Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biomédica
Print version ISSN 0120-4157On-line version ISSN 2590-7379
Biomédica vol.31 no.3 Bogotá July/Sept. 2011
ARTÍCULO ORIGINAL
1Facultad de Odontología, Universidad Autónoma de Campeche, Campeche, México
2Hospital de Especialidades Dr. Manuel Campos, Secretaría de Salud de Campeche, Campeche, México
3Área Académica de Odontología, Instituto de Ciencias de la Salud, Universidad Autónoma del Estado de Hidalgo, Pachuca, México
4Indiana University-Purdue University at Indianapolis School of Dentistry, Indianapolis, IN, United States
5The Regenstrief Institute, Inc., Indianapolis, IN, United States
6Facultad de Odontología, Universidad Autónoma de Yucatán, Mérida, México
7Universidad Politécnica del Estado de Morelos, Jiutepec, México
8Área de Medicina Preventiva, Unidad de Medicina Familiar del ISSSTE, Navolato, México
Recibido: 04/08/10; aceptado:19/05/11
Introduction. From an epidemiological point of view, non-syndromic orofacial clefts are the most common oral congenital deformities worldwide.
Objective. Family histories were traced and socioeconomic risk factors were identified for non-syndromic cleft lip with or without cleft palate.
Material and methods. A case-control study was carried out with 208 cases of non-syndromic cleft lip with or without cleft palate, and matched by age and sex with 416 controls. Cases were patients attending a referral clinic from 2002 through 2004 in Campeche, Mexico. A questionnaire was administered to collect sociodemographic and socioeconomic variables as well as familial background relevant to non-syndromic cleft lip with or without cleft palate. Conditional logistic regression models were used; adjusted odds ratios and 95% confidence intervals were calculated.
Results. In the multivariate model, the following risk factors were identified: 1) low socioeconomic status; 2) birth in the southern region of Campeche state; 3) home delivery or delivery in a publicly funded hospital; 4) occurrence of prior non-syndromic cleft lip with or without cleft palate cases in the father´s or mother´s family: 5) having a sibling with non-syndromic cleft lip with or without cleft palate; 6) the proband having another malformation, and 7) a history of infections during pregnancy. Prenatal care consisting of vitamin supplementation was a protective factor for non-syndromic cleft lip with or without cleft palate (odds ratio=0.29).
Conclusions. A “social gradient in health” was seen to link oral malformation with diet components, and several socioeconomic and socio-demographic factors broadly encompassed in low socioeconomic status. Further characterization of risk factors will guide the assemblage of a pro-active counseling and prevention program for families at risk for non-syndromic cleft lip and cleft palate.
Key words: Cleft lip, cleft palate, epidemiology, risk factors, socioeconomic factors, folic acid, Mexico.
Factores de riesgo hereditarios y socioeconómicos para labio o paladar hendido no asociados a un síndrome en México: estudio de casos y controles pareado
Introducción. Desde el punto de vista epidemiológico, las hendiduras faciales son las deformidades orales más comunes alrededor del mundo.
Objetivo. Identificar los factores de riesgo hereditarios y socioeconómicos relacionados con la presencia de labio o paladar hendido no asociados a un síndrome.
Materiales y métodos. Se hizo un estudio de casos y controles en el que se incluyeron 208 casos con diagnóstico de labio, paladar hendido o ambos no asociados a un síndrome, los cuales fueron pareados por edad y sexo con 416 controles. Se incluyeron todos los pacientes quirúrgicos atendidos durante el periodo 2002-2004 en el programa estatal de labio o paladar hendido de Campeche, México. Se aplicó un cuestionario en el que se recogió información sobre variables sociodemográficas y socioeconómicas, así como sobre antecedentes hereditarios de labio o paladar hendido no asociados a un síndrome en la familia. Debido a que el diseño fue pareado, el análisis se hizo con regresión logística condicionada.
Resultados. En el modelo multivariado para labio o paladar hendido no asociado a un síndrome se identificaron de forma significativa (p<0,05) los siguientes factores de riesgo: nivel socioeconómico bajo (razón de momios, RM=2,02), nacimiento en el sur del estado (RM=3,96), nacimiento en casa (RM=2,51) o nacimiento en hospital público (RM=4,08), antecedentes heredofamiliares paternos (RM=5,38), antecedentes heredofamiliares maternos (RM=4,11), tener otro hijo con labio o paladar hendido en la familia (RM=46,02), presentar algún otro defecto congénito asociado (RM=8,20) e infección en el embarazo (RM=2,90), y como factor protector, el cuidado prenatal y el uso de vitaminas (RM=0,29).
Conclusiones. El mayor riesgo en nuestra muestra para labio, paladar hendido o ambos, no asociados a un síndrome, radica en las variables relacionadas con los antecedentes familiares y hereditarios, y las indicadoras de la posición socioeconómica. Se observó un efecto protector del manejo prenatal con vitaminas.
Palabras clave: labio leporino, fisura del paladar, epidemiología, factores de riesgo, factores socioeconómicos, ácido fólico, México.
From an epidemiological perspective, non-syndromic orofacial clefts are the most common orofacial birth defects worldwide, and occur in 1 per 500 to 2,500 births depending on ancestry, geographic residential location, maternal age and prenatal exposures, and socioeconomic status. Their incidence varies markedly in terms of geographical location, being more common among Asians than in Caucasians and least common among Afro-Caribbean populations. In addition to the psychological, social and functional sequelae, this condition imposes an economic burden for families and health systems: treatment requires a multidisciplinary team, and a lengthy period of surgical and non-surgical intervention (1).
Cleft lip and/or palate is the second most common congenital malformation in Mexico. Between 2,300 and 2,600 new patients are encountered every year with this problem (2,3). Other estimates place the rate in Mexico at 1.7 (95% CI 1,3-2.2) per 1,000 live births for cleft lip, with or without cleft palate, with the following incidence for each malformation: place cleft lip 0.1-0.5, cleft lip with cleft palate 0.9-1.7, and cleft palate incidence 0.1-0.4 (4).
Although a developmental defect may occur at any time during gestation, only perturbations that occur during embryogenesis can produce major anatomical malformations of structures than develop from the neural tube and the neural crest. Defects arising from them are the most common and the most devastating in terms of mortality and morbidity, stillbirths, and spontaneous abortions. These include neural tube closure defects such as spina bifida, orofacial defects, and conotruncal heart defects (5).
Elevation and growth of palatal shelves is mostly driven by changes in the mesenchymal stroma, which is derived largely from neural crest cells that have migrated from the neural tube region into the craniofacial area (6). Past studies have identified some variables associated with cleft lip and/or palate. Vitamin supplements during pregnancy (especially folic acid with or without vitamins) appear to play an important role in non-syndromic cleft lip and/or palate prevention; other micronutrients have also been implicated be protective factors in orofacial clefts such as B1, B6, myo-inositol, zinc, iron and riboflavin (7-9).
However, other diverse conditions and maternal diseases (chronic or infectious, during or before pregnancy) are considered risk factors for cleft lip and/or palate. Some of these are influenza, common cold, orofacial herpes, gastroenteritis, sinusitis, bronchitis, epilepsy and angina pectoris (10), diabetes (11) and obesity (12). Additional reported factors are exposure to teratogenic agents (13), nicotine poisoning (14), prescription drug use during pregnancy (such as amoxicillin, phenytoin, oxprenolol, thiethylperazine, oxytetracycline, and carbamazepine) (15), and exposure to organic solvents (16).
Recent studies and reviews (17-20) identified genes that may play an important role in the etiology of cleft lip and/or palate, directly or through modifying the effect of environmental agents. Although several socio-demographic characteristics already have been identified as risk factors--such as father´s and mother´s age, sex, or even marital status (11,21,22), little information exists about the role of socioeconomic status (23-25).
In Mexico, only one study has been published showing that socioeconomic status is associated with cleft lip and/or palate occurrence; the authors, using several socioeconomic status indicators, observed an inverse dose-response relationship between cleft lip and/or palate and socioeconomic status (26). The lack of attention to these aspects is not surprising. The biomedical model has dominated dental research, focusing on the search of individual risk factors from a clinical and epidemiological perspective. The sparse evidence published on hypothesized links to social determinants of health has suggested that cleft lip and/or palate prevalence holds a negative gradient with schooling level of the parents (22) and increases with the experience of poverty (23).
Most research literature has reported findings and associations pertinent to industrialized countries, occurring in environments supporting unhindered access to largely equitable and appropriately established health services. In the current study, family histories were examined and socioeconomic risk factors were identified for non-syndromic cleft lip with or without cleft palate in an environment offering less sophisticated health systems, less equitable social structures, and living conditions more characteristic of a less developed country.
Material and methods
This study was approved by the Ethical and Research Committee of the School of Medicine of the Universidad Autónoma de Campeche. Assent was given by each participants or written consent provided by their parent or guardian.
Campeche is one of 32 states in Mexico, located in the southeast part of the country on the Gulf of Mexico. According to the state marginalization index, Campeche is classified as a state with high levels of poverty. In 2005, Campeche had a population of 754,730. Its main economic activity is oil extraction that accrues 60% of the national production. Other economic activities include farming, fishing, manufacturing, and tourism.
A case-control study was performed in a publicly funded hospital with a maxillo-facial specialty clinic where the State Cleft Lip and/or Palate Program is offered. The referral clinic for cleft lip and/or palate receives cases from across the state and implements treatment plans for patients, including surgical management..The patients included in the current study had a cleft lip and/or palate diagnosis registered in the maxillo-facial specialty clinic from September 2002 to August 2004 for surgical treatment or other management. All of the selected 208 cases were diagnosed as non-syndromic.
Cases were matched by age and sex with two controlsselected arbitrarily from patients who visited the hospital for other reasons and did not have diagnostic or clinical evidence of cleft lip and/or palate or its sequelae (information obtained from hospital administrative records). Their status was subsequently confirmed by directly questioning the patient or the parent/guardian, and also through clinical examination. The 416 controls selected were not brothers, sisters, or cousins of the cases.
Variables and data collection
Variables were divided in four groups:
a) sociodemographic (age of father and mother and patient´s birth order number among siblings);
b) socioeconomic (socioeconomic status, geographic region within the state where the participant was born rural or urban area of residence, and location of delivery of child);
c) family history background (whether mother and/or father had personal or family history of cleft lip and/or palate, sibling with cleft lip and/or palate, having any condition associated with cleft lip and/or palate in the proband or a sibling, and mother´s diabetes pregnancy history), and
d) gyneco-obstetric and perinatal variables (viral or bacterial vaginal or urinary tract infections during pregnancy, type of birth delivery, hormonal contraceptive use, prescription drug use, prenatal care and vitamin supplement use, single or twin pregnancy, and history of abortions and pre-eclampsia).
A maxillofacial surgeon diagnosed cleft lip and/or palate cases based on the Kernahan classification (27). Independent variables were determined through a questionnaire administered to a parent. The Bronfman Index (28) was used to evaluate socioeconomic status. This index has been validated in Mexico and other Latin American countries for epidemiological studies, and includes dwelling crowding, housing conditions and facilities, and maximum educational attainment of the head of the household. According to the Bronfman Index distribution, participants were stratified in high, medium, and low socioeconomic status.
Statistical analyses
For the univariate analysis, central tendency and dispersion measures were calculated for continuous variables as well as frequencies and percentages for categorical variables. Conditional logistic regression models, reported as odds ratio (OR) and 95% confidence intervals (CI95%), were used in the bivariate and multivariate analyses with case or control status as dependent variable. We chose this model due to the matched design of the study. Variables with a p value <0.20 in the bivariate analyses were used in the conformation of final model (29). The Variance Inflation Factor (VIF) test was used to account for and, if necessary, to avoid multicollineality between independent variables. Additionally, we used score test for trend of odds to demonstrate a monotonic relationship between the response variable and the categorized exposure variable. All analyses were undertaken in Stata 8.2® (30).
Results
Of the 208 cases, 81.3% (n=169) had cleft lip with cleft palate, distributed as follows: 36.1% (n=75) with left cleft lip and/or palate, 25.0% (n=52) with bilateral cleft lip and/or palate, and 20.2% (n=42) with right cleft lip and/or palate. Secondary cleft palate percentage was 6.3% (n=13), followed by unilateral right cleft lip with 4.3% (n=9). Finally, 8.1% (n=17) had other types of cleft. Table 1 shows the distribution of sociodemographic and socioeconomic characteristics for cases and controls, with crude odds ratio estimates.
The average age was the same for cases and controls, 10.7±6.7 and 10.7±6.7 years, respectively. Sex was distributed equally among cases and controls (61.1% were men and 38.9% women) indicating that matching was appropriate. No statistically significantly difference was observed (p>0.05) on parental ages, but controls were more likely to be older siblings (within family birth order) than cases (p<0.05) (table 1).
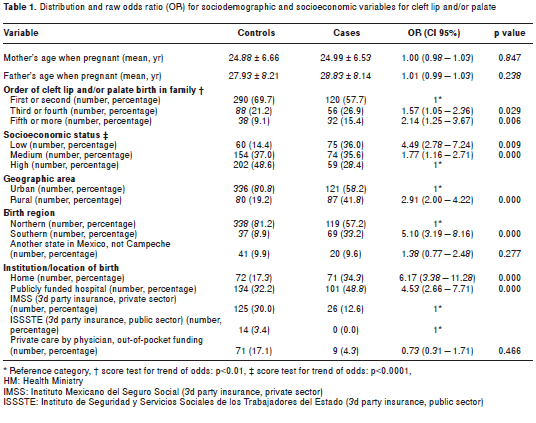
Regarding socioeconomic variables, cases were more likely to have low socioeconomic status than middle or high socioeconomic status (p<0.01), to live in a rural area (p<0.001), having been born in the southern region of Campeche state (p<0.001), or that they had been born at home or in a publicly funded hospitalin contrast to more affluent medical insurance/treatment options.
table 2 shows variables related to the family history background, as well as conditioned bivariate logistic regression analyses. Having a sibling with cleft lip and/or palate was a variable very strongly associated with the cleft lip and/or palate condition in the proband (OR=40.0; p<0.001). Although a cleft lip and/or palate background for the father or mother had a strong effect on the presence of cleft lip and/or palate in the proband (p<0.001), this condition was also more likely (OR=6.00) if another family history condition was concurrently identified. Maternal diabetes type 2 self-report (p>0.05) did not differ between cases and controls, whereas having any other child with any conditions different from cleft lip and/or palate approached marginal significance (p=0.089).
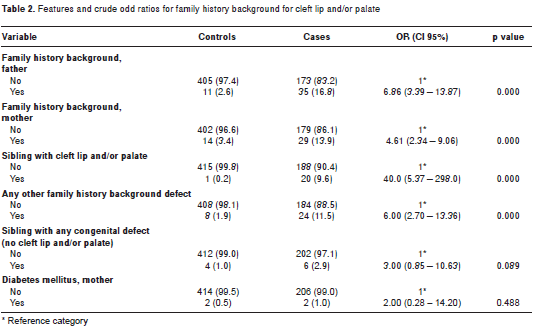
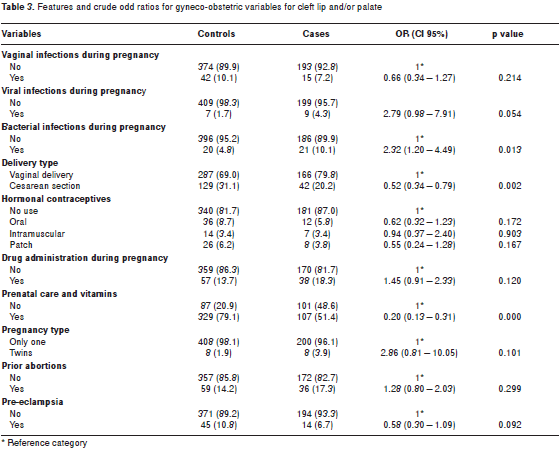
table 3 shows descriptive and bivariate results related to gynecological and perinatal variables. Infection during pregnancy (OR=2.32) was a risk factor for cleft lip and/or palate, whereas being born by c-section (OR=0.52) and having prenatal care and taking vitamins during pregnancy (OR=0.20) were protective factors against cleft lip and/or palate. Four variables that did not differ (p>0.05) between cases and controls were the following: vaginal/urinary tract infection during pregnancy, use of hormonal contraceptives, number of abortions, drug use during pregnancy, and type of delivery. Occurence of disease during pregnancy and pre-eclampsia approached marginal significance (p<0.10).
Three models were generated as a first step in the multivariate analysis, one for each variable group. These results are shown in table 4. table 5 presents the results of the conditional multivariate logistic regression analysis, showing that people of low socioeconomic status had higher risk of cleft lip and/or palate (OR=2.02; CI95%=1.14 3.57) than people with medium or high socioeconomic level. People born in the southern region of Campeche presented almost four times (OR=3.96; CI95%=2.09 7.50) the risk for cleft lip and/or palate compared to those born in the northern region.
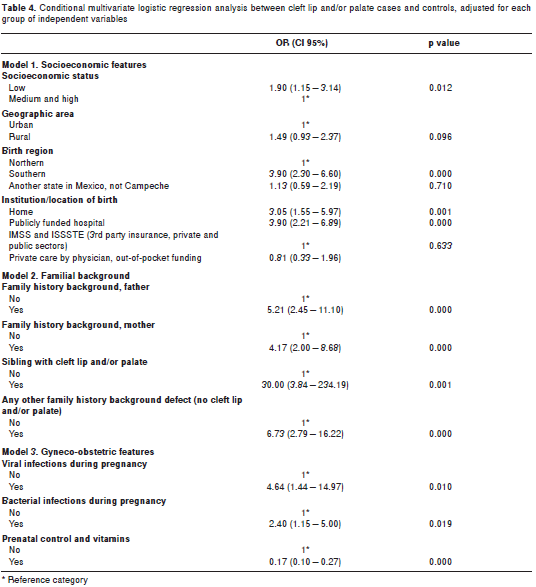
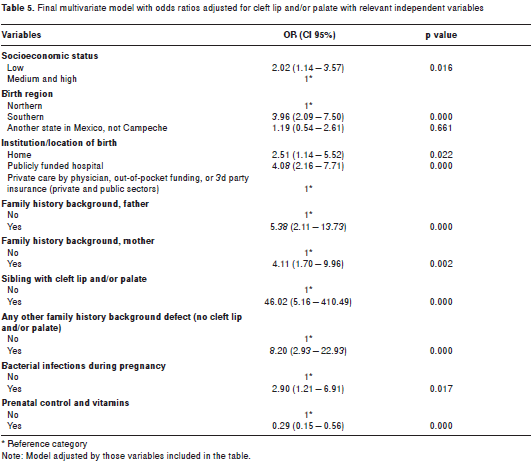
The location or institution where the delivery had taken place (another variable that can serve as a proxy for socioeconomic position) was related to cleft lip and/or palate: babies born at home (OR=2.51; CI95%=1.14 5.52) or in a publicly funded hospital (OR=4.08; CI95%=2.16 7.71) had higher cleft lip and/or palate risk than those born in a hospital funded by third-party payment schemes or personal funds.
A strong association characterized family history variables. Prior experiences of the father (OR=5.38; CI95%=2.11 13.73) and mother (OR=4.11; CI95%=1.70 9.96) were strong predictors of having cleft lip and/or palate. Presence of a sibling with cleft lip and/or palate was a very strong risk factor (OR=46.02; CI95%=5.16 410.49) for cleft lip and/or palate. Persons who also had other congenital defect(s) had 8 times higher probability of having cleft lip and/or palate than those who did not have any other defect.
Among the gyneco-obstetric and perinatal variables, bacterial infection during pregnancy was a risk factor for cleft lip and/or palate (OR=2.90; CI 95%=1.21 6.91), while availability of prenatal care and vitamin supplement use were protective factors against cleft lip and/or palate (OR=0.29; CI95%=0.15 0.56).
Discussion
Due to its impact on quality of life and function, as well as economic and clinical impacts over many years, orofacial defects are undoubtedly an important oral health issue. The present study established some of the most important factors related to cleft lip and/or palate in a Mexican population. This is a first account concerning the factors that seem to modify cleft lip and/or palate presentations and risk. Diverse studies on cleft lip and/or palate and other congenital malformations have been undertaken in Latin America, in particular, the productive output from the ECLAMC group (The Latin American Study of Congenital Malformations). Several of the current results were similar to reports from this pioneer group (31-33).
During the 1990´s the importance was recognized of prenatal dietary folate for prevention of neural tube defects (34). Folate is a one-carbon donor; as such, it is involved in the biosynthesis of purines and pyrimidines and in homocysteine remethylation, which produces methyl groups for methylation of DNA, proteins, and lipids (8). Whether folate can regulate directly gene expression is unknown, although several vitamins are known to do so by interacting with a nuclear receptors family of transcription factors, for example, retinoids and vitamin D (35). Folate may also regulate the expression of several essential genes for cellular multiplication and differentiation during embryogenesis, especially those involved in palate and lip formation. Although some controversy surrounds studies that have or have not found a risk reduction in cleft lip and/or palate and other types of congenital anomalies (7-9,31,36), it is indisputable that the use of multivitamins (containing principally folic acid) during the prenatal period is an effective public health measure to prevent malformations.
As in the current study, others have found some differences linked to physical location and cleft lip and/or palate risk (21). This finding may be related to parental economic activity and their habitation environment; this is perhaps best summarized in the rather diffuse array of factors encompassed in socioeconomic status. Environmentally associated factors can also be related to the regional economic development and hometown environmental conditions, because of the potential exposure to noxious agents (e.g., pollutants such as lead in paint from older housing).
Although the current study did not measure the specific genetic differences between cases and controls, the role of family history factors (coarsely measured through assessments of cleft lip and/or palate background in the family) was evident in the multivariate model. These results have been observed in other epidemiological studies focused on cleft lip and/or palate (21) and confirmed the strong influence of genetic background. Nevertheless, the statistical relationship between cleft lip and/or palate and close relatives may reflect not only genetic factors but also socioeconomic and/or environmental variables shared by the family members (e.g., low parent education, low socioeconomic level, or dietary folate deficiency). The importance of genetic background has been confirmed in research on specifical genetic alterations associated with non-syndromic cleft lip and/or palate (17-20). Therefore, the next step in our research program will be to ascertain specific genetic alterations that are intrinsically linked to cleft lip and/or palate in this Mexican population.
Ample evidence suggests that the position in the social structure is a strong predictor of morbidity and mortality. Furthermore, the existence of an association between health status and social status is generally acceptedâthose with higher economic position have better health generally (37,38). The “social gradient in health” implies that inequalities within population health status distribution are related to social status inequalities (39). The associations between socioeconomic position variables and various aspects of oral health have been consistently reported, including our own research on Mexican population groups (40-47).Although the exact mechanism governing these associations is not well understood, a first step toward addressing inequalities is assuming that socioeconomic factors are a multidimensional theoretical construct that covers a wide variety of social and financial circumstances (48).
In the present study, two indicator variables of socioeconomic position remained in the final model: socioeconomic status and location/institution of birth. This finding confirmed previous reports (23-26) (even using other socioeconomic position indicators) that showed that as socioeconomic position decreases, the risk of cleft lip and/or palate increases. Because mechanisms for each indicator and their impact on health status may not be the same for everyone (36), further research is needed to expand the knowledge base and ascertain if the cleft lip and/or palate phenomenon hinges upon (1) differential exposure to noxious agents more likely to be present in poor living conditions, or (2) nutritional deficiencies before and during pregnancy. Detailed study of these associations is necessary to inform preventive interventions tailored to maximize impact for these population groups.
Finally, our results supported previous reports suggesting that maternal infections during pregnancy can be risk factors for cleft lip and/or palate (10). Although the current study was unable to analyze each type of infection, additional studies are necessary to establish the biological mechanisms underlying this association. These studies must include larger samples so that these relationships can indeed be characterized.
Among the limitations of the current study is the potential recall bias that may affect any case and control study, since we were unable to exactly rebuild retrospectively the history of exposure. This bias may be higher in controls than in cases, thereby resulting in an overestimation of the effect of risk factors. We surmise that the variable more susceptible to this bias is infection during pregnancy and prenatal use of vitamins, but this bias may include less susceptible in variables such as family history and background, and socioeconomic position.
The highest risk for cleft lip and/or palate in the current study sample was associated with variables related to family history background, family history of cleft lip and/or palate, and socioeconomic indicator variables. We observed a protective effect of prenatal care and vitamin supplementation. The most significant risk factor (a sibling with cleft lip and/or palate) emphasized the importance of focusing on families that have already been affected by these alterations to offer support and counseling for mothers and families. This framework will promote family planning decisions based on an informed perspective, as well as implementing preventive and dietary health measures to minimize the risk of recurrence.
Acknowledgments
This report is part of the research outfit Bi-National/Cross-Cultural Health Enhancement Center.
Conflict of interest
None
Financial support
None
Correspondence: Carlo Eduardo Medina-Solís, Área Académica de Odontología, Instituto de Ciencias de la Salud, Universidad Autónoma del Estado de Hidalgo, Carretera Pachuca-Actopan, camino a Tilcuautla, ex Hacienda La Concepción, Pachuca de Soto, Hidalgo, M&ecute;xico Telefax: (+52) (981) 811 0215 cemedinas@yahoo.com
References
1. Wehby GL, Cassell CH. The impact of orofacial clefts on quality of life and health care use and costs. Oral Dis. 2010;16:3-10. [ Links ]
2. Armendares S, Lisker R. Análisis genético del labio y paladar hendidos y paladar hendido solo. Estudio en población mexicana. Rev Invest Clin. 1974;26:317-32. [ Links ]
3. Secretaría de Salud. Comunicado de prensa No. 213. 15/abril/2006. México, DF. Fecha de consulta: 3 y 4 de febrero de 2007. Disponible en: http://www.salud.gob.mx/ssa_app/noticias/datos/2006-04-15_2143.html [ Links ]
4. World Health Organization. International Collaborative Research on Craniofacial Anomalies. Typical orofacial clefts- cumulative data by register. Updated: February 2006. Fecha de consulta: 16 de febrero de 2007. Disponible en: http://www.who.int/genomics/anomalies/cumulative_data/en/index.html. [ Links ]
5. Rosenquist TH, Gelineau van Waes J, Shaw GM, Finnell R. Nutrient effects upon embryogenesis: Folate, vitamin A and iodine. In: Hornstra G, Uauy R, Yang X, editors. The impact of maternal nutrition on the offspring. Basel: Nestlé Nutrition Workshop Series Pediatric Program; 2005. p. 29-47. [ Links ]
6. Dudas M, Li WY, Kim J, Yang A, Kaartinen V. Palatal fusion Where do the midline cells go? A review on cleft palate, a major human birth defect. Acta Histochem. 2007;109:1-14. [ Links ]
7. Wehby GL, Murray JC. Folic acid and orofacial clefts: A review of the evidence. Oral Dis. 2010;16:11-9. [ Links ]
8. van Rooij IA, Vermeij-Keers C, Kluijtmans LA, Ocké MC, Zielhuis GA, Goorhuis-Brouwer SM, et al. Does the interaction between maternal folate intake and the methylenetetrahydrofolate reductase polymorphisms affect the risk of cleft lip with or without cleft palate? Am J Epidemiol. 2003;157:583-91. [ Links ]
9. Wilcox AJ, Lie RT, Solvoll K, Taylor J, McConnaughey DR, Abyholm F, et al. Folic acid supplements and risk of facial clefts: National population based case-control study. BMJ. 2007;334:464. [ Links ]
10. Metneki J, Puho E, Czeizel AE. Maternal diseases and isolated orofacial clefts in Hungary. Birth Defects Res A Clin Mol Teratol. 2005;73:617-23. [ Links ]
11. Carinci F, Rullo R, Farina A, Morano D, Festa VM, Mazzarella N, et al. Non-syndromic orofacial clefts in Southern Italy: Pattern analysis according to gender, history of maternal smoking, folic acid intake and familial diabetes. J Craniomaxillofac Surg. 2005;33:91-4. [ Links ]
12. Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: A systematic review and meta-analysis. JAMA. 2009;301:636-50. [ Links ]
13. Leite ICG, Paumgartten FJR, Koifman S. Chemical exposure during pregnancy and oral clefts in newborns. Cad Saude Publica. 2002;18:17-31. [ Links ]
14. Li Z, Liu J, Ye R, Zhang L, Zheng X, Ren A. Maternal passive smoking and risk of cleft lip with or without cleft palate. Epidemiology. 2010;21:240-2. [ Links ]
15. Puho EH, Szunyogh M, Metneki J, Czeizel AE. Drug treatment during pregnancy and isolated orofacial clefts in Hungary. Cleft Palate Craniofac J. 2007;44:194-202. [ Links ]
16. Chevrier C, Dananche B, Bahuau M, Nelva A, Herman C, Francannet C, et al. Occupational exposure to organic solvent mixtures during pregnancy and the risk of non-syndromic oral clefts. Occup Environ Med. 2006;63:617-23. [ Links ]
17. Jugessur A, Farlie PG, Kilpatrick N. The genetics of isolated orofacial clefts: From genotypes to subphenotypes. Oral Dis. 2009;15:437-53. [ Links ]
18. Vieira AR, McHenry TG, Daack-Hirsch S, Murray JC, Marazita ML. Candidate gene/loci studies in cleft lip/palate and dental anomalies finds novel susceptibility genes for clefts. Genet Med. 2008;10:668-74. [ Links ]
19. Scapoli L, Palmieri A, Martinelli M, Vaccari C, Marchesini J, Pezzetti F, et al. Study of the PVRL1 gene in Italian nonsyndromic cleft lip patients with or without cleft palate. Ann Hum Genet. 2006;70:410-3. [ Links ]
20. Riley BM, Mansilla MA, Ma J, Daack-Hirsch S, Maher BS, Raffensperger LM, et al. Impaired FGF signaling contributes to cleft lip and palate. Proc Natl Acad Sci USA. 2007;104:4512-7. [ Links ]
21. Harville EW, Wilcox AJ, Lie RT, Vindenes H, Abyholm F. Cleft lip and palate versus cleft lip only: Are they distinct defects? Am J Epidemiol. 2005;162:448-53. [ Links ]
22. Krapels IP, Zielhuis GA, Vroom F, de Jong-van den Berg LT, Kuijpers-Jagtman AM, van der Molen AB, et al. Periconceptional health and lifestyle factors of both parents affect the risk of live-born children with orofacial clefts. Birth Defects Res A Clin Mol Teratol. 2006;76:613-20. [ Links ]
23. Clark JD, Mossey PA, Sharp L, Little J. Socioeconomic status and orofacial clefts in Scotland, 1989 to 1998. Cleft Palate Craniofac J. 2003;40:481-5. [ Links ]
24. Carmichael SL, Ma C, Shaw GM. Socioeconomic measures, orofacial clefts, and conotruncal heart defects in California. Birth Defects Res A Clin Mol Teratol. 2009;85:850-7. [ Links ]
25. Puho E, Metneki J, Czeizel AE. Maternal employment status and isolated orofacial clefts in Hungary. Cent Eur J Public Health. 2005;13:144-8. [ Links ]
26. Escoffie-Ramírez M, Medina-Solís CE, Pontigo-Loyola AP, Acuña-González G, Casanova-Rosado JF, Colome-Ruiz GE. Asociación de labio y/o paladar hendido con variables de posición socioeconómica: un estudio de casos y controles. Rev Bras Saude Mater Infant. 2010;10:323-9. [ Links ]
27. Kernahan DA. The striped Y - a symbolic classification for cleft lips and palates. Plast Reconstr Surg. 1971;47:469-70. [ Links ]
28. Bronfman M, Guiscafré H, Castro V, Castro R, Gutiérrez G. La medición de la desigualdad: una estrategia metodológica, análisis de las características socioeconómicas de la muestra. Arch Invest Med. 1988;19:351-60. [ Links ]
29. Hosmer D, Lemeshow S. Applied logistic regression. 2nd edition. New York: Wiley-Interscience Publication; 2000. [ Links ]
30. Bagley SC, White H, Golomb BA. Logistic regression in the medical literature: Standards for use and reporting, with particular attention to one medical domain. J Clin Epidemiol. 2001;54:979-85. [ Links ]
31. López-Camelo JS, Castilla EE, Orioli IM; INAGEMP (Instituto Nacional de Genética Médica Populacional); ECLAMC (Estudio Colaborativo Latino Americano de Malformaciones Congénitas). Folic acid flour fortification: Impact on the frequencies of 52 congenital anomaly types in three South American countries. Am J Med Genet A. 2010;152A:2444-58. [ Links ]
32. Nazer J, Ramírez MC, Cifuentes L. Evolution of prevalence rates of orofacial clefts in a maternity of a Chilean clinical hospital. Rev Med Chil. 2010;138:567-72. [ Links ]
33. Rittler M, López-Camelo JS, Castilla EE, Bermejo E, Cocchi G, Correa A, et al. Preferential associations between oral clefts and other major congenital anomalies. Cleft Palate Craniofac J. 2008;45:525-32. [ Links ]
34. Tamura T, Picciano MF. Folate and human reproduction. Am J Clin Nutr. 2006;83:993-1016. [ Links ]
35. Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: Opening the X-files. Science. 2001;294:1866-70. [ Links ]
36. Johnson CY, Little J. Folate intake, markers of folate status and oral clefts: Is the evidence converging? Int J Epidemiol. 2008;37:1041-58. [ Links ]
37. Kawachi I. Income inequality in health. En: Berkman L, Kawachi I. Social epidemiology. New York: Oxford University Press; 2000. p. 76-93. [ Links ]
38. Lynch J, Kaplan G. Socioeconomic position. En: Berkman L, Kawachi I. Social epidemiology. New York: Oxford University Press; 2000. p. 13-35. [ Links ]
39. Kosteniuk JG, Dickinson HD. Tracing the social gradient in the health of Canadians: Primary and secondary determinants. Soc Sci Med. 2003;56:263-76. [ Links ]
40. Medina-Solís CE, Maupomé G, Pelcastre-Villafuerte B, Ávila-Burgos L, Vallejos-Sánchez AA, Casanova-Rosado AJ. Desigualdades socioeconómicas en salud bucal: caries dental en niños de 6 a 12 años de edad. Rev Invest Clin. 2006;58:296-304. [ Links ]
41. Bower E, Gulliford M, Steele J, Newton T. Area deprivation and oral health in Scottish adults: A multilevel study. Community Dent Oral Epidemiol. 2007;35:118-29. [ Links ]
42. Ferro R, Besostri A, Meneghetti B, Olivieri A, Benacchio L, Tabaccanti S, et al. Oral health inequalities in preschool children in North-Eastern Italy as reflected by caries prevalence. Eur J Paediatr Dent. 2007;8:13-8. [ Links ]
43. Jamieson LM, Parker EJ, Armfield JM. Indigenous child oral health at a regional and state level. J Paediatr Child Health. 2007;43:117-21. [ Links ]
44. Villalobos-Rodelo JJ, Medina-Solís CE, Maupomé G, Vallejos-Sánchez AA, Lau-Rojo L, Ponce de León-Viedas MV. Socioeconomic and socio-demographic variables associated with oral hygiene status in Mexican school children aged 6 to 12 years. J Periodontol. 2007;78:816-22. [ Links ]
45. Villalobos-Rodelo JJ, Medina-Solís CE, Maupomé G, Pontigo-Loyola AP, Lau-Rojo L, Verdugo-Barraza L. Caries dental en escolares de una comunidad del noroeste de México con dentición mixta, y su asociación con algunas variables clínicas, socioeconómicas y sociodemográficas. Rev Invest Clin. 2007;59:256-67. [ Links ]
46. Herrera MS, Lucas-Rincón SE, Medina-Solís CE, Maupomé G, Márquez-Corona ML, Islas-Granillo H, et al. Desigualdades socioeconómicas en salud bucal: factores asociados al cepillado dental en escolares nicaragüenses. Rev Invest Clin. 2009;61:489-96. [ Links ]
47. Villalobos-Rodelo JJ, Medina-Solís CE, Maupomé G, Lamadrid-Figueroa H, Casanova-Rosado AJ, Casanova-Rosado JF, et al. Dental needs and socioeconomic status associated with dental services´ utilization taking place in the presence of dental pain: A case control study. J Orofac Pain. 2010;24:279-86. [ Links ]
48. Laaksonen M, Rahkonen O, Martikainen P, Lahelma E. Socioeconomic position and self-rated health: The contribution of childhood socioeconomic circumstances, adult socioeconomic status, and material resources. Am J Public Health. 2005;95:1403-9. [ Links ]














