Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Biomédica
versão impressa ISSN 0120-4157versão On-line ISSN 2590-7379
Biomédica v.30 n.3 Bogotá jul./set. 2010
ARTÍCULO ORIGINAL
1LAPEX-Laboratorio de Parasitología Experimental, Departamento de Biología, Facultad de Ciencias, Universidad de Los Andes, Mérida, Venezuela.
Recibido: 09/12/09; aceptado:28/04/10
Introduction. Leishmaniasis is a vector-borne disease transmitted by the intradermal inoculation of Leishmania (Kinetoplastida: Trypanosomatidae) promastigotes together with saliva during the bite of an infected sand fly.
Objective. The salivary glands were compared from two vector species, Lutzomyia ovallesi (Ortiz,1952) and Lutzomyia migonei (França,1920) (Diptera: Psychodidae).
Material and methods. Protein profiles by SDS PAGE of salivary glands were compared among species as well as their development at several times post feeding. First, mice were immunized to salivary proteins by exposure to biting by L. ovallesi and of L. migonei. Antibodies in these mice against salivary gland-specific proteins were evaluated by immunoblotting.
Results. No apparent change was revealed in the kinetic expression of salivary proteins induced by the different physiological states post feeding. Qualitative and quantitative variations were detected in16-18 polypeptides with molecular weights ranging from 6 to 180 kDa. Species-specific proteins were demonstrated for L. migonei and L. ovallesi. In addition, antibodies against salivary gland specific proteins were found in mice immunized by the saliva of both species.
Conclusion. Basic information was obtained concerning the nature of salivary gland proteins of L. migonei and L. ovallesi. This information helps to elucidate the role of salivary proteins and their potential as effective tools in screening risk factors in human and other vertebrate hosts.
Key words: Lutzomyia, leishmaniasis, salivary proteins and peptides, saliva, Venezuela.
Las glándulas salivales de dos flebotominos vectores de Leishmania: Lutzomyia migonei (França) y Lutzomyia ovallesi (Ortiz) (Diptera: Psychodidae)
Introducción. La leishmaniasis es una enfermedad transmitida por la inoculación intradérmica de promastigotes de Leishmania (Kinetoplastida: Trypanosomatidae) junto con la saliva del vector durante la picada de un flebotomino infectado.
Objetivo. Comparar las glándulas salivales de Lutzomyia ovallesi (Ortiz, 1952) y Lutzomyia migonei (França, 1920) (Diptera: Psychodidae) vectores de Leishmania en América del Sur.
Materiales y métodos. Se analizaron los perfiles proteicos por SDS-PAGE de las glándulas salivales de estas dos especies en los diferentes grupos y tiempos posteriores a la alimentación. Se evaluó la presencia de anticuerpos producidos en los ratones inmunizados por la picaduras de L. ovallesi y L. migonei por inmunotransferencia.
Resultados. Los resultados mostraron que no hay cambio aparente en la cinética de expresión de las proteínas salivales, inducidas por los distintos estados fisiológicos, en las dos especies, presentándose variaciones cualitativas y cuantitativas. Los perfiles proteicos revelaron alrededor de 16 a 18 polipéptidos, con pesos moleculares entre 6 a 180 kDa. Los resultados mostraron proteínas específicas para L. migonei y L. ovallesi. También, se detectaron anticuerpos producidos en los ratones inmunizados por las picaduras deambas especies, contra proteínas específicas de las glándulas salivales.
Conclusión. Los resultados proveen información básica sobre las proteínas salivales de las especies L. migonei y L. ovallesi que podrían ser importantes en futuros estudios como posible herramienta para estudiar los factores de riesgos en la población y en otros huéspedes vertebrados.
Palabras clave: Lutzomyia, leishmaniasis, proteínas y péptidos salivales, saliva, Venezuela.
The saliva of sand flies (Diptera: Psychodidae) contains a diversity of bio-molecules with important functions. The saliva acts as a lubricant, as a cleaner for mouthparts, and as as a food moisturizer; its enzymes aid in the digestion of blood as well as food rich in carbohydrates, and its anticoagulants prevent blood from clotting blood feeding (1-7). The saliva also assists the establishment of the parasite within the vertebrate host, and it affects the vector competence of these insects to transmit Leishmania (Ross)(Kinetoplastida: Trypanosomatidae) (6,8-14).
Some researchers have regarded the saliva of sand flies as a "pharmacological cocktail" because it contains a variety of molecules such as proteins, enzymes, prostaglandins, nucleotides and nucleosides, which can act as anti-haemostatic, anti-inflammatory and immune modulator agents (8,12,15-18). These molecules modify the physiology of their host at the location of the bite, facilitating the acquisition of blood meal by the sand fly, as well as providing a suitable microenvironment for the development of Leishmania (19,20).
However, substantial variation has been reported in the immunosuppresive and anti-haemostatic components in saliva for different populations and species of sand flies (4,6,21). Antibodies against sand fly saliva in hamsters (10,22), in mice (11,14) and in humans have been demonstrated after exposure to feeding sand flies or salivary gland extracts (13,14,23).
Most studies of sand fly saliva have been undertaken with the species of Phlebotomus (Rondani & Berté) and Lutzomyia longipalpis (Lutz & Neiva). The current objective is to determine whether species-specific proteins occur in the saliva from two other important neotropical vectors, L. ovallesi (Ortiz) and L. migonei (França) that may affect their vectorial capacity (24-27). Both species have been incriminated as vectors of cutaneous leishmaniasis in the north-central and west-central regions of Venezuela (28-32).
Material and methods
Sand flies
Lutzomyia ovallesi and L. migonei female sand flies, 5 to 7 days old, were used from a laboratory colony originating from specimens captured in Arenal, Ejido, Mérida State, Venezuela (8°35´´N,71°9´W), at 1,360 m above sea level. These colonies were maintained in an incubator at 25±1°C and with a relative humidity of 80±10% (33), in the Laboratorio de Parasitología Experimental (LAPEX), de la Universidad de Los Andes, Mérida, Venezuela.
Parasites
The parasite species usedwas Leishmania amazonensis (Lainson & Shaw) (IFLA/BR/1967/PH8) WHO reference strain) maintained by periodic passage through experimental infections in hamsters Mesocricetus auratus (Waterhouse) at LAPEX.
Salivary glands obtained
Females of L. ovallesi and L. migonei were dissected with stylets under a stereoscopic microscope (Zeiss-Stemi, 2000-C), in phosphate-buffered saline (PBS), pH 7.2 at 4°C. The dissected salivary glands were collected in polypropylene vials containing 20 µl of a Tris buffer (Tris 20 mM, NaCl 150 mM) pH 7.6 and stored in groups of 20 pairs of salivary glands at -20°C until later analysis.
Salivary glands measurements
Diameter and length of salivary gland lobules of L. ovallesi and L. migonei were measured with an Axiostar microscope (Zeiss) with objective lenses calibrated with a micrometer.
Proteins determination by the Lowry method
The modified Lowry method was used for the protein determination in salivary glands of female L. ovallesi and L. migonei, using the reagent of Folin Ciocalteu (34). Absorbance was determined in a Microwell System reader at 660 nm. For each protein determination, 40 pairs of salivary glands were homogenized in 20 µl Tris buffer pH 7.6. Each protein determination was repeated three times.
Preparation of L. ovallesi and L. migonei mouse anti-saliva antiserum
Two groups of 12 mice, Mus musculus (Linnaeus) BALB/c, were immunized; one group exposed to biting L. ovallesi females and the other exposed similarly to L. migonei. The mice were immobilized by intraperitoneal injection of sodium pentobarbital. Nineteen feeding episodes were administered to the mice using L. ovallesi and twenty-two episodes using L. migonei. Each feeding episode was approximately 2 h long, with the episodes distributed over a 6-month period. Each group was bitten by 90 to 150 sand flies, once a week. A week after the last biting exposures, the mice were bled by heart puncture, and the serum separated and stored at -20°C until use. At the same time, control sera were obtained from Balb/c mice which had never been exposed to sand fly bites.
Artificial feeding
Females sand flies were allowed to feed to repletion on blood provided by a membrane feeder, using chicken skin membrane. Young and healthy mice (Mus musculus), strain NMRI, provided the blood. The membrane feeder was maintained at a temperature of 37 ± 1°C. The fed females fed were placed in an incubator with a supplementary diet of sucrose solution. Salivary gland samples were dissected from these flies at 0, 24, 72, and 120 hours post blood feeding.
Artificial infection
To produce infections in the sand fly, the blood in the membrane feeder was mixed with amastigotes of L. amazonensis from foot lesion tissue of experimentally infected hamsters. Tissues were homogenized in a mortar with buffered saline PBS, pH 7.2 at 4°C, and then centrifuged at 12,000 g for 20 min. The number of parasites was estimated in a Neubauer chamber and a 2x107 amastigotes/blood ml inoculum was used. Afterwards, fed females were maintained as above. The salivary glands from the sand flies were obtained at 0, 24, 72, and 120 hours post blood feeding.
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
Salivary gland proteins from L. ovallesi and L. migonei females were isolated by the SDS-PAGE technique using a discontinuous gel system described by Laemmli (35). Gels of 4% were used for the stacking gel and of 10% for the resolving gel. Protein samples were prepared with sample buffer, using 10-15 ug of protein per lane. Electrophoresis runs were performed at 300 mA with a constant voltage of 200V for 90 min. Gels were fixed with ethanol solution, washed and stained with silver nitrate. After staining and the appearance of bands, gels were placed on a transilluminator and photographed with a 5.0 megapixels digital camera Minolta Dimage F300.
Molecular weight determination
Salivary gland lysates from L. ovallesi and L. migonei were compared on the SDS-PAGE with markers of known molecular weight ranging from 6-180 kDa Sigma USA, and to provide calibrated curves for each gel.
Immunoblotting of the proteins from L. ovallesi and L. migonei salivary glands
To determine the antigenicity of proteins from salivary glands of L. ovallesi and L. migonei, the immunoblotting technique of Towbin et al. (36) was used. After electrophoretic fractioning by SDS-PAGE to 10%, the gel was transferred to nitrocellulose membranes (0.2 µM), in presence of transfer buffer at 70V for 90 min. These membranes were incubated at room temperature for one h in blocking solution with gentle agitation. The membranes were incubated (1 h, gentle agitation) with serum from the mice exposed to sand fly females of L. ovallesi and L. migonei, diluted 1/50 in TBS buffer plus 5% low-fat dried milk. Three 5 min TBS buffer washes followed, each with gentle agitation. The membranes were incubated with the secondary anti-IgG antibody from mice conjugated with peroxidase, in presence of TBS buffer plus 5% skim milk. After three washes in PBS, the membranes were incubated with fresh buffer and developing solution. When the bands appeared, the reaction was stopped with running water.
Statistical analysis
The arithmetic means of the diameters of L. ovallesi and L. migonei salivary glands and the mean values for protein content were tested for significance by the Mann-Whitney test (37), with significance set at p<0.05.
Ethical clearance. The study was reviewed and approved by the Bioethical Committee of the University of The Andes for its use of laboratory animals.
Results
Lutzomyia ovallesi salivary glands (figures 1 and 2) were smaller in size compared to those of L. migonei, with a mean for the major lobe of 150.1 ± 18.6µm in length and 108.2 ± 17.8 µm in width; and for the minor lobe a mean of 133.3 ± 22.0 µm in length and 96.3 ± 16.4 µm in width. Whereas L. migonei salivary glands (figures 3 and 4) presented a mean of 177.9 ± 15.3 µm in length and 114.4 ± 12.7 µm in width for the major lobe; and of 143.9 ± 26.1 µm in length and 94.7 ± 20.6 µm in width for the minor lobe. Statistical analysis showed significant differences for salivary gland size between the two species (p= 0.040). The salivary glands of L. ovallesi and L. migonei after the blood feeding presented a statistically significant diminution of size in both lobes, compared to post sucrose feeding. Lutzomyia ovallesi glands were consistently smaller for both lobes in comparison with L. migonei, (p=0.028) (table 1).
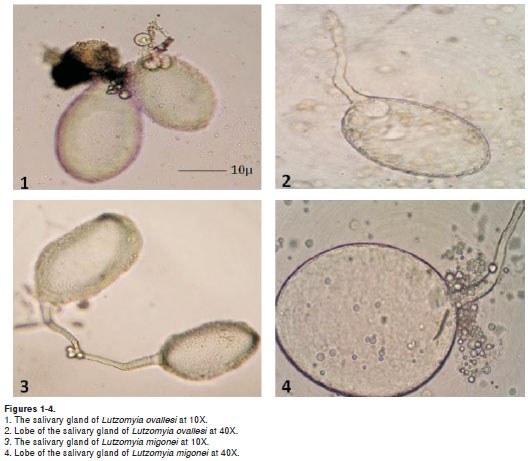
The results for the protein content in L. migonei salivary glands fed with sucrose showed the greatest protein concentration with a mean of 0.84 ± 0.02 ug, whereas L. ovallesi presented a mean of 0.59 ± 0.10 ug of protein detected, with a significant difference between the concentration of both groups of salivary glands of females (p=0.025).
The profile for the protein expression in salivary glands of the sugar-fed L. ovallesi consisted of 18 protein bands within the range of 6 to 180 kDa and for L. migonei females, 16 protein bands with that range.
However, most of the proteins in the L. ovallesi saliva (whether fed with sugar solution, blood or Leishmania infected blood), were also present in L. migonei saliva. Despite the similarity in expression in both species, qualitative and quantitative interspecific variation was present, such as appearance and disappearance of polypeptides, intensity and thickness of protein bands, and the detection of additional polypeptides with molecular weights >100 kDa (table 2). Fourteen protein bands were present in both species, corresponding to the following molecular weights: 6, 14, 18, 20, 29, 45, 48, 52, 66, 92, 112, 116, 125 and 180 kDa. Four bands were specific to L. ovallesi (15, 99, 100 and 170 kDa) and two bands specific to L. migonei (25 and 32 kDa).
Evaluation of L. ovallesi anti-saliva protein by immunoblotting showed the presence of 5 protein bands with molecular weights of 6, 14, 18, 20 and 29 kDa. The L. migonei anti-saliva serum recognized 5 protein bands with molecular weights of 6, 14, 20, 25 and 29 kDa. The 18 kDa band was unique to L. ovallesi and the 25 kDa unique to L. migonei.
Strong cross-reactivity was observed between the L.ovallesi anti-saliva serum and the protein bands for L. migonei saliva; the serum recognized 4 protein bands with molecular weights of 6, 14, 25 and 29 kDa in the L. migonei salivary gland homogenate. Similarly, the L. migonei anti-saliva serum recognized one protein of 29 kDa in the L. ovallesi homogenates (table 3).

Discussion
Statistically significant differences were demons-trated between the protein content and size of L. ovallesi and L. migonei salivary glands. Lutzomyia ovallesi salivary glands were of smaller dimension and contained fewer proteins compared with L. migonei. The differences were in accordance with the published accounts of protein contents of a variety of sand fly species (7,21,38,39). Bloodmeal analysis indicated that these species were opportunistic feeders, with little attraction to a particular host species (32). Lutzomyia migonei is a widespread sand fly in South American and is a suspected Leishmania vector in several Brazilian states, as well as in Venezuela. It can be infected with either Leishmania (Viannia) braziliensis or L. (Leishmania) amazonensis, indicating this species to be a competent host and potential vector (25). Recently, this species was also implicated as a vector of visceral leishmaniasis (Leishmania infantum) in Argentina (40). In Venezuela, Lutzomyia ovallesi is the primary vector of cutaneous leishmaniasis (L. braziliensis) in the north-central area of Venezuela (41,42). Overall, leishmaniasis in Venezuela is endemic and focal, and the knowledge of the role of the many sand fly species as vectors is fragmentary. The present study has provided data on the biological characteristics of two probable vectors in this country.
A similar difference in lobe size of salivary glands has been observed mosquito females, which exhibit three lobes of different sizes (43,44). Moreover, the results show a decrease in gland size between these two species of Lutzomyia post blood feeding in comparison with sugar solution feeding. These differences are statistically significant (p<0.05). Other authors report that after being fed with blood or carbohydrates, the total protein content of sand fly saliva can decrease by half or more of its original quantity (7,39,45,46). These observations provide support for the differences in gland size described above.
These differences may be due to several factors, such as the amount and composition of the food ingested, preference for a specific vertebrate host, behavior or feeding habits of the different species (7,47-50), and are factors that can play mitigating roles in L. ovallesi and L. migonei vectorial capacity. These differences may have co-evolved among the different sand fly salivary proteins and the host in order (1) to avoid homeostasis of the vertebrate host and (2) to ease the ingestion by presenting specific activities such as coagulation cascade inhibition, platelet aggregation and vasodilatation.
Furthermore, the results show that most of the proteins of the L. ovallesi saliva fed with sugar solution, blood and Leishmania infected blood were also present in L. migonei saliva. Changes in the kinetic expression of salivary proteins were not apparent in the different physiological states for both species, although the results showed qualitative and quantitative variation appearance and disappearance of polypeptides, intensity and thickness of protein bands, and the detection of polypeptides with molecular weights higher to 100 kDa.
Differences have been found in protein profiles of salivary gland homogenates of sand flies belonging to the genus Phlebotomus and Lutzomyia fed with sugar solution, for which protein profiles ranged from 8 to 17 protein bands with a molecular weight range of 6-80 kDa (6,14,23,51).
The different L. ovallesi and L. migonei salivary gland homogenates, under the different physiological processes and experimental times, showed nine (9) main polypeptides ranging from 6 to 66 kDa common to both of these species. However, two polypeptide products of 25 and 32 kDa for L. migonei were not found in L. ovallesi saliva; and four (4) polypeptides with molecular weights of 15, 99, 100 and 170 kDa, can be found only in L. ovallesi. Although saliva protein determinations for other sand fly species have been already reported, no comparative studies Lutzomyia species have been done (3,5,6). The differences between L. ovallesi and L. migonei may involve differences in the activity of the molecules involved in the process of feeding or in the digestion process. Small differences in protein expression or small changes in the post-transductional polypeptides may explain the parasite-vector specificity that occurs in nature (50,52).
Antigenic components in sand fly salivary proteins have been reported to induce the production of immune globulins in animals exposed to sand fly bites (10,13,14). In this regard, Volf and Rohousova (23), found important differences in P. papatasi (Scopoli), P. perniciosus (Newstead)and P. halepensis (Theodor) salivary antigen components. These authors detected a weak cross-reaction between P. perniciosus and P. halepensis. These observations contrast to some extent the stronger cross-reactions observed in the current study. These results are also in accordance with the observations made by Morris et al. (53), who demonstrated immunity in animals vaccinated with the salivary protein maxadilan, a vasodilatory peptide and immune modulator of 6.5 kDa of L. longipalpis. A high production of antibodies was induced that was able to protect animals from the infection by L. major (Yakimoff & Schokhor).
Moreover, Barral et al. (13) reported the presence of six main antigens in L. longipalpis saliva, 3 of which were often recognized by the serum from individuals with a low immune response to antigens of Lutzomyia. Antigens with molecular weights of approximately 6, 12, 36 and 96 kDa were recognized only by the serum from individuals with IgG antibodies for antigens of Lutzomyia. Similarly, Gomes et al. (24) identified 5 salivary antigens by immunoblotting with molecular weights of 45, 44, 43, 35 and 17 kDa in L. longipalpis saliva. These salivary antigens were recognized by the serum from children who lived in an endemic area of visceral leishmaniasis in Brazil.
Furthermore, Barral et al. (13), found a significant correlation between L. longipalpis IgG anti-saliva levels and the Leishmania chagasi (Cunha & Chagas) delayed-type hypersensitivity response (DTH), showing that the study of anti-saliva antibodies of sand flies must be considered to be an integral part of the epidemiology of leishmaniasis.
The results obtained herein demonstrate the presence of specific proteins in L. ovallesi and L. migonei salivary glands, as well as the presence of saliva polypeptides common to both species. These polypeptides have potential as epidemiological markers in endemic zones and as possible molecular targets for the vaccine-type control alternatives. As potential for vaccine development, the constant proteins in the 2 species as well as specific proteins that facilitate the entrance of the parasite into the host vertebrate are best candidates for a saliva-based anti-Leishmania vaccine. Additionally, it is suggested to use new technologies, such as the genomic and the proteomic, in an effort to produce recombinant proteins.
Acknowledgments
We are grateful to Irlanda Márquez for assistance.
Conflict of interests
We did not identify any conflict of interest.
Financing
This study was supported by grant of CDCHT-ULA (Cod: C-1606-08-03-B) and project LOCTI-ULA (Cod: L-C-13-07-03.2007-MRW).
Correspondencia: Elsa Nieves, Laboratorio de Parasitología Experimental, Departamento de Biología, Facultad de Ciencias, Universidad de Los Andes, Mérida, Venezuela. Telephone: (0274) 2401244. nevelsa@ula.ve
References
1. Foster WA. Mosquito sugar feeding and reproductive energetics. Annu Rev Entomol. 1995;40:443-74. [ Links ]
2. Holiday-Hanson ML, Yuval B, Washino RK. Energetics and sugar-feeding of field-collected anopheline females. J Vector Ecol. 1997;22:83-9. [ Links ]
3. Charlab R, Valenzuela JG, Rowton ED, Ribeiro JM. Toward an understanding of the biochemical and pharmacological complexity of the saliva of a hemathophagous sand fly Lutzomyia longipalpis. Proc Natl Acad Sci USA.1999;96:15155-60. [ Links ]
4. Elnaiem DE, Meneses C, Slotman M, Lanzaro GC. Genetic variation in the sand fly salivary protein, SP-15, a potential vaccine candidate against Leishmania major. Insect Mol Biol. 2005;14:145-50. [ Links ]
5. Anderson JM, Oliveira F, Kamhawi S, Mans BJ, Reynoso D, Seitz AE, et al. Comparative salivary gland transcriptomics of sandfly vectors of visceral leishmaniasis. BMC Genomics. 2006;7:52. [ Links ]
6. Wahba M, Riera C. Salivary gland composition of some Old World vector sand fly. J Egypt Parasitol. 2006;36:289-96. [ Links ]
7. Prates DB, Santos LD, Miranda JC, Souza AP, Palma MS, Barral-Netto M, et al. Changes in amounts of total salivary glands proteins of Lutzomyia longipalpis Diptera: Psychodidae according to age and diet. J Med Entomol. 2008;43:409-13. [ Links ]
8. Ribeiro JM. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect Agents Dis. 1995;4:143-52. [ Links ]
9. Stark KR, James AA. Anticoagulants in vector arthropods. Parasitol Today.1996;12:430-7. [ Links ]
10. Ghosh KN, Mukhopadhyay J. The effect of anti-sandfly saliva antibodies on Phlebotomus argentipes and Leishmania donovani. Int J Parasitol. 1998;28:275-81. [ Links ]
11. Belkaid Y, Kamhawi S, Modi G, Valenzuela J, Noben-Trauth N, Rowton ED, et al. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J Exp Med. 1998;188:1941-53. [ Links ]
12. Belkaid Y, Valenzuela JG, Kamhawi S, Rownton E, Sacks DL, Ribeiro JM. Delayed-type hypersensitivity to Phlebotomus papatasi sand fly bite: an adaptive response induced by the fly? Proc Natl Acad Sci USA. 2000;97:6704-9. [ Links ]
13. Barral A, Honda E, Caldas A, Costa J, Vinhas V, Rowton ED, et al. Human immune response to sandfly salivary gland antigens: a useful epidemiological marker?. Am J Trop Med Hyg. 2000;62:740-5. [ Links ]
14. Rohousova I, Ozensoy S, Ozbel Y, Volf P. Detection of species-specific antibody response of humans and mice bitten by sandflies. Parasitol. 2005;130:493-9. [ Links ]
15. Ribeiro JM. Role of saliva in blood-feeding by arthropods. Ann Rev Entomol. 1987;32:463-78. [ Links ]
16. Champagne DE. The role of salivary vasodilators in blood-feeding and parasite transmission. Parasitol Today. 1994;10:430-3. [ Links ]
17. Bowman AS, Dillwith JW, Saber JR. Tick salivary prostaglandins: presence, origin and significance. Parasitol Today. 1996;12:388-96. [ Links ]
18. Bowman, JD, Waddell D, Hanson BD. Biochemical mechanism of the antileishmanial activity of sodium stibogluconate. AntimicrobAgents Chemother. 1997;27: 916-20. [ Links ]
19. Champagne DE, Valenzuela JG. Pharmacology of Haematophagous arthropod saliva. En: Wikel SK, editor. The immunology of host-ectoparasitic arthropod relationships. Wallingford, UK: CAB International; 1996. p. 107-30. [ Links ]
20. Wikel SK, Ramachandra RN, Bergman DK. Arthropod modulation of host inmune responses. En: Wikel SK, editor. The immunology of host-ectoparasitic arthropod relationships. Wallingford, UK: CAB International; 1996. p. 107-30. [ Links ]
21. Volf P, Tesarova P, Nohynkova E. Salivary proteins and glycoproteins in phlebotomine sandflies of various species, sex and age. Med Vet Entomol. 2000;14:251-6. [ Links ]
22. Volf P, Rohousova I. Species-specific antigens in salivary glands of phlebotomine sandflies. Parasitology. 2001; 122:37-41. [ Links ]
23. Gómez RB, Brodskyn C, De Oliveira CI, Costa J, Miranda JC, Caldas A, et al. Seroconversion against Lutzomyia longipalpis saliva concurrent with the development of anti-Leishmania chagasi delayed - type hypersensitivity. J Infect Dis. 2002;186:1530-4. [ Links ]
24. Nieves E, Pimenta PF. Development of Leishmania (Vianna) braziliensis and Leishmania (Leishmania) amazonensis in the sand fly Lutzomyia migonei (Diptera: Psychodidae). J Med Entomol. 2000;37:134-40. [ Links ]
25. Nieves E, Pimenta PF. Influence of vertebrate blood meals on the development of Leishmania (Vianna) braziliensis and Leishmania (Leishmania) amazonensis in the sand fly Lutzomyia migonei (Diptera: Psychodidae). Am J Trop Med Hyg. 2002;37:640-7. [ Links ]
26. Nieves E, Dávila-Vera D, Palacios-Prü E. Daño ultraestructural del intestino medio abdominal de Lutzomyia ovallesi (Ortiz) (Diptera: Psychodidae) ocasionado por Leishmania (Leishmania) amazonensis. Parasitol Latinoam. 2004;59:115-22. [ Links ]
27. Noguera P, Rondón M, Nieves E. Caloric content of the sand fly Lutzomyia ovallesi (Diptera: Psychodidae) vector of Leishmania. Revista Colombiana de Entomología.2006;32:57-60. [ Links ]
28. Bonfante-Garrido R, Urdaneta R, Urdaneta I, Alvarado J. Natural infection of Lutzomyia ovallesi (Diptera: Psychodidae) with Leishmaniasis in Duaca, Lara State, Venezuela. Trans R Soc Trop Med Hyg. 1991;85:61. [ Links ]
29. Bonfante-Garrido R, Spinetti H, Cupillo E, Momen H, Grimaldi G. Lutzomyia ovallesi (Diptera: Psychodidae) as a vector of cutaneous leishmaniasis in Venezuela.Parassitologia. 1991;33:99-104. [ Links ]
30. Feliciangeli MD. Vectors of leishmaniasis in Venezuela. Parassitologia. 1991;33:229-36. [ Links ]
31. Añez N, Nieves E, Cazorla D. Epidemiology of cutaneous leishmaniasis in Mérida, Venezuela: III. Altitudinal distribution, age structure, natural infection and feeding behaviour of sandflies and their relation to the risk of transmission. Ann Trop Med Parasitol. 1994;88:279-87. [ Links ]
32. Nieves E, Villarreal N, Rondón M, Sánchez M, Carrero J. Factores de riesgo y evaluación de conocimiento sobre la leishmaniasis tegumentaria en un área endémica de Venezuela. Biomédica. 2008;28:347-56. [ Links ]
33. Killick-Kendrick R, Leaney AJ, Ready PD. The establishment, maintenance and productivity of laboratory colony of Lutzomyia Longipalpis (Diptera: Psychodidae). J Med Entomol. 1977;13:429-40. [ Links ]
34. Lowry OH, Rosenbrough N, Farr A, Randall R. Protein measurement with the Folin Phenol reagent. J Biol Chem. 1951;193:265-75. [ Links ]
35. Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-5. [ Links ]
36. Towbin J, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350-4. [ Links ]
37. Wayne WD. Bioestadística base para el análisis de las Ciencias de la Salud. Cuarta edición. México: Limusa Wiley; 2002. [ Links ]
38. Ribeiro JM, Rossignol PA, Spielman A. Blood finding strategy of a capillary - feeding sandfly Lutzomyia longipalpis. Comp Biochem Physiol. 1986;83:683-6. [ Links ]
39. Cerná P, Mikes L, Volf P. Salivary gland hyaluronidase in various species of phlebotomine sandflies (Diptera: Psychodidae). Insect Biochem Biol. 2002;32:1691-7. [ Links ]
40. Salomón OD, Quintana MG, Bezzi G, Morán ML, Betbeder E, Valdéz DV. Lutzomyia migoneias putative vector of visceral leishmaniasis in La Banda, Argentina. Acta Trop. 2010;113:84-7. [ Links ]
41. Feliciangeli MD, Reyes RM, Limongi JE. Natural infection of Lutzomyia ovallesi (Diptera: Psychodidae) with parasites of the Leishmania braziliensis complex in a restricted focus of cutaneous leishmaniasis in northern Venezuela. Mem Inst Oswaldo Cruz. 1988; 83: 393-4. [ Links ]
42. FeliciangeliMD, Rodríguez N, Bravo A, Arias F, GuzmánB.Vectors of cutaneous leishmaniasis in north-central Venezuela. Med Vet Entomol. 1994;5:317-24. [ Links ]
43. Moreira-Ferro CK, Marinnotti O, Bijovsky AT. Morphological and biochemical analyses of the salivary glands of the malaria vector, Anopheles darlingi. Tissue Cell. 1999;31:264-73. [ Links ]
44. Nascimento EP, Dos Santos Malafronte R, Marinnotti O. Salivary gland proteins of the mosquito Culex quinquefasciatus. Arch Insect Biochem Physiol.2000;43:9 -15. [ Links ]
45. Cerná P, Mikes L, Volf P. Salivary gland hyaluronidase in various species of phlebotomine sandflies (Diptera: Psychodidae). Insect Biochem Biol. 2002;32:1691-7. [ Links ]
46. Kato H, Jochim RC, Lawyer PG, Valenzuela JG. Identification and characterization of a salivary adenosine deaminase from the sand fly Phlebotomus duboscqi, the vector of Leishmania major in subsaharan Africa. J Exp Biol. 2007;210:733-40. [ Links ]
47. Ribeiro JM, Rowton ED, Charlab R. Salivary amylase activity of the phlebotomine sand fly, Lutzomyia longipalpis. Insect Biochem Mol Biol. 2000;30:271-7. [ Links ]
48. Katz O, Waitumbi JN, Zer R, Warburg A. Adenosine, AMP, and protein phosphatase activity in sandfly saliva. Am J Trop Med Hyg. 2000;62:145-50. [ Links ]
49. Jacobson RL, Schlein Y. Phlebotomus papatasi and Leishmania major parasites express α- amylase and α-glucosidase. Acta Trop. 2001; 78: 41-9. [ Links ]
50. Valenzuela JG, Belkaid Y, Rowton ED, Ribeiro JM. The salivary apyrase of the blood-sucking sand fly Phlebotomus papatasi belongs to the novel Cimex family of apyrases. J Exp Biol. 2001;204:229-37. [ Links ]
51. Valenzuela JG, Garfield M, Rowton ED, Pham VM. Identification of the most abundant secreted proteins from the salivary glands of the sandfly Lutzomyia longipalpis, vector of Leishmania chagasi. J Exp Biol. 2004;207:3717-29. [ Links ]
52. Freire T, Robillo C, Casaravilla C, Álvarez DE, Medeiros AC, Carmona C, et al. Antígenos mucínicos de O -glicosilación simple: nuevas similitudes moleculares entre células cancerosas y parásitos. Actas Fisiol. 2002;8:89-107. [ Links ]
53. Morris RB, Shoemaker CB, David JR, Lanzaro GR, Titus RG. Sandfly Maxadilan exacerbates Infection with Leishmania major and vaccinating against it protects against L. major infection. J Immunol. 2001;167:5226-30. [ Links ]














