a conclusion based on prior experience with NTM inflammations (13).
Additional study of the patients. None of the six patients had an underlying disease or had tuberculosis, nor was a history of tuberculosis noted their families. Tuberculin tests were strongly positive in five of patients, and ranged from 17 to 20 mm in diameter. For five patients, the thoracic X rays were normal; no information was available for the sixth patient. None had developed satellite adenopathies. Five were cured with strictly supervised antituberculosis treatment, and one patient was cured of four lesions without antituberculosis treatment. None of the patients had presented recurrence of lesions after more than one year of follow up.
Discussion
Lesions appeared in patients a few months after mesotherapy and were limited to the injection site. They were in the form of multiple non painful erythematous papules and nodules, some of them ulcerated and draining, without satellite adenopathy. The lesions were diagnosed as caused by NTM upon consultation with dermatologists and after several failing treatments. After culture of lesions and skin biopsies, the expected NTM, such as M. abscessus, M. chelonae or M fortuitum (2-7) were not isolated. Instead M. tuberculosis was identified by its phenotype in culture. Therefore,molecular methods were employed to confirm the presence of M. tuberculosis. The PRA test of the skin biopsies embedded in paraffin confirmed that they contained DNA of M. tuberculosis. This test further confirmed that the isolated M. tuberculosis is not a laboratory contaminantt (14). Isolates were obtained without difficulty, with defined colonies, and in different periods of time; this provided additional evidence that sample contamination was not a factor (14). All the phenotypical and genotypical evidence pointed to M. tuberculosis as the correct identification.
Only one of the isolated bacilli was isoniacide-resistant. The tuberculosis-specific treatment was successful in five of the six patients, who, nevertheless, were left with noticeable, hyper pigmented, depressed scars. One patient (Case 4), healed spontaneously after the largest nodule was removed. These cases of cutaneous tuberculosis occurred in healthy persons which were treated with mesotherapy for esthetic reasons. Because the tuberculosis was localized at the lesion site, immunity probably developed due to previous exposure to the bacilli. Prior immunity may have permitted the spontaneous cure in one of the tuberculosis cases. Some level of immunity may be expected in older people whose lifetime has been spent a in tuberculosis-endemic country.
This is a new inoculation form of M. tuberculosis. Only one other case had been reported, also in Colombia (15). The origin of these bacteria is, at present, speculative.They probably were not present in the injected product, noting that the composition of these products is not publically available (15). If the product were contaminated with M. tuberculosis, the number of infected patients will be greater. If the product were contaminated with NTM due to poor hygienic practices such as non-sterile needles, syringes, or distilled water, the introduction of the NTM in the product flask will have resulted in its distribution to a larger patient population (2,5-7,13).Local health authorities have been informed of these cases by the Colombia National Institute of Health, as these officials are responsible for further investigations of the conditions under which the mesotherapy was performed.. Another source of infection may be the person that performs the injections; this possibillity was not investigated.
Cutaneous tuberculosis inoculation through injection is a rare event, first reported in in the 1950s, and only occasionally more recently (16,17). It has been shown in patients that received corticosteroid intramuscular injections (18), in children vaccinated against pertussis -where both nurse and physician had pulmonary tuberculosis (19), and in medical doctors, nurses and laboratory personnel injured by needles contaminated with blood or tissues from tuberculosis or AIDS patients (20-22). Cutaneous inoculation with tuberculosis in people without previous contact with the bacilli (usually children), a primary tuberculosis occurs, with satellite adenopathy (23,24). Possibly, the injection site becomes a locus minoris resistentiae, where the tuberculosis bacilli have better growing conditions (25). The most recent form of cutaneous tuberculosis through inoculation was associated with neural therapy (15) or mesotherapy, such as the patients introduced herein.
Nonetheless, skin lesions that appear at injection sites following therapeutic or cosmetic interventions (17,26) must be examined by culture for Mycobacterium and other microorganisms and cannot be treated as a common bacterial infection. Occasionally, these examinations demonstrate that the cutaneous lesions are sterile, even though they have clinical and histopathological features similar to those reported here (27).
In conclusion, mesotherapy is a widely used cosmetic treatment administered through injections on abdomen, buttocks and face.These can produce complications caused by NTM that consist of deep tissue cutaneous papules and nodules that drain to the skin surface and which, after healing, leave noticeable scarring (2-7). In the current case report, similar lesions were produced by M. tuberculosis, as identified by phenotypical and genotypical methods. When cutaneous lesions occur on intradermotherapy injection sites, inflammation caused by mycobacteria is suspect. Culture of the skin biopsy or secretion is essential to identify causal agents, however. Antibiotic treatment has proved to be effective. Information concerning these complications is recommended for distribution among personnel performing this kind of intervention and recipient patients.The current study further recommends that health authorities schedule periodicinspections of the protocols used in mesotherapeutic clinics, including strict biosecurity regulation, drug usage and stringent asepsis of implements,
Acknowledgements
Figure 1 was kindly provided by Dr. Hugo Herrera.
Conflict of interests
Authors declare that no conflict of interests exist in the elaboration and writing of this paper.
Financial support
This work was supported by Instituto Nacional de Salud, Bogotá, Colombia and Universidad de La Sabana, Chía, Cundinamarca, Colombia.
Corresponding author: Gerzaín Rodríguez, Grupo de Microbiología Molecular, Facultad de Medicina, Universidad de La Sabana, Chía, Cundinamarca, Colombia. Teléfono: (571) 861 5555; fax: 8615555, extensión 2626 gerzain_rodriguez@yahoo.com
References
1. d´Orsi LM. Introduçao a intradermoterapia. En: Villarejo KM, Sabatovitch O, editors. Dermatología estética. Sao Paulo: Atheneu; 2003. p. 371-4. [ Links ]
2. Camargo D, Saad C, Ruiz F, Ramírez ME, Lineros M, Rodríguez G, et al. Iatrogenic outbreak of M. chelonae skin abscesses. Epidemiol Infect. 1996;117:113-9. [ Links ]
3. Villanueva A, Villanueva R, Acosta B, Ruiz F, Agüero S, Zhang Y, et al. Report of an outbreak of postinjection abscesses due to Mycobacterium abscessus, including management with surgery and clarithromycin therapy and comparison of strains by random amplified polymorphic DNA polymerase chain reaction. Clin Infect Dis. 1997;24:147-53. [ Links ]
4. Prado AC, Castillo PF. Lay clinics and epidemic outbreak of mycobacterium skin and soft-tissue infection. Plast Reconstr Surg. 2004;113:800-1. [ Links ]
5. Del Solar M, Salomón M, Bravo F, Seas C, Gotuzzo E, Culqui D. Infección cutánea por micobacterias atípicas de crecimiento rápido (MACR) debido a mesoterapia cosmética. Reporte de casos y revisión de la literatura. Folia Dermatol Perú. 2005;16:127-35. [ Links ]
6. Rivera-Olivero IA, Guevar A, Escalona A, Oliver M, Pérez-Alfonzo R, Piquero J, et al. Infecciones en tejidos blandos por micobacterias no tuberculosas secundarias a mesoterapia. ¿Cuánto vale la belleza? Enferm Infecc Microbiol Clin. 2006;24: 302-6. [ Links ]
7. Sañudo A, Vallejo F, Sierra M, Hoyos JG, Yepes S, Wolf JC, et al. Nontuberculous mycobacteria infection after mesotherapy: preliminary report of 15 cases. Int J Dermatol. 2007;46:649-53. [ Links ]
8. Kent P, Kubica G. Public health mycobacteriology. A guide for the level III laboratory. Atlanta, Georgia: US Department Services, Centers for Disease Control; 1985. [ Links ]
9. Canneti G, Wallac FA, Khomenko A. Advances in techniques of testing mycobacterial drugs sensitivity and the use of sensitivity tests in tuberculosis control programs. Bull World Health Organ. 1969;41:21-43. [ Links ]
10. van Soolingen DV, De Hass PE, Kremer K. Restriction fragment length polymorphism (RFLP) typing of Mycobacteria. Bilthoven, The Netherlands: National Institute of Public Health and the Environment; 2002. [ Links ]
11. Burgos MV, Méndez JC, Ribón W. Molecular epidemiology of tuberculosis: methodology and applications. Biomédica 2004;24(Suppl.1):188-201. [ Links ]
12. Telenti A, Marchesi F, Balz M, Bally F, Bottger EC, Bodmer T. Rapid identification of Mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31:175-8. [ Links ]
13. Rodríguez G, Ortegón M, Camargo D, Orozco LC. Iatrogenic Mycobacterium abscessus infection: histopathology of 71 patients. Br J Dermatol. 1997;136:214-8. [ Links ]
14. Burman WJ, Reves RR. Review of false-positive cultures for Mycobacterium tuberculosis and recommendations for avoiding unnecessary treatment. Clin Infect Dis. 2000;31:1390-5. [ Links ]
15. Díaz BM, Muñoz OA, Klinger JC. Mycobacterium tuberculosis responsible for cutaneous disease after percutaneal inoculation of solutions: a case report. Int J Dermatol. 2003;42:564-6. [ Links ]
16. Ebrill D, Elek S. Tuberculous abscess following intramuscular penicillin. Lancet 1946;ii:379-80. [ Links ]
17. Tomar RP, Gupta A, Wilkhoo NS, Bhalla PJ. Tubercular abscess following intramuscular injections. Armed Forces Med J India. 2007;63:374-5. [ Links ]
18. de Jong JW, van Altena R. Non-respiratory tuberculosis with M. tuberculosis after penetrating lesions of the skin: five case histories. Int J Tuberc Lung Dis. 2000;12:1184-7. [ Links ]
19. Oka S, State M. 13 years follow up study of an epidemic inoculation tuberculosis in children caused by vaccination against Pertussis. Am Rev Resp Dis. 1963;88:462-5. [ Links ]
20. Genné D, Siegrist HH. Tuberculosis of the thumb following a needlestick injury. Clin Infect Dis. 1998;26:210-1. [ Links ]
21. Kramer F, Sasse SA, Simms JC, Leedom JM. Primary cutaneous tuberculosis after a needlestick injury from a patient with AIDS and undiagnosed tuberculosis. Ann Intern Med. 1993;119:594-5. [ Links ]
22. Oymak SF, Gülmez I, Demir R, Ozesmi M. Transmission of Mycobacterium tuberculosis by accidental needlestick. Respiration. 2000;67:696-7. [ Links ]
23. Barbagallo J, Tager P, Ingleton R, Hirsch R, Weinberg JM. Cutaneous tuberculosis. Diagnosis and treatment. Am J Clin Dermatol. 2002;3:319-28. [ Links ]
24. Grayson W, Calonge E, McKee P. Infectious diseases of the skin. Tuberculosis. In: McKee P, Calonge E, Granter SR, editors. Pathology of the skin with clinical correlation. Third edition. Philadelphia: Elsevier Mosby; 2005. p. 894-902. [ Links ]
25. Vidal D, Barnadas M, Pérez M, Coll P, Alomar A. Tuberculous gumma following venepuncture. Br J Dermatol. 2001;144:601-3. [ Links ]
26. Wong HW, Tay YK, Sim CS. Papular eruption on a tattoo: a case of primary inoculation tuberculosis. Australas J Dermatol. 2005;46:84-7. [ Links ]
27. Davis M, Wright T, Shehan JM. A complication of mesotherapy: noninfectious granulomatous panniculitis. Arch Dermatol. 2008;144:808-9. [ Links ]














