Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biomédica
Print version ISSN 0120-4157On-line version ISSN 2590-7379
Biomédica vol.31 no.3 Bogotá July/Sept. 2011
ARTÍCULO ORIGINAL
1Laboratorio de Investigación en Productos Naturales Vegetales, Departamento de Química, Facultad de Ciencias, Universidad Nacional de Colombia, Bogotá, D.C., Colombia
2Laboratorio de Farmacogenética del Cáncer, Departamento de Farmacia, Facultad de Ciencias, Universidad Nacional de Colombia, Bogotá, D.C., Colombia
Recibido: 24/09/10; aceptado:28/04/11
Introduction. The antiproliferative effect of eleven neolignans, two lignans and one diterpene isolated from three Lauraceae plants, four benzofurans and two bicyclooctanes synthetic derivatives was evaluated in vitro on a set of five human cancer cells from solid tumors with a high incidence in Colombia.
Objective. To evaluate the cytotoxic effect of twenty compounds on the tumor cell lines HeLa, A-549, Hep-2, PC-3, and MCF-7.
Materials and methods. Fourteen natural compounds were isolated by chromatographic techniques from three native colombian plants (Pleurothyrium cinereum, Ocotea macrophylla and Nectandra amazonum), whose structures were established by spectroscopic methods; six synthetic derivatives were prepared by oxyarylation and diazomethane methylation. Antiproliferative effect and cell recovery were performed by means of in vitro treatment of tumor cell lines with test compounds, evaluating cell viability by resazurin staining.
Results. Among test compounds, only neolignans ocophyllal A, cinerin D, kaurenoic acid, two benzofuran-derivatives, and synthetic (-)-cinerin A were found to have antiproliferative effect at different levels. Bicyclooctanoids as well as kaurenoic acid exhibited activity against all human cancer cells while benzofuranoids showed selective activity against HeLa. Furthermore, compounds (-)-cinerin A and kaurenoic acid exhibited total lethal effect against all-five cell lines and PC-3, Hep-2, and A549 cell lines, respectively.
Conclusion. Test compounds exhibiting antiproliferative activity showed interesting results, which would promote their use as lead compounds on further studies for anticancer agents development.
Key words: biological products, Lauraceae, straining, diterpenes, in vitro.
Efecto citotóxico de algunos compuestos naturales aislados de plantas Laureaceae y derivados sintéticos
Introducción. El efecto contra la proliferación celular de once neolignanos, dos lignanos y un diterpeno, aislados de tres plantas de la familia Lauraceae, y cuatro benzofuranos y dos biciclooctanos sintéticos, fue evaluado in vitro sobre cinco líneas celulares derivadas de tumores sólidos de alta incidencia en Colombia.
Objetivo. Evaluar el efecto citotóxico de veinte compuestos sobre las líneas tumorales HeLa, A-549, Hep-2, PC-3 y MCF-7.
Materiales y métodos. Los 14 compuestos de origen natural fueron aislados de tres plantas nativas colombianas (Pleurothyrium cinereum, Ocotea macrophylla y Nectandra amazonum) por técnicas cromatográficas y se establecieron sus estructuras por métodos espectroscópicos, y los seis derivados sintéticos fueron preparados mediante reacción de oxiarilación y metilación con diazometano. El efecto contra la proliferación y la recuperación celular se hicieron mediante tratamiento in vitro de las líneas tumorales con los compuestos , evaluando la viabilidad celular por tinción con resazurina.
Resultados. Entre los compuestos evaluados, solamente ocofilal A, cinerina D, ácido kaurenoico, dos benzofuranos y la (-)-cinerina A sintética presentaron actividad contra la proliferación celular en diferentes niveles. Los biciclooctanos, así como el ácido kaurenoico, fueron activos contra todas las líneas celulares, mientras que los benzofuranoides mostraron actividad selectiva contra HeLa. Además, la (-)-cinerina A exhibió un efecto letal total contra todas las líneas celulares, mientras que el ácido kaurenóicopresentó efecto letal total contra PC-3, Hep-2 y A549.
Conclusión. Los compuestos evaluados que exhibieron actividad contra la proliferación celular mostraron resultados interesantes, lo cual sugiere su potencial uso como cabezas de serie o moléculas plantilla en el desarrollo de agentes anticancerígenos.
Palabras clave: productos biológicos, Lauraceae, cribado, diterpenos, in vitro.
Drug-seeds discovery from natural products has led to novel medical advances, mostly including research on anti-cancer agents through exploration for sources of novel cytotoxic compounds (1). Cytotoxicity studies can be regarded when compounds exhibit a potential biological activity on cell damage, whose causes might be related to either agent concentration or exposure time for cells to these agents, which compromises cell viability (2).
Cancer is one of the leading causes of death worldwide, being the main reason to searching for new antineoplastic agents, mostly from natural origin, which has became an important research approach (3). Currently, the drugs used to treat cancer may have two limitations: reduced activity, on the one hand, or acquired resistance by tumor cells, on the other (4). In Colombia, recent statistics indicated that the highest incidences of cancer disease correspond to breast, cervix, stomach, leukemia, colon, and prostate cancer (5). Therefore, performing in vitro screening in order to discover cytotoxic agents against tumor cell lines still remains as an important objective of anticancer research (6).
Lignans and neolignans (C6C3 dimers to the shikimate biosynthetic pathway) (7), are well-recognized for their wide-range of important biological activities, because they have been used as lead compounds for the development of new drugs (8). An important bioactive class of these type of natural products is related to the benzofuran moiety, which has exhibited antitumor (8), antiangiogenic (8), cytotoxic (9-11), antileishmanial (11), antitrypanosomal (12), antibacterial (13), PAF-antagonistic (14), among others, the cytotoxicity being a significant property of this type of compounds (8-11). Lauraceae plants are well-known to contain benzofuran neolignans as well as bicyclo 3.2.1octane neolignans (7), which are considered as benzofuran-derived (15), and they have exhibited excellent PAF-antagonistic activity (16).
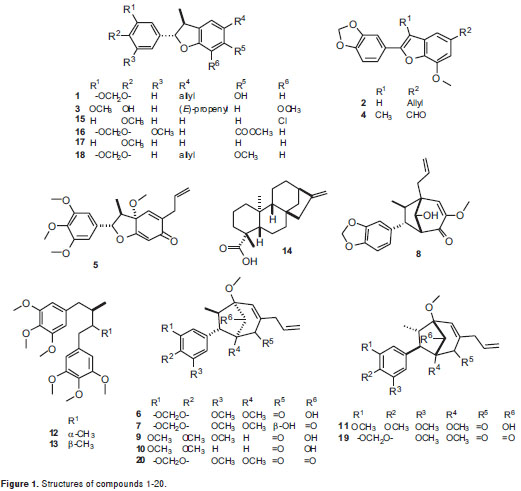
As part of our research on bioactive natural compounds, the phytochemical exploration carried out on the leaves of three Lauraceae plants (Pleurothyrium cinereum, Ocotea macrophylla and Nectandra amazonum) yielded five benzofuran neolignans 1-5, six bicyclo 3.2.1octane neolignans 6-11, two diastereomeric dibenzylbutane lignans 12-13and a diterpene 14. Including a group of six synthetic neolignan derivatives 15-20, the naturally-occurring compounds were screened in order to determine their in vitro antiproliferative effect on a set of five human cancer cells consisting of cervical carcinoma HeLa, lung carcinoma A-549, larynx carcinoma Hep-2, prostate adenocarcinoma PC-3, and breast carcinoma MCF-7. Structures of test compounds were determined by extensive spectroscopic analyses, and their absolute configuration was defined by circular dichroism (CD) comparing with data reported in the literature for related compounds.
Materials and methods
General experimental procedures
CD spectra were obtained using a JASCO J-720 spectropolarimeter. 1H and 13C Nuclear Magnetic Resonance (NMR) spectra were recorded on a Bruker Avance 400 spectrometer, using tetramethylsilane (TMS) as internal shift reference. High Resolution Mass Spectrometry (HRMS) was determined on a Shimadzu LCMS-IT-TOF (with an electrospray (ESI) source and in the positive ion mode) mass spectrometer. Cell growth indications were performed by resazurin method using a TECAN GENios plate reader with excitation wavelength at 535nm and emission at 595nm.
Plant material
Whole plants of Pleurothyrium cinereum, Ocotea macrophylla and Nectandra amazonum were collected in different regions of Colombia: Tumaco county, Department of Nariño, on the NocaimaVergara road at Nocaima county, Department of Cundinamarca, and near the biological station “El Zafire”, 6 hours from Leticia city, Department of Amazonas, respectively. Reference specimens (numbers COL518334 for P. cinereum, COL517191 for O. macrophylla, COL519818 for N. amazonum) are kept at Herbario Nacional Colombiano, Universidad Nacional de Colombia.
Extraction and isolation
Detailed extraction and isolation processes, and spectroscopic features for the test compounds were previously reported (19-24) (figure 1) including liliflol A 1 (17), 3´-methoxy-3,4-methylendioxy-4´,7-epoxy-nor-8.5´-neolignan-7.8´-diene 2 (12), (+)-licarin A 3 (18), ocophyllal A 4 (19), (+)-mirandin A 5 (20), cinerin C 6 (21), (-)-cinerin D 7 (21), 2´-epi-guianin 8 (19), ocophyllol C 9 (19), kadsurenin C 10 (22), nectamazin C 11 (23), meso- and threo-3,4,5,3´,4´,5´-hexamethoxy-8.8´-neolignan 12-13 and kaurenoic acid 14 (24). Compounds 2, 5-7 were isolated from P. cinereum, compounds 4, 8-9 from O. macrophylla, and compounds 1, 3, 10-11 from N. amazonum, using conventional chromatographic methods.
Chemical synthesis
Benzofurans 15-17 were synthesized via metal-catalyzed oxyarylation reaction (25). Benzofuran 18 was obtained by direct O-methylation of liliflol A using diazomethane prepared in situ by decomposition of N-methyl-N-nitrosourea, following the reported methodology (26). Bicyclooctanes 19-20 were obtained by HPLC kinetic resolution on a racemic product (±)-cineric A, which was synthesized following a reaction protocol developed by us, starting from the corresponding benzofuran precursor (25,35). Semi-preparative Kinetic resolution was performed in a HPLC system consisting of a Jasco PU-980 pump and Jasco multiwavelength detector MD-910 equipped with a Chiralcel OD-H semi-preparative column (100x10 mm) (Daicel Chemical Industries LTD) isocratically eluted with hexane: iPrOH 93:7 at a flow rate of 5 ml/min. Levorotatory compound, 20 was eluted for 11.3 min, and dextrorotatory 19, for 13.2 min.
Cytotoxicity assay
The cytotoxic effect of test compounds 1-20 was evaluated in vitro against a panel of five human cancer cell-lines including cervical carcinoma HeLa, lung carcinoma A-549, larynx carcinoma Hep-2, prostate adenocarcinoma PC-3 and breast carcinoma MCF-7 (all adherent growth), derived from human solid tumors, obtained from the Cell Bank of the Laboratorio de Inmunología at Instituto Nacional de Cancerología de Colombia in Bogotá. All tumor cell-lines were cultured in Minimum Essential Medium (MEM) supplemented with 5% fetal bovine serum (FBS), gentamicin (50 mg/ml) and sodium bicarbonate (2.2 g/L), in 75 cm2 boxes for sterile cultures, and incubated under standard conditions (27). Medium was changed every two days for maintaining the culture by washing cells with phosphate buffer solution (PBS) 298 mOsm/L, pH= 7.3. Cultures with 90% confluence were treated with 0.025% trypsin solution and 0.03% EDTA for 5 minutes at 37°C, obtaining a cell suspension which was counted in a Neubauer chamber by trypan blue exclusion method. Cell growth was ensured with minimal essential medium (MEM) supplemented with bovine fetal serum (FBS) and preserved with antibiotic to achieve the appropriate cell density according to each cell line, using 96-well plates (100 µl/well). Incubation conditions were maintained in a moistened atmosphere at 37°C and 5% CO2.
After adjusting the cell densities established by us in previous studies (28-32), cells were seeded into 96-well plates, incubated for 24 hours in standard conditions to allow cell adhesion to the well surface, and after this time, test compounds 1-20 were placed as treatments (at three concentrations: 6, 18 and 54 µg/ml, using three replicates per concentration) and doxorrubicin HCl (Ebewe Pharma®) as positive control, by up to a volume of 100 µl per well, obtained by serial dilution in MEM supplemented with 5% FBS (28). After 48 hours of treatment, the viable cell population on the plates was determined using 100 µl of rezasurin (0.25 mg/ml in PBS), which was placed in each well with medium without supplement to avoid interferences during test. Plates were incubated for 4 hours under standard conditions, and then read using a TECAN GENios reader. Resorufin formed by rezasurin reduction contains suitably both chromophoric and fluorophoric moieties, allowing performing absorbance (at 570 nm) and/or fluorescence (excitation at 535 nm and emission at 595 nm) measurements, in a non-destructive testing. It shows almost no cytotoxic activity, allowing discrimination of the cytotoxic activity of the cytostatic. Supernatant was then carefully removed and 100 µl of medium supplement was added, whose plates were further incubated for 48 hours, repeating again the procedure described above. Resulting values were taken as an indirect measure of viable cell mass.
Results
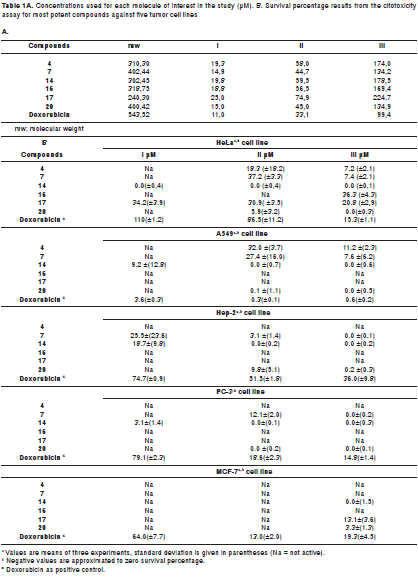
Figure 1 shows the chemical structures of isolated compounds 1-20, whose antiproliferative effects were established using resazurin, which exhibited slightly more sensitivity than MTT for proliferative estimation. However, only compounds 4, 7, 14, 16, 17, and 20 showeda considerable activity against the tumor cell-lines at the tested concentrations. Table shows the antiproliferative results as survival percentage at three concentrations (6, 18, and 54 µg/ml) for those compounds as a dose-dependent behavior. The most important results are described below.
Liliflol A, 1, a dihydrobenzofuran neolignan isolated from N. amazonum, exhibited no significant antiproliferative activity against all the cell lines. An O-methylation, using diazomethane prepared in situ by decomposition of N-methyl-N-nitrosourea, was performed starting from compound 1 to yield the synthetic derivative 18(Figure 2a). The antiproliferative effect of 18 remained identical to that of 1, indicating no important effect when the structure was slightly transformed.
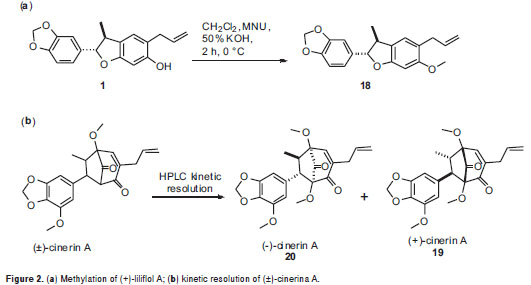
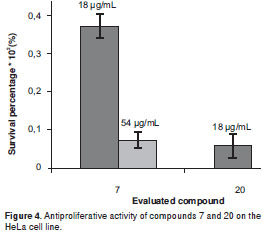
Kaurenoic acid 14was found to be the most potent antiproliferative compound,showing activity against the five evaluated human cancer cells lines at three concentrations, whose relative survival percentages were estimated at 0%. Remarkably, kaurenoic acid presented activity against the PC-3 and A-549 cell lines, which exhibited resistance as compared with the positive control (doxorubicin HCl), Similarly, (+)-cinerin D 7 and (-)-cinerin A 20 also showed activity against all the tested human cancer cells at different levels. Relative survival percentages of 7 were 37.2% and 7.4% at 18 and 54 ug/ml, respectively, while compound 20 displayed 5.0 and 0% survival percentages at 18 and 54 µg/ml, respectively, against the HeLa cell line. Synthetic (±)-cinerin A was separated into its enantiomers through semi-preparative HPLC kinetic resolution, obtaining (+)-cinerin A 19, and (-)-cinerin A 20 (figure 2b). Interestingly, the dextrorotatory derivative 19, showed no significant activity against all cell lines, while the levorotatory enantiomer 20, was active against all the tumor cell lines tested.
On the other hand, benzofurans 16, 17 and 4 exhibited selective activity against the HeLa cell line. Compound 17 was found to be the most potent antiproliferative compound among the benzofuran-type compounds, whose survival percentage values were similar at the concentrations tested. Furthermore, ocophyllal A, 4, having a benzofuran aldehyde moiety, presented activity against A549 cell line with survival values of 32.0% and 11.2 % at 18 and 54 ug/ml, respectively. However, allyl, propenyl and chlorobenzofuran 1-3, 15, 18 and cyclohexadienone 5 derivatives, bicyclooctane neolignans 6, 9, 10 and diarylbutane lignans 12-13 showed no significant antiproliferative activity against the cell lines evaluated with survival values of >50%.
After 48 h, medium was replaced by fresh medium, to determine the cell recovery by using new resazurin. Thus, cell recovery of tested human tumor cell lines was qualitatively determined after evaluation of the antiproliferative effect. Compounds 20 and 14 exhibited total lethal effect against four cell lines and PC-3, Hep-2, and A549 cell lines, respectively. However, it is suggested to carry out trials in shorter times to establish the recovery effect, avoiding raised additional effects in the development of the test.
In addition, the effects of the most active compounds 4, 7, 14, 16, 17 and 20, were also tested against the MCF-7 breast carcinoma cell line, which has been extensively studied by the Pharmacogenetics of Cancer Group. This cell line showed survival values above 50 percent, indicating that the cytotoxic effect on this cell line is lower in comparison to the effect against other tumor cell lines. Susceptibility of the MCF-7 cell line was exhibited in the presence of 14, 17 and 20 (table 1).
Discussion
At the Pharmacogenetics of Cancer laboratory the methods for indirect assessment of cell survival using MTT and resazurin (29) were previously compared. Resazurin was found to be slightly more sensitive than MTT for proliferative estimation, leading to use the resazurin method with more reliable results, even during recovery time after treatment (29). Furthermore, different types of controls, as medium, vehicle (DMSO), and growth were previously optimized in order to ensure the test quatlity (29-32).
Test compounds from natural sources, 1-14, and synthetic benzofurans, 15-17, were obtained in previous studies (19-25). Here the O-methylation of liliflol A, 1, affording 18, and the racemic resolution of synthetic (±)-cinerin A into 19 and 20 are decribed. In a previously reported cytotoxic evaluation against the tumor cell lines MCF-7 and HCT-8, compound 14 was found to exhibit a 45% survival rate at 78 µM (33), and an effect against the PC-3 cell-line at 3.7 µg/ml (34); compound 14 also showed an IC50 of 1.6 µg/ml in KB cells derived from nasopharyngeal cancer (35), results which are in agreement with those of the present work. By means of electrophoretic analyses, the mode of action of compound 14 was established as nucleosomal fragmentations, indicating apoptotic activity of 14 involved on differentiation of protein expression associated with the regulation of apoptosis (34). Our results also indicate that compound 14 exhibits significant activity against HeLa, A-549, Hep-2, and PC-3 tumor cell lines, showing enhanced activity and behavior against HeLa as compared to that of doxorubicin HCl (figure 3), which indicated an excellent antitumor parameter. These results point to the promising activity of compound 14 for antitumor research purposes as a leading compound for structural optimization. Likewise, Perseal D, a compound isolated from Persea obovatifolia (Lauraceae), having a benzofuran aldehyde moiety (like compounds 4, 16, 17), also exhibited antiproliferative activity against the A549 cell line with ED50 of 0.59 µg/ml (10), which indicates this chemical skeleton could be a good candidate to be used for further antitumor studies.
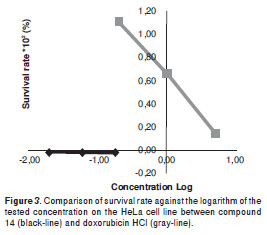
Conversely, compounds 7 and 20 are related to the bicyclooctane core (21), and they share a very similar chemical structure (differenced solely by the functional group at C-2´). Activity exhibited by these two compounds on the HeLa cell line indicated that 20 possesses a more forceful cytotoxic profile as compared to that of 7 (figure 4). Both compounds showed similar effect against the PC-3 and A549 cell lines, while the antiproliferation of compound 7 was greater to that of 20 against the Hep-2 cell line. Interestingly, no significant antiproliferative activity was found for the neolignans having an OH group at C-4´, as in compounds 6, 8-11 and 19,with survival values of >50%. Additionally, in order to determine the configuration-dependent antiproliferative activity of bicyclo 3.2.1octane nuclei, (±)-cinerin A, prepared in a previous work by total synthesis from commercially available materials (36), were separated into compounds 19 and 20, enantiomers with a significantly different antiproliferative effect. Dextrorotatory enantiomer 19 showed no relevant cytotoxic activity against four cell lines, while levorotatory enantiomer 20 which shares an identical absolute configuration to that of natural product (-)-cinerin A, which was not suitable to be included on this study due to a low isolated amount (21), displayed activity towards four cell lines at 54 µg/ml, having the greatest antiproliferative effect on cell lines PC-3, A549 and Hep-2 at 18 µg/ml. This fact indicated the importance of the absolute configuration determination in rational drug design.
Although some compounds showed activity above the selection criteria, further biological studies are suggested to complete their activity profile. Additionally, we found some compounds with no antiproliferative activity like 8, 17, 19, since they exhibited no significant effect on the cell growth, indicating either no promising anticancer activity or the lack of previous bioactivation.
In summary, the antiproliferative effect of twenty compounds, related to the bicycle 3.2.1octane and benzofuran neolignans, kaurane diterpene and diarylbutane lignans classes, obtained by isolation from three lauraceae plants and synthesis, was evaluated in vitro against six human cancer cell lines. Several of them showed theiranticancer potential because they have a CL50 under 10 mM, the reference value recommended by NCI for compounds (37). Three benzofuran-type compounds exhibited selective activity against HeLa cell lines, which might be used in further optimization studies to improve their action on the treatment of cervical carcinoma diseases. Kaurenoic acid and (-)-cinerin A were found to be the most potent antiproliferative compounds at reasonable levels, which would be good candidates to be used as lead compounds for further SAR studies for anticancer agent development. However, it is necessary to perform many other studies both in vitro and in vivo to determine their true potential for the development of medicines
Acknowledgements
Authors thank the Chemistry and Pharmacy Departments and the División de Investigación, Sede Bogotá (DIB) at Universidad Nacional de Colombia, Bogotá, for the financial support. Our gratitude is extended to L. M. Escobar and C. P. Cordero for their support on the development and optimization of the used methodology.
Conflict of interests
Authors declare no conflicts of interest.
Financial support
This work was carried out with financial support by Colciencias and “División de Investigaciones de Bogotá (DIB) at Universidad Nacional de Colombia” (Call 2009, Project Code No. 8003383).
Corresponding author: Fabio Ancízar Aristizábal, Laboratorio de Farmacogenética del Cáncer, Departamento de Farmacia, Universidad Nacional de Colombia, Carrera 30 N° 45-03, Edificio 450, Oficina 206, AA 14490, Bogotá, D.C., Colombia Teléfono: (571) 314 5000 extensión 14643; fax: (571) 316 5060 faaristizabalg@unal.edu.co
References
1. Bailly C. Ready for a comeback of natural products in oncology. Biochem Pharmacol.2009;77:1447-57. [ Links ]
2. Lieberman MM, Patterson GM, Moore RE. In vitro bioassays for anticancer drug screening: Effects of cell concentration and other assay parameters on growth inhibitory activity. Cancer Lett. 2001;173:21-9. [ Links ]
3. Nobili S, Lippi D, Witort E. Natural compounds for cancer treatment and prevention. Pharmacol Res. 2009;59:365-78. [ Links ]
4. Mans DR, Da Rocha A, Schwartsmann G. Anti-cancer drug discovery and development in Brazil: Targeted plant collection as a rational strategy to acquire candidate anti-cancer compounds. Oncologist. 2000;5:185-99. [ Links ]
5. Organización Mundial de la Salud. Descriptive note number 297: Cancer. Geneva: WHO; 2009. [ Links ]
6. Instituto Nacional de Cancerología. Anuario estadístico 2005: Por el control del cáncer. Volumen 3. Bogotá: Legis; 2007. [ Links ]
7. Whiting DA. Lignans, neolignans and related compounds. Nat Prod Rep. 1985;2:191-211. [ Links ]
8. Apers S, Vlietinck A, Pieters L. Lignans and neolignans as lead compounds. Phytochem Rev. 2003;2:201-17. [ Links ]
9. Tsai IL, Hsieh CF, Duh CY, Chen IS. Cytotoxic neolignans from Persea obovatifolia. Phytochemistry. 1996; 43: 1261-3. [ Links ]
10. Tsai IL, Hsieh CF, Duh CY. Additional cytotoxic neolignans from Persea obovatifolia. Phytochemistry. 1998;48:1371-5. [ Links ]
11. van Miert S, van Dyck S, Schmidt TJ, Brun R, Vlietinck A, Lemiére G, et al. Antileishmanial activity, cytotoxicity and QSAR analysis of synthetic dihydrobenzofuran lignans and related benzofurans. Bioorg Med Chem. 2005;13:661-9. [ Links ]
12. Cherigo L, Polanco V, Ortega-Barria E, Heller MV, Capson TL, Rios LC. Antitrypanosomal activity of a novel norlignan purified from Nectandra lineata. Nat Prod Res. 2005;19:373-7. [ Links ]
13. Pessini GL, Filho BPD, Nakamura CV, Cortez DA. Antibacterial activity of extracts and neolignans from Piper regnellii (Miq.) C. DC. var. pallescens (C. DC.) Yunck. Mem Inst Oswaldo Cruz. 2003;98:1115-20. [ Links ]
14. Ma Y, Han GQ, Wang YY. PAF antagonistic benzofuran neolignans from Piper kadsura. Acta Pharm Sin. 1993;28:370-3. [ Links ]
15. Sefkow M. The stereoselective synthesis of neolignans. Synthesis. 2003;17:2595-625. [ Links ]
16. Han G. PAF receptor antagonistic principles from Chinese traditional drugs. Progr Nat Sci. 1995;5:299-306. [ Links ]
17. Iida T, Ito K. Four phenolic neolignans from Magnolia liliflora. Phytochemistry. 1983;22:763-6. [ Links ]
18. Nascimento IR, Lopes LM. 2,3-dihydrobenzofuran neolignans from Aristolochia pubescens. Phytochemistry. 1999;52:345-50. [ Links ]
19. Coy-Barrera ED, Cuca-Suárez LE, Sefkow M. PAF-antagonistic bicyclo 3.2.1octanoid neolignans from leaves of Ocotea macrophylla Kunth. (Lauraceae). Phytochemistry. 2009;70:1309-14. [ Links ]
20. Coy ED, Cuca LE. Chemical constituents from Pleurothyrium cinereum (van der Werff) (Lauraceae) from Colombia. Biochem Syst Ecol. 2008;36:674-7. [ Links ]
21. Coy ED, Cuca LE, Sefkow M. Macrophyllin-type bicyclo 3.2.1octanoid neolignans from the leaves of Pleurothyrium cinereum. J Nat Prod. 2009;72:1245-8. [ Links ]
22. Han GQ, Dai P, Xu L, Wang SC, Zheng QT. Bicyclooctanoid neolignans from Piper kadsura. Chin Chem Lett. 1992;3:521-4. [ Links ]
23. Coy ED, Cuca LE. Three New 7.3´,8.5´-connected bicyclo 3.2.1octanoids and other neolignans from leaves of Nectandra amazonum NEES. (Lauraceae). Chem Pharm Bull. 2009;57:639-42. [ Links ]
24. Patra A, Mitra AK, Mitra SR, Kirtaniya CL, Adityachaudhury N. Carbon-13 NMR spectra of kauranoid diterpenes. Org Magn Reson. 1980;14:58-60. [ Links ]
25. Coy ED, Jovanovic L, Sefkow M. One-pot, Pd-catalyzed synthesis of trans-dihydrobenzofurans from o-aminophenols. Org Lett. 2010;12:1976-9. [ Links ]
26. Arndt F. Diazomethane. In: Organic syntheses. Collection Volume 2. New York: Wiley; 1943. p. 165. [ Links ]
27. Freshney I. Culture of animal cells: A manual of basic technique. 4th edition. England: John Wiley and Sons Inc.; 2000. [ Links ]
28. León CJ, Gómez SM, Morantes SJ, Cordero CP, Aristizabal FA. Caracterización del perfil de sensibilidad de un panel de líneas celulares para valoración de citotoxicidad in vitro. Biomédica. 2006;26:161-8. [ Links ]
29. Escobar L, Rivera A, Aristizabal F. Comparison of resazurin and MTT methods on studies of citotoxicity in human tumor cell lines. Vitae. 2010;17:67-74. [ Links ]
30. Escobar L, Aristizabal F. Fluorometric assay for cell proliferation in human tumor cell lines application. Vitae. 2010;17:173-80. [ Links ]
31. Cordero CP, Aristizabal FA. Evaluación preliminar in vitro de citotoxicidad de extractos vegetales sobre líneas derivadas de tumores humanos, empleando dos métodos colorimétricos. Rev Colomb Biotecnol. 2002;4:100-6. [ Links ]
32. Cordero CP, Gómez S, León CJ, Morantes SJ, Aristizabal FA. Cytotoxic activity of compounds isolated from Colombian plants. Fitoterapia. 2004;75:225-7. [ Links ]
33. Costa-Lotufo LV, Cunha GM, Farias PA, Viana GS, Cunha KM, Pessoa C, et al. The cytotoxic and embryotoxic effects of kaurenoic acid, a diterpene isolated from Copaifera langsdorffii oleo-resin. Toxicon. 2002;40:1231-4. [ Links ]
34. Ruiz Y, Rodríguez J, Arvelo F, Usubillaga A, Monsalve M, Galindo-Castro I. Cytotoxic and apoptosis-inducing effect of ent-15-oxo-kaur-16-en-19-oic acid, a derivative of grandiflorolic acid from Espeletia schultzii. Phytochemistry. 2008;69:432-8. [ Links ]
35. Mongelli E, Pomilio AB, Sánchez JB, Guerra FM, Massanet GM. ent-Kaur-16-en-19-oic acid, a KB cells cytotoxic diterpenoid fromElaeoselinum foetidum. Phytother Res. 2002;16:387-8. [ Links ]
36. Coy ED, Cuca LE, Sefkow M. The first diastereoselective synthesis of cinerins AC, PAF-antagonistic macrophyllin-type bicyclo 3.2.1octane neolignans, using a novel Pd-catalysed oxyarylation. Org Biomol Chem. 2010;8:2003-5. [ Links ]
37. Boyd M. The NCI in vitro anticancer drug discovery screen. Concept, implementation, and operation, 1985-1995. In: Teicher B, Totowa NJ, editors. Anticancer drug development guide: Preclinical screening, clinical trials and approval. New York; Humana Press: 1997. p. 23-42. [ Links ]














