Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Biomédica
versión impresa ISSN 0120-4157versión On-line ISSN 2590-7379
Biomédica v.30 n.3 Bogotá jul./sep. 2010
PRESENTACIÓN DE CASO
1 Departamentos de Investigación y Médico, Fundación Neumológica Colombiana, Bogotá, D.C., Colombia
2 Servicio de Neumología, Fundación Cardioinfantil-Instituto de Cardiología, Bogotá, D.C., Colombia
3 Facultad de Medicina, Universidad de La Sabana, Chía, Colombia
4 Fundación Cardiovascular de Colombia, Bucaramanga, Colombia
5 Grupo de Micobacterias, Instituto Nacional de Salud, Bogotá, D.C., Colombia
6 Laboratorio de Refrencia e Investigación en Enfermedades Tropicales, Dirección de Sanidad del Ejército, Bogotá, D.C., Colombia
7 Grupo de Inmunología y Epidemiología Molecular, Universidad Industrial de Santander, Bucaramanga, Colombia.
Recibido: 03/02/10; aceptado:27/05/10
Rapidly growing mycobacteria are non-tuberculous mycobacteria amply present in the environment. Although they are not usually pathogenic for humans, they are opportunistic in that they can cause disease in people with disadvantageous conditions or who are immunocompromised. Mycobacterium peregrinum, an opportunistic, rapidly growing mycobacteria, belongs to the M. fortuitum group and has been reported as responsible for human cases of mycobacteriosis.
A case of M. peregrinum type III is herein reported as the first in Colombia. It presented as a disseminated disease involving a prosthetic aortic valve (endocarditis) in a seventeen-year-old girl with a well-established diagnosis of prosthetic aortic valve endocarditis who was referred for a surgical replacement. Due to a congenital heart disease (subaortic stenosis with valve insufficiency), she had two previous aortic valve implantation surgeries. One year after the second implantation, the patient presented with respiratory symptoms and weight lost indicative of lung tuberculosis.
A chest X-ray did not show parenchymal compromise but several Ziehl-Neelsen stains were positive. An echocardiography showed a vegetation on the prosthetic aortic valve. In blood and sputum samples, M. peregrinum type III was identified through culture, biochemical tests and hsp65 gene molecular analysis (PRA). The patient underwent a valve replacement and received a multidrug antimycobacterial treatment. Progressive recovery ensued and further samples from respiratory tract and blood were negative for mycobacteria.
Key words: Mycobacterium, Mycobacterium peregrinum infections, aortic valve, endocarditis, Colombia.
Micobacteriosis diseminada con compromiso de válvula aórtica protésica: primer caso de Mycobacterium peregrinum de tipo III reportada en Colombia
Las micobacterias de rápido crecimiento son microorganismos pertenecientes a las micobacterias no tuberculosas que tienen amplia distribución ambiental. Aunque usualmente no son patógenas para los humanos, en condiciones desfavorables, pueden causar enfermedad en la población general o en huéspedes inmunocomprometidos, por lo cual se consideran oportunistas. Mycobacterium preregrinum es una micobacteria de rápido crecimiento perteneciente al complejo fortuitum que ha sido reportado como responsable de casos de micobacteriosis en humanos.
Se presenta el caso de una micobacteriosis por M. peregrinum de tipo III, el primero reportado en Colombia, en una paciente de 17 años de edad con una endocarditis de una válvula aórtica protésica, implantada inicialmente por estenosis subaórtica congénita con insuficiencia y, posteriormente, por estenosis aórtica relacionada con la válvula inicialmente implantada. Un año después del segundo implante, presentó sintomas respiratorios y pérdida de peso sugestivos de tuberculosis pulmonar.
Las coloraciones de Ziehl-Neelsen del esputo fueron positivas aunque la radiografía de tórax no mostró compromiso del parénquima. En el ecocardiograma se encontró una vegetación en la válvula aórtica. En las muestras de sangre y de esputo, se identificó M. peregrinum de tipo III por cultivo, pruebas bioquímicas y análisis molecular del gen hsp65 por PCR-restriction pattern analysis (PRA).
La paciente se sometió a cambio de válvula y recibió tratamiento combinado contra la micobacteria, con rápida recuperación. Las muestras tomadas del sistema respiratorio y sanguíneo se tornaron negativas para micobacterias.
Palabras clave: Mycobacterium, Mycobacterium peregrinum, válvula aórtica protésica, endocarditis, micobacterias de rápido crecimiento, infecciones por Mycobacterium, Colombia.
The genus Mycobacterium comprises strictly pathogenic, opportunistic (potentially but not usually pathogenic), and non-pathogenic species. Opportunistic mycobacteria are commonly recovered from natural and human-influenced environments and can cause disease in humans and animals, especially birds. These characteristics reference them as environmental opportunistic mycobacteria or non-tuberculous mycobacteria (1,2). Environmental opportunistic mycobacteria include slowly growing mycobacteria (colonies usually visible after seven days, such as the Mycobacterium avium intracellulare complex) and rapidly growing mycobacteria (colonies usually visible less than seven days) (1,3).
Rapidly growing mycobacteria are classified in three groups: M. fortuitum, M. chelonae-M. abscessus and M. smegmatis (3). In clinical conditions, rapidly growing mycobacteria have been related to skin and soft tissue infections (frequently postsurgical wound infections), pulmonary disease, and colonization of implanted materials such as prosthetics, catheters and sutures; these infections could result in sepsis and disseminated disease (3-7). Clinical cases of environmental opportunistic mycobacteria, including rapidly growing mycobacteria, have been reported in Colombia (8-11).
Mycobacterium peregrinum, a rapidly growing mycobacterium belonging to the M. fortuitum group, has been reported as causing opportunistic disease in humans (3,12). Several past cases have had M peregrinum designated as the causative agen as a consequence of the reclassification of microorganisms initially described as M. fortuitum (3).
Herein, the first report of mycobacteriosis caused by M. peregrinum type III in Colombia is presented and, to the best of our knowledge, the first one affecting a prosthetic cardiac valve.
Clinical case
A seventeen-year old female patient, born in Arauca (Colombia), was referred to the Fundación Neumológica Colombiana-Fundación Cardioinfantil-Instituto de Cardiología (Bogotá, Colombia) with a diagnosis of prosthetic aortic valve endocarditis. Fifteen years before, the patient had been diagnosed with a persistent ductus arteriosus and a mild perimembranous interventricular communication and 5 years later, with a subaortic stenosis with moderate insufficiency of the aortic valve. These abnormalities were corrected by surgical closure of the ductus and interventricular communication, subaortic ring widening and implantation of a mechanical aortic valve.
Four years later, she began to develop a progressive relative aortic valve stenosis, and at age 14, 12 years after her initial diagnosis, her aortic valve was replaced by a mechanical valve. Two years later, the patient consulted because of persistent cough with hemoptoic sputum. A serial Ziehl-Neelsen (ZN) stain of the sputum was positive and an antituberculosis treatment with isoniazid (H), ethambutol (E) and rifampicin (R) was started. However, since the chest X-ray showed no parenchymal abnormalities and the ZN stain and culture of bronchoalveolar lavage was negative, the antituberculosis treatment was withdrawn. Eleven months later she presented with weakness and cough. A new ZN stain of the sputum was positive, again without parenchymal abnormalities. Antituberculosis treatment was restarted but complicated by a medicamentous hepatitis.
Six months later, the patient consulted because of lower limb pain, purpuric lesions suggesting vasculitis, left eye amaurosis, convulsive crisis, cough, and hemoptoic sputum. She was hospitalized at Fundación Cardiovascular de Colombia in Bucaramanga. An infectious endocarditis of her prosthetic valve was suspected and later confirmed by echocardiography. Direct stains and cultures for common microorganisms in blood, cerebral spinal fluid and sputum samples were negative. Collagen diseases were ruled out. A drug-resistant tuberculosis or non-tuberculous mycobacteria infection were suspected. A treatment with ciprofloxacine, claritromicine and trimethoprim-sulfamethoxazole was initiated, and due to non- medical reasons, the patient was referred to the Fundación Neumológica Colombiana-Fundación Cardioinfantil-Instituto de Cardiología for a new aortic valve replacement. A clinical diagnosis of infectious endocarditis was evident.
The physical examination showed a patient with deteriorated general condition, tachycardia, cardiac murmur, normal breath sounds, painful edema of the right knee, and purpuric lesions in the legs. No neurological abnormalities were found.
The hemogram showed normocytic normochromic anemia and normal count of white blood cells. Creatinine and transaminases levels were normal. The immunological profile and HIV serology were negative. A serial ZN stain of the sputum and blood cultures for common bacteria were also negative. The chest X-ray showed mild cardiomegaly with a mechanical aortic valve; neither lung abnormalities nor pleural effusions were found. The echocardiogram showed a 5 x 5 mm vegetation on the mechanical aortic valve, paravalvular leak and a saccular image suggesting abscess. The fiberoptic bronchoscopy was normal. The routine stains, including ZN stain, of the bronchoalveolar lavage were negative, but a significant lymphocytosis (81%) was found.
A rapidly growing mycobacteria was isolated in cultures from blood and sputum samples and sent to the Colombian National Institute of Health for phenotypical identification; pigment production was determined, as well as growth speed at 45° C, 37°C, 32°C and 22°C. Enzymatic tests for catalases, nitrates, arylsulphatase, pyrazinamidase, urease, acid phosphatase, tween hydrolysis and niacin detection were performed. Additionally, growth or inhibition capacity was determined in MacConkey medium, 0.2% picric agar and Lowestein Jensen (LJ) medium for 10 ug/L tiofen-carboxylic acid hydrazide (TCH), 250 ug/L hydroxylamine (HA), and 5% sodium chloride; Molecular identification was done through gene hsp65 restriction analysis (PRA) (13,14), the patterns found for the BstEII enzyme were 240/130/85 and 145/140/100/60 for the HaeIII enzyme, resulting in M. peregrinum type III identification in blood and sputum samples.
A disseminated mycobacteriosis with aortic valve endocarditis due to M. peregrinum type III was diagnosed.
The treatment was adjusted including amikacin, imipenem, clarithromycin, trimethoprim-sulfametho-xazole, rifampicin, and doxicicline and, a few days after, the patient was undergone to an implantation of a biological aortic valve with replacement of the ascending aorta and coronary re-implantation. Histopathologic findings of the resected material were compatible with mycobacteria infection but ZN stains were negative.
The patients condition slowly improved and she was finally discharged at the end of 2006. She continued improving but appearing hypoacusia probably due to amikacin which was withdrawn. She completed the antimycobacterial treatment prescribed for twelve months. Subsequent bacteriological tests, during and after the treatment, were negative (follow-up until 2008). During 2008, she required a reintervention related with her previous ascending aortic replacement.
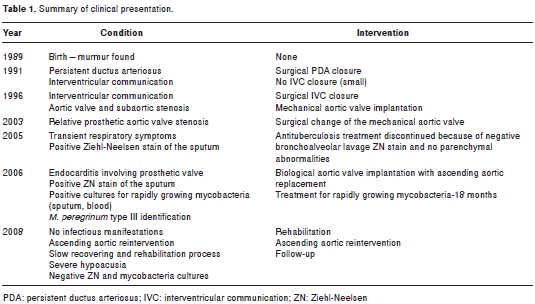
Table 1 shows a summary of the clinical presentation and management of the patient.
Discussion
Rapidly growing mycobacteria are microorganisms belonging to the non-tuberculous mycobacteria amply present in natural (water, soil) or human-influenced environments (3). Rapidly growing mycobacteria are not usually pathogenic for humans but they can cause several type of infections including skin and soft tissue infections, osteomyelitis, lymphadenitis, disseminated disease, meningitis, postsurgical wound infections, infections of prostethic devices, and chronic lung disease (3-7,15-18). Some cases have been described in Colombia (8,11).
M. peregrinum type III is a rapidly growing mycobacteria belonging to the M. fortuitum group (3) which also includes M. fortuitum, M. fortuitum third variant (19), M. mucogenicum, and M. senegalense. Although M. peregrinum was first proposed in 1962, only in the last years has been systematicly included (20-22). Like other rapidly growing mycobacteria, M. peregrinum has been reported as causing skin and soft tissue infections, peritonitis (after cancer gastric surgery), primary bacteremia, pneumonia, tonsillar abscess, catheter-related bacteremia, and implantable cardioverter device infection (12,23-27). Recently, Nagao informed 11 cases reported in the literature (12). However, it is possible that some other cases had been misclassified as M. fortuitum and previously reported (3).
The presence of foreign artificial material (catheter, implantable devices) or immunocompromising conditions (HIV infection, infliximab treatment) confirm the oportunistic character of M. peregrinum and suggest that those conditions favour the adherence, colonization, and posterior progression of the mycobacteria infection.
Non-tuberculous mycobacteria colonization and infection of intracardiac devices such as prosthetic valves, catheters, pacemaker wires or defibrillators have been known for long time (15-18). The presence in water sources and soil favors the colonization and infection of predisposed patients. M. fortuitum group may represent 1% to 2% of sporadic community-acquired or health care-associated infections due to rapidly growing mycobacteria (3) and up to 2% of in-hospital opportunistic infections (4).
The M. fortuitum group is more sensitive to several drugs than other rapidly growing mycobacteria making the treatment generally efective (3,7,12,28). Some of the effective drugs are: amikacin, imipenem, ciprofloxacin, gatifloxacin, levofloxacin, linezolid, clarithromycin, trimethoprim-sulfamethoxazole, cefo-xitin, and doxycycline (3,7,12,28). However, the best treatment for M. peregrinum has not been established and significant differences may be expected among the M. fortuitum group. For example, in the case reported by Nagao (12) the M. peregrinum was resistant to clarithromycion and minocycline. Rapidly growing mycobacteria are usually resistant to the common antituberculous drugs (isoniazide, for example).
As it has been described for disseminated cases (3), our patient was treated with two inyectable drugs: amikacin and imipenem, in addition to clarithromycin, trimethoprim-sulfamethoxazole, rifampicin, and doxicicline.
This is the first case of mycobacteriosis due to M. peregrinum type III described in Colombia and the first one reported as resulting in a prosthetic valve endocarditis, accepting that it is possible that some cases previously atributed to M. fortuitum may have been caused by M. peregrinum.
In our report, the disseminated condition was demonstrated by the isolation of the microorganism in sputum and blood samples. The repeatedly positive ZN stains of the sputum, with lymphocytosis in the bronchoalveolar lavage, and withouth lung parenchymal abnormalities suggests a colonization and infectious compromise of the upper respiratory tract or the tracheobronchial tree (tracheobronchitis). The conventional treatment for tuberculosis, as expected, was not effective, favoring the subsequent dissemination with colonization and infection of the prosthetic aortic valve (endocarditis due to M. peregrinum). Other manifestations such as the purpuric lesions in the legs, arthritis (right knee), amaurosis and convulsive crisis can be attributed to the endocarditis rather than direct infection due to the mycobacteria. It is difficult to establish the exact moment when the respiratory colonization evolved into a disseminated disease affecting mainly the prosthetic valve (endocarditis).
The persistent presence of positive ZN stain without positive cultures for M. tuberculosis, should suggest a diagnosis of microorganism containing mycolic acids in their walls like non-tuberculous mycobacteria, Corynebacterium, Nocardia and Rodhococcus (22).
Non-tuberculous mycobacteria, especially rapidly growing mycobacteria including M. peregrinum, should be considered in patients with insidious conditions and chronic use of catheters, prosthetic valves, or implantable devices.
Conflict of interest
None of the authors declare conflicts of interest.
Financing
Instituto Nacional de Salud de Colombia; Fundación Neumológica Colombiana; Fundación Cardioinfantil-Instituto de Cardiología; Fundación Cardiovascular de Colombia.
Correspondencia: Wellman Ribón, Universidad Industrial de Santander, Carrera 32 Nº 29-31, oficina 106, edificio Roberto Serpa, Bucaramanga, Colombia Teléfono: (057) 634 4000, extensión 3140 wribon@uis.edu.co, wellmanribon@yahoo.es
References
1. Falkinham III JO. Nontuberculous mycobacteria in the environment. Clin Chest Med. 2002;23:529-51. [ Links ]
2. Heifets L. Mycobacterial infections caused by nontuberculous mycobacteria. Semin Respir Crit Care Med. 2004;25: 283-95. [ Links ]
3. Brown-Elliott BA, Wallace RJ Jr. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev. 2002;15:716-46. [ Links ]
4. Raad II, Vartivarian S, Khan A, Bodey GP. Catheter-related infections caused by the Mycobacterium fortuitum complex: 15 cases and review. Rev Infect Dis. 1991;13:1120-5. [ Links ]
5. Griffith DE, Girard WM, Wallace RJ Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria: analysis of 154 patients. Am Rev Respir Dis. 1993;147:1271-8. [ Links ]
6. Wallace RJ Jr., Musser JM, Hull SI, Silcox VA, Steele LC, Forrester GD, et al. Diversity and sources of rapidly growing mycobacteria associated with infections following cardiac bypass surgery. J Infect Dis. 1989;159:708-16. [ Links ]
7. Daley CL, Griffith DE. Pulmonary disease caused by rapidly growing mycobacteria. Clin Chest Med. 2002;23:623-32. [ Links ]
8. Garzón MC, Orjuela D, Naranjo O, Llerena C. Micobacterias no tuberculosas en Colombia 1995-2003. Inf Quinc Epidemiol Nac. 2005;10:168-73. [ Links ]
9. Camargo D, Saad C, Ruiz F, Ramírez ME, Lineros M, Rodríguez G, et al. Iatrogenic outbreak of M. chelonae skin abscesses. Epidemiol Infect. 1996;177:113-9. [ Links ]
10. Ortegón M, Rodríguez G, Camargo D, Orozco LC. Mycobacterium chelonae y Mycobacterium abscessus: patógenos emergentes. Biomédica. 1996;16:217-38. [ Links ]
11. Murcia-Aranguren MI, Gómez-Marín JE, Alvarado FS, Bustillo JG, de Mendivelson E, Gómez B, et al. Frequency of tuberculous and non-tuberculous mycobacteria in HIV infected patients from Bogotá, Colombia. BMC Infect Dis. 2001;1:21. [ Links ]
12. Nagao M, Sanobe M, Bando T, Saito T, Shirano M, Matsushima A, et al. Surgical site infection due to Mycobacterium peregrinum: a case report and literature review. Int J Infect Dis. 2009;13:209-11. [ Links ]
13. Telenti A, Marchesi F, Balz M, Bally F, Bötter EC, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31:175-8. [ Links ]
14. Castro C, Puerto G, García L, Orjuela D, Polo C, Garzón M, et al. Identificación molecular de micobacterias no tuberculosas mediante análisis de los patrones de restric-ción, Colombia 1995-2005. Biomédica. 2007;27:439-46. [ Links ]
15. Wallace R Jr., Swenson J, Silcox V, Good RC, Tschen JA, Stone MS. Spectrum of disease due to rapidly growing mycobacteria. Rev Infect Dis. 1983;5:657-79. [ Links ]
16. Hogg GG, Schinsky M, McNeil M, Lasker BA, Silcox VA, Brown J. Central line sepsis in a child due to a previously unidentified Mycobacterium. J Clin Microbiol. 1999;37:1193-6. [ Links ]
17. Chua J, Wilkoff B, Lee I, Juratli N, Longworth D, Gordon S. Diagnosis and management of infections involving implantable electrophysiologic cardiac devices. Ann Intern Med. 2000;133:604-8. [ Links ]
18. Karchmer AW, Longworth DL. Infections of intracardiac devices. Cardiol Clin. 2003;21:523-71. [ Links ]
19. Wallace RJ Jr., Brown BA Jr., Silcox VA, Tsukamura M, Nash DR, Steele LC, et al.Clinical disease, drug susceptibility, and biochemical patterns of the unnamed third biovariant complex of Mycobacterium fortuitum. J Infect Dis. 1991;163:598-603. [ Links ]
20. Kusunki S, Elaki T. Proposal of Mycobacterium peregrinum sp.nov., nom. rev., and elevation of Mycobacterium chelonae subs. abscessus (Kubical et al.) to especies states: Mycobacterium abscessus comb.nov. Int J Syst Bacteriol. 1992;42:240-5. [ Links ]
21. Herdman AV, Steele JC. The new mycobacterial species-emerging or newly distinguished pathogens. Clin Lab Med. 2004;24:651-90. [ Links ]
22. Brown JM, McNeil MM, Desmond EP. Nocardia, Rhodococcus, Gordona, Actinomadura, Streptomyces, and other actinomycetes of medical importance. In: Murray PR, Baron EJ, Pfaller MA, Tenover FC, Yolken RH, editors. Manual of clinical microbiology. Seventh edition. Washington, D.C.: American Society for Microbiology; 1999. p. 370-92. [ Links ]
23. Rodríguez-Gancedo MB, Rodríguez-González T, Yague G, Valero-Guillen PL, Segovia-Hernández M. Mycobacterium peregrinum bacteremia in an immunocompromised patient with a Hickman catheter. Eur J Clin Microbiol Infect Dis. 2001;20:589-90. [ Links ]
24. Short WR, Emery C, Bhandary M, O JA. Misidentification of Mycobacterium peregrinum, the causal organism of a case of bacteremia and automatic implantable cardioverter defibrillator-associated Infection, due to its unusual acid-fast staining characteristics. J Clin Microbiol. 2005; 43: 2015-7. [ Links ]
25. Heliot MP, Roussel F, Herve F, Muir JF, Levesque H. Fatal Mycobacterium peregrinum pneumonia in refractary polymiositis treated with infliximab. Reumathology. 2005;44:1202-3. [ Links ]
26. Rivera-Olivero IA, Guevara A, Escalona A, Oliver M, Pérez-Alfonzo R, Piquero J, et al. Infecciones en tejidos blandos por micobacterias no tuberculosas secundarias a mesoterapia. ¿Cuánto vale la belleza? Enferm Infecc Microbiol Clin. 2006;24:302-6. [ Links ]
27. Sakai T, Kobayashi C, Shinohara M. Mycobacterium peregrinum infection in a patient with AIDS. Intern Med. 2005;44:266-99. [ Links ]
28. DeGroote MA, Huit G. Infections due to rapidly growing Mycobacteria. Clin Infect Dis. 2006;42:1756-63. [ Links ]














