Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biomédica
Print version ISSN 0120-4157
Biomédica vol.33 no.3 Bogotá july/Sept. 2013
https://doi.org/10.7705/biomedica.v33i3.770
ARTÍCULO ORIGINAL
doi: http://dx.doi.org/10.7705/biomedica.v33i3.770
Departamento de Análises Clínicas e Toxicológicas, Faculdade de Farmácia, Universidade Federal da Bahia, Bahia, Brasil
Authors' contributions:
Márcia Cristina Aquino Teixeira designed the study.
Márcia Cristina Aquino Teixeira, Robson Paixão Souza and Neci Matos Soares wrote the manuscript.
Robson Paixão Souza and Leda Maria Alcântara analyzed the results.
Joelma Nascimento Souza and Joelma Figueiredo Menezes performed the laboratory work.
Recibido: 23/07/12; aceptado:08/05/13
Introduction: Nematodes of Trichostrongylus genus are mainly parasites of herbivores, although sporadic human infections have been reported in many countries.
Objective: To describe the frequency and seasonality of Trichostrongylus spp. infection in individuals attended at a public clinical laboratory.
Materials and methods: Fecal samples of 9,283 individuals were evaluated by spontaneous sedimentation (Lutz) in the Parasitology Laboratory of the Pharmacy College, Federal University of Bahia, Brazil, from January of 2006 to May of 2008. The positive samples for either Trichostrongylus spp. or hookworms were further examined to evaluate the morphometry of nematode eggs.
Results: One-hundred and ten patients (1.2%) were confirmed to be infected by Trichostrongylus spp. The positive cases were significantly more frequent in females (1.6%; p <0.05), with higher distribution in the age group between 11-20 years (1.9%), compared to those aged 51-60 (0.8%) and older than 60 years (0.9%)( p <0.05), independent of gender. Trichostrongylus spp. infections were more common from March to May (40 cases) and showed a homogeneous distribution over the other periods of the year (21-25 cases). The hematological analyses of 60 Trichostrongylus -infected patients showed normal levels of eosinophils in most of the positive cases.
Conclusions: The data reveal that the occurrence of infection by Trichostrongylus spp. in residents of Salvador is more frequent than those reported in other urban regions and that it is essential to distinguish the parasite from other nematodes in routine parasitological examination.
Key words: Trichostrongylus , infection; diagnosis, differential; eosinophilia, seasons.
doi: http://dx.doi.org/10.7705/biomedica.v33i3.770
Infección humana por Trichostrongylus spp. en residentes de zonas urbanas de la ciudad de Salvador, Bahia, Brasil
Introducción. Los nematodos del género Trichostrongylus son parásitos principalmente herbívoros, aunque se hayan descrito en muchos países infecciones humanas esporádicas.
Objetivo. Describir la frecuencia y estacionalidad de la infección por Trichostrongylus spp. en individuos atendidos en un laboratorio clínico público.
Materiales y métodos. Las muestras fecales de 9.283 individuos fueron analizadas mediante sedimentación espontánea (Lutz) en el Laboratorio de Parasitología de la Facultad de Farmacia de la Universidad Federal de Bahía, Brasil, desde enero de 2006 a mayo de 2008. Las muestras positivas para Trichostrongylus o Ancylostoma fueron reexaminadas para evaluar la morfometría de los huevos de nematodos.
Resultados. La infección por Trichostrongylus spp. se confirmó en 110 pacientes (1,2 %), con mayor frecuencia en el sexo femenino (1,6 %; p<0,05). La frecuencia fue mayor en el grupo de edad de 11 a 20 años (1,9 %), independientemente del sexo, en comparación con las personas de 51 a 60 (0,8 %) y con los mayores de 60 años (0,9 %) (p < 0,05). Las infecciones por Trichostrongylus spp. fueron más frecuentes entre marzo y mayo (40 casos) con una distribución homogénea en otros periodos del año (21 a 25 casos). Los análisis hematológicos de 60 pacientes infectados con Trichostrongylus spp. presentaron niveles normales de eosinófilos en la mayoría de los casos positivos.
Conclusiones. Los datos revelan que la incidencia de la infección por Trichostrongylus spp. en individuos residentes en Salvador es más frecuente que las descritas en otras áreas urbanas y, por ello, es esencial distinguir dicho parásito de otros nematodos en los exámenes parasitológicos de rutina.
Palabras clave: Trichostrongylus , infección, diagnóstico diferencial, eosinofilia.
doi: http://dx.doi.org/10.7705/biomedica.v33i3.770
Nematodes of the genus Trichostrongylus are ubiquitous parasites of the digestive tract or respiratory system of several animals, with large distribution among the herbivores (1-3). Abebe and colleagues showed that sheep and goats are important reservoirs of these parasites (3). Moreover, studies conducted in the forest of Ulu Gombak, Kuala Lumpur, showed that the helminth Trichostrongylus , along with Taenia and Hymenolepis, accounted for roughly 41% of infection in rodents (4). The parasite has also been observed in chickens, with a prevalence of 4% (5).
Parasites of the family Trichostrongylidae have a tendency to colonize sites such as the abomasum, stomach, or small intestine of ruminants, horses, and swine, depending on the species. The life cycle takes place when eggs are passed in the feces of infected animals. Subsequently, first-stage larvae (L 1 ) hatch from mature eggs and develop into the second stage (L 2 ) and then into the third stage (L 3 ) infective larvae, protected by environmental variations by an impermeable sheath. The L 3 form may survive winter or dry periods due to hypobiosis and produce clinical outbreaks in ruminants in early spring or after rainy periods by re-hydrating (6).
Epidemiological studies indicate a global distribution and low prevalence of infections in humans (7-13). Accidental infections by T. orientalis have been reported in Korea, China, Japan and Armenia (9). Other species of Trichostrongylus associated with infections in humans have also been described, such as T. orientalis , T. colubriformis , T. vitrinus , T. axei , T. capricola , T. probolurus and T. skrjabin. Nevertheless, the species T. axei , T. colubriformis and T. orientalis are the most common parasites in human infections; most frequently acquired through contact with herbivorous animals (8-12).
The weather is one of the major factors influencing the transmission of the parasite, which usually occurs in regions that have warm temperatures (22 °C to 26 °C) and high humidity (80-100%). Thus, inter-tropical humid areas offer the most conducive conditions, while arid environments present the least conducive conditions for larval development. Sandy soil and large-size grasses also contribute to the spread of Trichostrongylus , by facilitating the movement of larvae and their survival, respectively (2,14). The transmission of the parasite to humans is often related to the ingestion of water or unwashed vegetables contaminated with infective larvae, especially where manure is used for soil fertilization (7-9,15).
Trichostrongylus spp. human infections are usually asymptomatic. However, in the most severe cases, the hematophagism of the L 4 larvae and adult forms can cause inflammation of the bowel mucosa and tissue damage. The clinical symptoms are cramping, abdominal pain, flatulence, nausea and diarrhea. Infections with a heavy worm load can progress to eosinophilia and anemia (12,13,16). Treatment is similar to that used for other helminthes: albendazole, mebendazole, pyrantel pamoate or ivermectin (15-19).
The diagnosis of Trichostrongylus infection is mainly performed by detecting eggs in stool examinations using spontaneous sedimentation or formalin-ether methods (15,17,20). While there is a similarity between Trichostrongylus and hookworm eggs, the former are longer and elongated (6,15,20). Despite the vast literature in veterinary medicine on parasites of ruminants, scientific reports on the prevalence of Trichostrongylus spp. in humans are scarce. Reported frequencies after 1990's are usually very low, ranging from 0.01% to 2.6%, usually associated with rural workers who work directly with cattle, goats and sheep (13,21-23). In Brazil, there are very few reports of the frequency of Trichostrongylus , especially in the northeastern region of the country. This is most likely due to difficulties in distinguishing the parasite eggs from other nematodes, such as hookworms. Therefore, the aim of this study was to evaluate the frequency and seasonality of Trichostrongylus infection in individuals living in urban areas of the city of Salvador in northeastern Brazil and to discuss the importance of its differential diagnosis.
Materials and methods
Samples and parasitological analysis
A total of 9,283 fecal samples from patients seen at the Clinical Analysis Laboratory of the Pharmacy College, Federal University of Bahia, were analyzed from January 2006 to May 2008 using spontaneous sedimentation (Lutz method). Approximately three grams of feces were dissolved in water and filtered through gauze into a 200 ml tapered glass cup for spontaneous sedimentation. After two hours, the supernatant was discarded and the pellet was suspended in water. The sedimentation process was repeated twice to clarify the sediment. For each sediment sample, three slides were examined under light microscopy at 100x and 400x magnification, using an iodine stain. Positive samples for hookworms, Trichostrongylus spp or Meloydogyne spp. eggs were re-analyzed using an ocular micrometer lens to measure the eggs. Trichostrongylus eggs are elongated and range in size from 75-95 µ m long by 40-50 µ m wide. They are similar to hookworm eggs, however, the eggs of hookworms are 60-75 µ m long by 35-40 µ m wide and have a shorter length:width ratio. Hookworm eggs also show a clear space between the morula and egg shell, decreasing over the egg maturation. The eggs of Meloidogyne spp are ovoid or ellipsoid, measuring 82-120 µ m in length and 24-43 µ m in width. They may present rounded or oval corpuscles, which resemble the drops of fat found between the morula and the shell.
Analysis of data
Data referencing age, gender, address, physical complaints, levels of blood eosinophilia of Trichostrongylus -infected individuals and month of fecal analysis were collected from patient laboratory records. Comparisons of frequencies of Trichostrongylus -positive cases, according to the age and gender of patients and the month of detection of infection were performed using the chi-square test. Values of p <0.05 were considered significant. We evaluated the Trichostrongylus infection seasonality by distributing the diagnosed cases into four periods of three months: September to November, December to February, March to May and June to August (with the June to August period corresponding to the rainy season in Salvador).
Ethical considerations
All adult individuals and parents or legal guardians of minors with confirmed Trychostrongylus infection were required to sign an informed consent upon receiving laboratory results. This work was approved by the Ethics Committee of the Gonçalo Moniz Research Center, Oswaldo Cruz Foundation.
Results
The parasitological examination of 9,283 fecal samples detected 110 (1.2%) Trichostrongylus infected patients. All cases were confirmed by further analysis of the size and shape of eggs in order to differentiate them from other smooth, thin-shelled, ellipsoidal nematode eggs found in human feces (figure 1). The positive cases were significantly ( p <0.05) more frequent in females (1.6%) than in male patients (0.7%) as seen in table 1. The age group between 11-20 years old was the most affected by Trichostrongylus infection (1.9%). The positivity observed in this cluster was significantly higher than those found in people aged 51-60 and above 60 years ( p <0.05) (table 1).
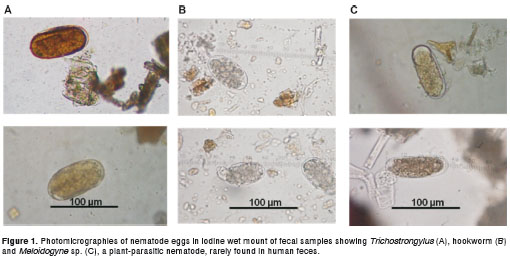
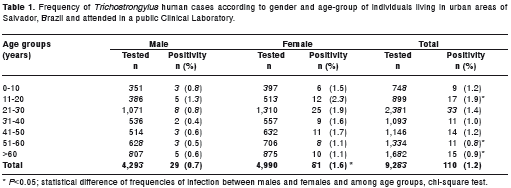
The majority of Trichostrongylus -infected patients (62.7%; 69/110) were mono-infected. Co-infection with other helminths included: Trichuris trichiura (n=4), Strongyloides stercoralis (n=2), Schistosoma mansoni (n=2), hookworm (n=1) and Enterobius vermicularis (n=1). The hematological analyses of 60 Trichostrongylus -infected patients showed normal relative levels of eosinophils (1-6%) in 48 individuals and discrete to intense eosinophilia in the 12 remaining cases (table 2). This final group had Trichostrongylus associated with Trichuris trichiura (n=1) and Strongyloides stercoralis (n=2) co-infection (table 2).
Trichostrongylus spp. human infection in Salvador was more common from March to May (40 cases) and showed a homogeneous distribution over the other periods analyzed, with 21 cases from December to February, 25 cases from June to August and 24 cases from September to November, as shown in table 3. However, no statistical difference was found in the distribution of Trichostrongylus infection in this population according to the period of year.
Most Trichostrongylus -infected individuals (67 cases) lived in urban areas close to the laboratory location, which is considered a middle-class neighborhood. The other 43 positive cases resided in distant urban boroughs (more than 10 Km distance from the laboratory) of lower socioeconomic characteristics.
Discussion
Human infection by Trichostrongylus spp. varies according to the population and geographical region studied, with rates of prevalence higher in rural areas (9). In addition to greater contact with animals, the socioeconomic conditions and hygiene in rural zones tend to be lower than those found in urban areas (11-13).
In comparison with the urban network of other Brazilian cities, Salvador is considered a national metropolitan center. Salvador has 2.6 million inhabitants, making it the third most populous city in Brazil and the eighth most populous city in Latin America. In this study, all subjects evaluated were from boroughs of Salvador or metropolitan areas, all with basic urban amenities: treated potable water supply, sewage system, rubbish disposal, public lighting, and sidewalks. Nevertheless, other epidemiological risk factors for Trichostrongylus infection, such a past history of visiting rural areas was not collected or analyzed in this study.
The prevalence of 1.2% of Trichostrongylus infection in this study was higher than that reported in humans living in urban areas in South Africa (0.1%) (24), Egypt (0.2%) (21) and Brazil (0.2% and 0.05%) (25), and lower than those reported in an irrigation zone in Peru (2.1%) (26), in a livestockbreeding area of a southeast Brazilian city (2.1%) (20), and in patients attending the Mansoura University Hospital in Egypt (2.6%) (21). The heterogeneity in environmental, socioeconomic and personal hygiene habits and characteristics of the populations studied may account for the differences observed in Trichostrongylus infection frequencies.
The source of Trichostrongylus spp. human infection in urban areas is uncertain. Disturbances in water reservoirs for human consumption caused by contamination with feces of ruminants, rodents or even humans might be implicated (9). Furthermore, water used for irrigation of leaves of lettuce, green onions, parsley, and other greens frequently used to prepare salads and sandwiches, might be contaminated with infective larvae.
Although Trichostrongylus spp. infection occurs across all ages and in both genders, women were significantly the most affected group with 1.9% of cases with age between 11-20 years. This may suggest an influence of dietary habits in this cluster (higher intake of fruits or other raw vegetables) predisposing them to infection (27,28), with the development of resistance leading to reduction of the prevalence in older people, as observed herein. Nevertheless, there is no published data about the role these factors play in the susceptibility of Trichostrongylus infection. This is most likely due to the low prevalence of this parasite in humans.
In this work, a higher number of Trichostrongylus cases were diagnosed between March and May, the months immediately preceding the rainy season, which corresponds with the autumn months. Salvador has a tropical rainforest climate with no discernible dry season. Temperatures are relatively constant throughout the year, with hot and humid weather conditions. The driest month of the year is January and the rainy season is mainly between May and July, with 789 mm of rainfall. In another study conducted in Brazil, most of the L 3 larvae of Trichostrongylus colubriformis were recovered from the forage apex of the Panicum maximum ( Aruana grass ) during autumn months, while in springtime, the biggest L 3 recovery occurred at the 21-28 cm stratum from both Aruana and Brachiaria decumbens (14). The results of this study suggest that the migration of T. colubriformis larvae is more influenced by weather conditions than by forage species. The increased vertical migration of larvae on vegetation during March to May might have favored the spread of the parasite among animals and then on to areas with water reservoirs or vegetable plantations intended for human consumption in Salvador. In fact, in tropical and subtropical areas, clinical infections in animals with weight loss and diarrhea can be observed right after the summer, during periods of rain due to rehydration of larvae in hypobiosis (1,2,6).
Concerning the differentiation of the species of the genus Trichostrongylus , this usually can be accomplished by evaluating the morphology of larvae, by analyzing the spicules and copulatory bursa of male adults, or by molecular techniques (10,15). In this study, the only evolutionary forms detected in fecal samples were eggs. Therefore, it was not possible to determine the species involved in the positive cases since it would have been necessary to either analyze the morphology of infective larvae (L 3 ) obtained from fecal culture, or the morphology of adult worms, which are rarely found in feces.
Studies on the epidemiology and diversity of the genus Trichostrongylus in human infections are still very limited. The real prevalence may be underestimated because most of the patients are asymptomatic or develop mild gastrointestinal disorders (13,16). Patients infected with Trichostrongylus may be asymptomatic, with normal eosinophilia. Conversely, a high worm burden can lead to marked eosinophilia and development of symptoms, including epigastric pain and diarrhea (12). Most Trichostrongylus human cases diagnosed in our study had no complaints of gastrointestinal symptoms and presented normal levels of blood eosinophils and low worm burden, as observed by the few parasite eggs detected in feces. These data suggest that Strongyloides stercoralis coinfection was the likely etiology of eosinophilia in two patients with discrete and intense blood eosinophil levels, respectively, as the correlation between asymptomatic persistent eosinophilia and strongyloidiasis is well documented (29). Furthermore, the parasite eggs may be confused with those from hookworms, and, therefore, the use of an ocular micrometer to measure the eggs for a parasitological diagnosis is necessary. In fact, in a recent study conducted in a rural village in Laos, 93.5% (43 from 46) of parasitological cases initially diagnosed as hookworm were later confirmed as Trichostrongylus colubriformes by analyzing adults worms eliminated after treatment with albendazole (30). These results show a high transmission of Trichostrongylus in the Laos Province and point to a misdiagnosis of the nematode.
Further studies on the Trichostrongylus spp. prevalence in human hosts should be carried out using more sensitive diagnostic techniques, such as the agar plate culture, which also permits the identification of L 3 infective larvae. Finally, the appropriate differentiation between hookworms and the genus Trichostrongylus is crucial in order to adopt specific measures to control widespread parasitic infections.
The authors confirm that there are no conflicts of interest.
This work was supported by the Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) and Universidade Federal da Bahia (UFBA), Brazil.
Corresponding author:
Márcia Cristina Aquino Teixeira, Av. Barão de Jeremoabo, Nº 147, Campus Universitário de Ondina 40170-115, Salvador, Bahia, Brasil Telephone: (5571) 3283 6950; fax: (5571) 3283 6949 marciat@ufba.br
1. Khan MN, Sajid MS, Khan MK, Iqbal Z, Hussain A. Gastrointestinal helminthiasis: Prevalence and associated determinants in domestic ruminants of district Toba Tek Singh, Punjab, Pakistan. Parasitol Res. 2010;107:787-94. http://dx.doi.org/10.1007/s00436-010-1931-x [ Links ]
2. Morgan ER, Torgerson PR, Shaikenov BS, Usenbayev AE, Moore AB, Medley GF, et al . Agricultural restructuring and gastrointestinal parasitism in domestic ruminants on the rangelands of Kazakhstan. Vet Parasitol. 2006;139:180-91. http://dx.doi.org/10.1016/j.vetpar.2006.02.016 [ Links ]
3. Abebe R, Gebreyohannes M, Mekuria S, Abunna F, Regassa A. Gastrointestinal nematode infections in small ruminants under the traditional husbandry system during the dry season in southern Ethiopia. Trop Anim Health Prod 2010;42:1111-7. http://dx.doi.org/10.1007/s11250-010-9532-3. [ Links ]
4. Paramasvaran S, Krishnasamy M, Lee HL, John J, Lokman H, Naseem B M, et al . Helminth infections in small mammals from Ulu Gombak Forest Reserve and the risk to human health. Trop Biomed. 2005;22:191-4. [ Links ]
5. Orunc O, Bicek K. Determination of parasite fauna of chicken in the Van region. Turkiye Parazitol Derg. 2009;33:162-4. [ Links ]
6. Johnstone C, Guerrero J, Chou S, Hodday M, Howe-Smith R, Eisenberg A. Parasites and parasitic diseases of domestic animals. Fecha de consulta: 12 de mayo de 2011. Disponible en: http://cal.vet.upenn.edu/projects/merial/index.html [ Links ]
7. Cancrini G, Boemi G, Iori A, Corselli A. Human infestations by Trichostrongylus axei , T. capricola and T. vitrinus : 1st report in Italy. Parassitologia. 1982;24:145-9. [ Links ]
8. Ghadirian E, Arfaa F. Present status of trichostrongyliasis in Iran. Am J Trop Med Hyg. 1975;24:935-41. [ Links ]
9. John DT, Petri WA. The intestinal nematodes. En: Markell and Voge's Medical Parasitology. 9th edition. St. Louis, USA: Elsevier; 2006. p. 266-7. [ Links ]
10. Yong TS, Lee JH, Sim S, Lee J, Min DY, Chai JY, et al. Differential diagnosis of Trichostrongylus and hookworm eggs via PCR using ITS-1 sequence. Korean J Parasitol. 2007;45:69-74. http://dx.doi.org/10.3347/kjp.2007.45.1.69 [ Links ]
11. Millington MA, Costa CH, Tavares AM, Dourado H, Reid WA, Macedo V, et al. Detection of helminthiasis with the Kato-Katz, Baermann-Moraes and Harada methods, in Tefe and various villages by the Japura-Caqueta River, Amazonas. Rev Soc Bras Med Trop. 1989;22:217-8. [ Links ]
12. Ralph A, O'Sullivan MV, Sangster NC, Walker JC. Abdominal pain and eosinophilia in suburban goat keepers –trichostrongylosis. Med J Aust. 2006;184:467-9. [ Links ]
13. Boreham RE, McCowan MJ, Ryan AE, Allworth AM, Robson JM. Human trichostrongyliasis in Queensland. Pathology. 1995;27:182-5. [ Links ]
14. da Rocha RA, Bricarello PA, da Rocha GP, Amarante AF. Trichostrongylus colubriformis larvae recovery from different Brachiaria decumbens and Panicum maximum strata . Rev Bras Parasitol Vet. 2007;16:77-82. [ Links ]
15. Centers for Disease Control and Prevention. Trichostrongylosis . In: Parasites and Health. 2011. Fecha de consulta: 1 de abril de 2011. Disponible en: http://www.dpd.cdc.gov/dpdx/html/Trichostrongylosis.htm [ Links ]
16. Wall EC, Bhatnagar N, Watson J, Doherty T. An unusual case of hypereosinophilia and abdominal pain: An outbreak of Trichostrongylus imported from New Zealand. J Travel Med. 2011;18:59-60. http://dx.doi.org/10.1111/j.1708-8305.2010.00474.x [ Links ]
17. Thibert JB, Guiguen C, Gangneux JP. Human trichostrongyloidosis: Case report and microscopic difficulties to identify ancylostomidae eggs. Ann Biol Clin. 2006;64:281-5. [ Links ]
18. Crompton DWT, Montresor A, Nesheim MC, Savioli L. Controlling disease due to helminth infections. Geneva: WHO; 2003. Fecha de consulta: 13 junio del 2010. Disponible en: http://whqlibdoc.who.int/publications/2003/9241562390.pdf [ Links ]
19. Bradbury RS. An imported case of trichostrongylid infection in Tasmania and a review of human trichostrongiloidiosis. Annals of the Australasian College of Tropical Medicine. 2006;72:25-8. [ Links ]
20. Zoppas BC, Soligo DS, Santos I, Paganella MP. Primeira experiência no diagnóstico laboratorial de trichostrongylose humana na região nordeste do Rio Grande do Sul. Rev Bras Anal Clin. 2005;37:105-6. [ Links ]
21. el-Shazly AM, el-Nahas HA, Soliman M, Sultan DM, Abedl Tawab AH, Morsy TA. The reflection of control programs of parasitic diseases upon gastrointestinal helminthiasis in Dakahlia Governorate, Egypt. J Egypt Soc Parasitol. 2006;36:467-80. [ Links ]
22. Shin EH, Guk SM, Kim HJ, Lee SH, Chai JY. Trends in parasitic diseases in the Republic of Korea. Trends Parasitol. 2008; 24:143-50. http://dx.doi.org/10.1016/j.pt.2007.12.003 [ Links ]
23. Youn H. Review of zoonotic parasites in medical and veterinary fields in the Republic of Korea. Korean J Parasitol. 2009;47:133-41. http://dx.doi.org/10.3347/kjp.2009.47.S.S133. [ Links ]
24. Adams VJ, Markus MB, Adams JF, Jordaan E, Curtis B, Dhansay MA , et al. Paradoxical helminthiasis and giardiasis in Cape Town, South Africa: Epidemiology and control. Afr Health Sci. 2005;5:276-80. [ Links ]
25. Fleury GC, Correa MO, Amato-Neto A. Identification of Trichostrongylus colubriformis as a human parasite. Rev Inst Med Trop Sao Paulo. 1970;12:288-92. [ Links ]
26. Esteban JG, González C, Bargues MD, Angles R, Sánchez C, Naquira C. High fascioliasis infection in children linked to a man-made irrigation zone in Perú. Trop Med Int Health. 2002;7:339-48. http://dx.doi.org/10.1046/j.1365-3156.2002.00870.x [ Links ]
27. Fagerli RA, Wandel M. Gender differences in opinions and practices with regard to a "healthy diet". Appetite. 1999;32:171-90. http://dx.doi.org/10.1006/appe.1998.0188 [ Links ]
28. Johansson L, Thelle DS, Solvoll K, Bjørneboe GA, Drevon CA. Healthy dietary habits in relation to social determinants and lifestyle factors. Br J Nutr. 1999;81:211-20. [ Links ]
29. Goswami ND, Shah JJ, Corey GR, Stout JE. Persistent eosinophilia and Strongyloides infection in Montagnard refugees after presumptive albendazole therapy. Am J Trop Med Hyg. 2009;81:302-4. [ Links ]
30. Sato M, Yoonuan T, Sanguankiat S, Nuamtanong S, Pongvongsa T, Phimmayoi I. Short report: Human Trichostrongylus colubriformis infection in a rural village in Laos. Am J Trop Med Hyg. 2011;84:52-4. http://dx.doi.org/10.4269/ajtmh.2011.10-0385. [ Links ]













