Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biomédica
Print version ISSN 0120-4157
Biomédica vol.34 no.1 Bogotá Jan./Mar. 2014
https://doi.org/10.7705/biomedica.v34i1.1446
ARTÍCULO ORIGINAL
doi: http://dx.doi.org/10.7705/biomedica.v34i1.1446
1 Fundación Salutia, Centro de Investigaciones en Economía, Gestión y Tecnologías en Salud, Bogotá, D.C., Colombia
2 External Advisory Consultant in Hematology, Bogotá, D.C., Colombia
3 Grupo de Investigación en Economía de la Salud, Universidad de Cartagena, Cartagena, Colombia
Author contributions:
Milton Fabián Suárez-Ortegón participated in designing the project, in field work, statistical analysis and in drafting the document.
Alejandra Arbeláez, Mildrey Mosquera, Cecilia Aguilar-De Plata participated in designing the project, in field work and in correcting the document.
Robinson Ramírez-Vélez participated in drafting the document.
Martín Romero: Team leader, research design and development, analysis and discussion of results.
Diana Chávez: Data gathering and management, support in the building of the model.
Magaly De los Ríos: Review process, discussion of drug facts.
Nelson Álvis and Martín Romero: Revision of the model, participation in the discussion.
All authors participated in the drafting and approval of the final manuscript.
This study was conducted at Fundación Salutia, Centro de Investigaciones en Economía, Gestión y Tecnologías en Salud in Bogotá, D.C., Colombia
Recibido: 02/11/12; aceptado: 08/08/13
Introduction: New tyrosine kinase inhibitor treatments for chronic myeloid leukemia based on nilotinib, dasatinib and imatinib have improved patient quality of life and have turned chronic myeloid leukemia from a fatal disease into a chronic disease.
Objective: To evaluate the cost-effectiveness of nilotinib, 600 mg, and dasatinib, 100 mg, each compared to imatinib, 400 mg, as first-line therapy in chronic myeloid leukemia in Colombia from a third-party payer´s perspective.
Materials and methods: A cost-effectiveness analysis was performed using a Markov model to evaluate a hypothetical cohort of one hundred 55 year-old patients with newly diagnosed chronic myeloid leukemia in the chronic phase, and the time horizon for the baseline case was established as being until the end of life. Progression-free life-years saved were considered the primary outcome. Transition probabilities for major molecular response, disease progression to accelerated phase or blast crisis, and chronic myeloid leukemia related deaths were analyzed in the model for each arm. A 3% discount rate was applied to all costs and patient outcomes. Model robustness was evaluated using both univariate and multivariate Montecarlo sensitivity analysis.
Results: Nilotinib was higher in expected progression-free life-years saved (15.21 vs. 12.64 for imatinib), followed by dasatinib (14.91 vs. 14.54 for imatinib). Imatinib had lower total lifetime costs. The incremental cost-effectiveness ratio was US$ 33,120.36 in the nilotinib arm and US$ 514,939.08 in the dasatinib arm per progression-free life-years (PF-LYs) saved, each compared to imatinib. When analyzing nilotinib versus dasatinib indirectly, nilotinib was found to be dominant due to higher efficacy (2.25 PF-LYs) and lower costs (US$ 44,674) in the baseline case. The average estimated cost to manage disease progression per year was US$ 101,978.78, considered to be the threshold.
Conclusion: In Colombia, using PF-LYs as the efficacy outcome, nilotinib is highly cost-effective when compared to imatinib and dominant vs. dasatinib in first-line therapy for CML in chronic phase.
Key words : Protein-tyrosine kinases; leukemia, myelogenous, chronic, BCR-ABL positive; costs and cost analysis; drug evaluation.
doi: http://dx.doi.org/10.7705/biomedica.v34i1.1446
Análisis de costo-efectividad de nilotinib, dasatinib e imatinib como terapia de primera línea en leucemia mieloide crónica en Colombia, 2012
Introducción. Los nuevos inhibidores de la tirosina cinasa para tratar la leucemia mieloide crónica basados en nilotinib, dasatinib e imatinib, mejoraron la calidad de vida de los pacientes y la tornaron en enfermedad crónica.
Objetivo. Evaluar el costo-efectividad de nilotinib, 600 mg, y dasatinib, 100 mg, comparados con imatinib, 400 mg, como terapia de primera línea en leucemia mieloide crónica desde la perspectiva del tercero pagador en Colombia.
Materiales y métodos. Se analizó el costo-efectividad mediante un modelo de Markov con ciclos trimestrales, que evaluó una cohorte hipotética de 100 pacientes de 55 años recién diagnosticados con leucemia mieloide crónica en fase crónica en un horizonte temporal hasta el final de la vida. El desenlace primario fueron los años de vida ganados libres de progresión. Se analizaron las probabilidades de transición para respuesta molecular mayor, progresión de la enfermedad y muerte relacionada con la leucemia mieloide crónica en el modelo para cada grupo. Se aplicó una tasa de descuento de 3 % a los costos y resultados de los pacientes. La solidez del modelo se evaluó por medio de un análisis de sensibilidad de tipo Montecarlo.
Resultados. Nilotinib fue mayor en años de vida ganados libres de progresión esperados (15,21 Vs . 12,64 para imatinib), seguido por dasatinib (14,91 Vs . 14,54 para imatinib). El grupo tratado con imatinib fue la opción menos costosa y menos efectiva. La relación costo-efectividad incremental´ (sic.) fue de US$ 33.120 en el grupo de nilotinib y de US$ 514.939,08 en el grupo de dasatinib por año de vida ganado libre de progresión comparados con imatinib. Al comparar indirectamente nilotinib con dasatinib, nilotinib fue dominante debido a su mayor eficacia (2,25 años de vida ganados libres de progresión) y menor costo (US$ 44.674). El costo promedio estimado para manejar la progresión de la enfermedad por año fue US$ 101.978,78 considerado como umbral.
Conclusión. Usando como medida de efectividad los años de vida ganados libres de progresión, en Colombia el nilotinib es altamente costo-efectivo frente al imatinib y dominante cuando se compara con dasatinib para el tratamiento de primera línea de pacientes con leucemia mieloide crónica en fase crónica.
Palabras clave: proteínas tirosina quinasas, leucemia mielogenosa crónica BCR-ABL positiva, costos y análisis de costo, evaluación de medicamentos.
doi: http://dx.doi.org/10.7705/biomedica.v34i1.1446
Chronic myeloid leukemia (CML) was the first malignant disease in which an acquired genetic anomaly was demonstrated as the final trigger in a chronic myeloproliferative syndrome characterized by a translocation between chromosomes 9 and 22, giving rise to the formation of the so-called Philadelphia chromosome (Ph) and the creation of a new fused gene, BCR-ABL . This gene codifies a chimeric BCR-ABL protein that presents an elevated tyrosine kinase (TK) activity, which increases the survival and proliferation of cells and inhibits apoptosis (1).
CML represents approximately 10 to 15% of all leukemias, and the average risk that a person has of developing CML during his or her lifetime is estimated at 1 in 625. This disease is slightly more common in men than in women and more common in white subjects than in black subjects (2,3) with an average diagnosis age of 55 years (4). In the United States, the incidence is 1 to 1.5 cases per 100,000 inhabitants (5,6).
Disease clinical progression can be divided into three primary phases: The chronic phase (1st), during which 90% of patients are diagnosed, may last between three and eight years. Blood cells retain their capacity to differentiate themselves normally, until the illness progresses to the accelerated phase (2nd), during which immature cells begin to be detected during blood circulation, and lastly, the blast crisis phase (3rd), characterized by +30% of immature cells (blasts) in circulation, including extramedullary infiltrates associated with an increase in splenomegaly. During blast crisis, or the terminal phase of the disease, the disease transforms into acute myeloid leukemia (AML) in 85% of cases and into acute lymphoblast leukemia (ALL) in 10-15% of cases (7). During this phase, a patient´s survival period decreases to months and even weeks (8).
The main purpose of treatment for CML includes eradicating the leukemic clone from the bone marrow or maintaining the chronic phase with sustained remission and minimal toxicity from the treatment (7). Current treatment focuses on counteracting and slowing the disease progression, stopping tyrosine kinase activity, blocking the ATP- joining region, blocking dimerization, and generating antibodies against the tyrosine receptor (9).
The introduction into clinical practice of tyrosine kinase inhibitors (TKI) that specifically block the enzymatic action of the fusion protein BCR- ABL became a significant contribution to the management of CML (10). The use of new treatments based on imatinib, nilotinib and dasatinib have improved the patients´ quality of life and changed the natural history of the disease, transforming it from a disease that tended to be fatal to a chronic disease (8).
The history of CML treatment has allowed for partial understanding of the biology of cancer and the development of highly specific inhibitors directed against some of the genetic abnormalities of the disease. Until a few years ago, there were limitations in the treatment of this condition; nevertheless, the progress of basic and clinical research has allowed for changes in response rates, quality of life and the survival of affected subjects. However, imatinib mesylate and second- generation tyrosine kinase inhibitors (nilotinib, dasatinib and bosutinib) have not managed to stop disease progression, but they have been able to modify the progression of the blast crisis (11).
The annual mortality rate for CML is less than 10% for the first two years after diagnosis (10,11). Survival after five years in low, intermediate and high-risk groups is 76%, 55% and 25%, respectively, and for subjects in the accelerated and blast phases it is lower than 10% and 5%, respectively (10,11).
Nilotinib and dasatinib were initially developed for the treatment of patients resistant or intolerant to imatinib. In recent studies these have separately demonstrated superiority to imatinib in terms of progression free survival (PFS) and major molecular response (MMR) as 1st-line treatment in newly diagnosed patients (12-16) . These efficacy outcomes created the need for a health economics evaluation in Colombia to help in decision making.
The aim of this study was to evaluate the cost-effectiveness of nilotinib, 600 mg, and dasatinib, 100 mg, each compared to imatinib, 400 mg, as first line therapy in CML in Colombia from a third- party payer´s perspective.
Materials and methods
Markov model
A cost-effectiveness analysis was performed from a third-payer perspective using a Markov chain model (figure 1) with quarterly cycles, based upon a decision-making tree that simulates the natural history of the disease from a temporary standpoint until the end of life.
As a case study, a patient with an average age of 55 years (average age of disease onset) (4), recently diagnosed with CML in the chronic phase, who had not received any other treatment in each branch of treatment was considered.
The model assumes that all patients admitted are newly diagnosed and found to be in the chronic stage of disease and may or may not reach major molecular response (MMR). Those not in MMR may progress (to accelerated phase or blast crisis phase). Patients in the accelerated phase may remain so or go on to the blast phase and, once in the accelerated or blast phase, may die as a result of the disease. During any of the stages, patients may experience adverse events. Lastly, patients may die at any moment for any other reason (figure 2). The model does not include a state of voluntary withdrawal or suspension of treatment, since this would represent implications that would adversely affect the model.
Clinical data
As a starting point, the literature available in the Pubmed, LILACS, and Cochrane Library databases, as well as in the Trip Database and gray literature, were reviewed using the following keywords: nilotinib, dasatinib, imatinib, chronic myeloid leukemia, BCR-ABL, tyrosine kinase, as well as the expressions Cost-effectiveness analysis, costs and economic assessment, in order to find information on effectiveness and safety in the treatment of the disease and the selected comparisons. Phase III studies, systematic reviews, economic assessments and medical practice guides were selected. We did not include expert opinions, or narrative non-systematic reviews or phase-I/II clinical trials. We excluded studies evaluating the molecular composition of the medications, as well as case reports or case series.
Search strategies identified 442 studies, starting in 2000 and with a cut-off date of November 2011; when applying the inclusion criteria, 191 studies were selected on Pubmed, and from these, the 102 most pertinent publications in terms of their subject and study objective were selected. These were: Six clinical practice guides; 17 cost-effectiveness analyses and other economic assessments; 19 systematic reviews; 60 clinical trials.
Studies were included if they involved:
Randomized controlled trials or systematic reviews of randomized controlled trials.
Adults with chronic phase chronic myeloid leukemia resistant to any treatment specifically directed against chronic myeloid leukemia.
Dasatinib, nilotinib or imatinib treatments (standard dose).
Imatinib or nilotinib comparators where the treatment is dasatinib; imatinib or dasatinib where the treatment is nilotinib, dasatinib, or nilotinib.
For the cost-effectiveness review, the inclusion and exclusion criteria were the same as for the clinical effectiveness review, except in the case of study design, where full cost-effectiveness analyses, cost-utility analyses, and cost-benefit analyses were included.
Publications about two fundamental studies were also selected, which served as the source for this assessment: ENESTnd (12,13) and DASISION (14,15). Likewise, publications were identified that served as a basis for knowledge about the natural history of the disease and the technologies assessed. Publications were found that had the objective of assessing recent phase III clinical trials that compared nilotinib or dasatinib with imatinib in recently diagnosed CML (16-18).
No simultaneous studies were found and the duration of the clinical trials selected (24 months) was short (compared to the natural history of disease) but showed convincing results. There are other studies that correspond to second-line management that are not applicable for this analysis.
The ENESTnd study evaluated nilotinib versus imatinib and, at the same time, the DASISION study evaluated dasatinib versus imatinib; both studies published their results at 12 (12-14), 18 (presented as abstracts in ASH 2010) and 24 (13, 15) months. Although the populations studied in these two studies were similar, the manner of measuring efficacy was different in that, for the ENESTnd study, major molecular response (MMR) was the primary point for results, whereas in the DASISION study, this result was the final secondary point (best response reached). Both trials reported MMR by risk group category at 12, 18 and 24 months.
The American Cancer Society clinical practice guide (3), the ESMO Guidelines Working Group (19), and the Consensus Guide for the Diagnosis and Treatment of CML in Colombia (20) recommend imatinib at a dose of 400 mg/QD as a first treatment option. On the other hand, the results of the ENESTnd and DASISION studies showed efficacy benefits for first-line nilotinib and dasatinib in doses of 300 mg/BID and 100 mg/QD, respectively. In fact, this treatment regimen has been recommended by the National Comprehensive Cancer Network (2, 19,21) as an alternative choice for patients in the chronic phase.
Due to a lack of head-to-head trials of safety and efficacy on first-line treatment between nilotinib and dasatinib for CML, a four-branch parallel analysis was performed (comparing nilotinib vs. imatinib and dasatinib vs. imatinib), using the information from these two available clinical studies. A final comparison between nilotinib and dasatinib was made indirectly using the Boucher method (22), considering the similarity of the results of each analysis for the group receiving imatinib.
Progression-free time measured in progression-free life-years ( PF-LYs ) was selected as the outcome of the analysis, considering that the analysis emphasizes first-line treatment and the primary success is getting the patient to remain in the chronic phase. Health-related quality of life was not considered as an outcome because in Colombia there is no available information to date in that regard, and its extrapolation between countries is arguable.
Transition probabilities for major molecular response (MMR), disease progression to the accelerated phase or blast crisis (AF/BC), and CML-related death were included in the model for each branch, considering the duration of each cycle (quarterly) and the stage within the model to which it was applied according to the literature review, and estimated according to information from clinical trials ENESTnd (12,13) (12, 18 and 24 months), and DASISION (14-15) (12, 18 and 24 months), which was validated by clinical experts. In all branches, the probabilities of presenting with grade 3/4 hematological adverse events were included for each type of treatment obtained from those studies (12-15) as shown in table 1.
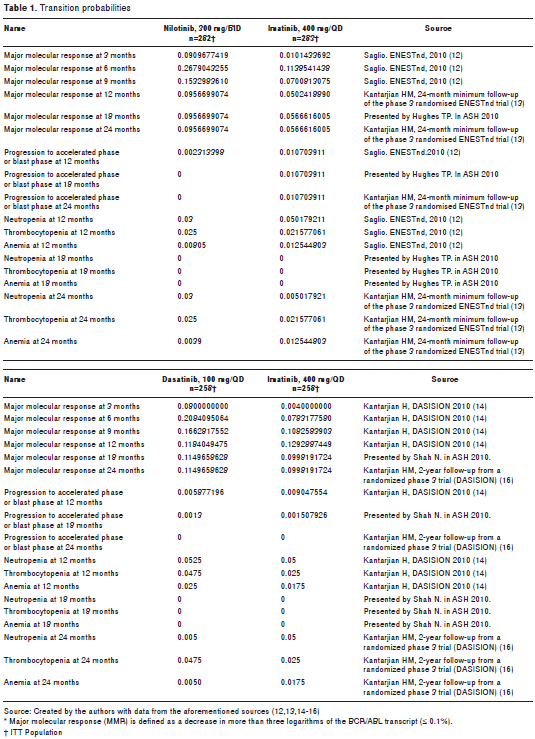
Knowing that effectiveness is given for 24 months, a comparative analysis was performed with the IRIS study, which has reported the results of a 7-year follow-up (23). Keeping in mind that the trend in the three studies was similar, it was assumed that performance over time from an efficacy point of view would be maintained with equal tendency as it figures into the probabilities for subsequent cycles.
Assessment of costs
The costs of the medications were calculated by taking the average sales price to the distributor of nilotinib, of imatinib and dasatinib, and the prices regulated by the State as the maximum chargeable price according to Resolution 2569 of 2012 (24) (table 2).
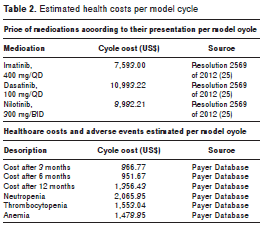
Each stage included the cost of healthcare as established according to the 2011 insurance price databases and validated with grounds on the recommendations of the European Leukemia Net (25,26) for the treatment of chronic myeloid leukemia and the accepted clinical recommendation guidelines (2,20,22) (table 2).
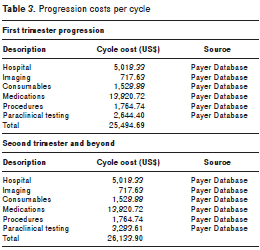
In order to establish the cost of the stage of progression (adsorbent stage), the total average cost was estimated to include: hospital costs, medications, diagnostic imaging, consumables, procedures, laboratory tests and medical care, differentiating them from their beginning (first trimester) and subsequent periods (table 3). Bone marrow transplant costs were not included in the progression costs due to the age of the patients in the baseline case.
According to DANE (27) vital statistics registries (2000-2008), sepsis occurred as a direct cause of death in 16.54% and hemorrhagic shock, in 19.60%, and according to the data collected from the service usage databases, the average cost estimates for care prior to death would be US$ 8,713.
Discount rate
Most pharmacoeconomic analyses agree that costs should be discounted in any study with a time horizon longer than one year (28). The discount is used to convert future costs into present values, allowing for the creation of useful economic data, taking into account costs and benefits for several years. Although the appropriate discount rate is controversial and may vary over time, according to the economic environment, the costs and health benefits were chosen objectively.
The results were presented as total values reached from a temporary standoint, and the incremental cost- effectiveness ratios (ICER) were analyzed by applying an annual discount rate of 3% to both costs and outcomes, keeping in mind the recommendations of different international guidelines.
Premises
Due to the absence of an official cost-effectiveness threshold in Colombia for the analyzed outcome, all comparisons were made by taking average estimated costs for the treatment of patient disease progression for one trimester (cycle) as a reference, understanding that this is the therapeutic goal and the profits earned over time could be comparable to the annual cost of US$ 101,978.78.
In order to determine the robustness of the model and control over uncertainty, a univariable and multivariable Montecarlo sensitivity analysis was performed with variations of ±20% in all of the variables, assuming that all of them behaved normally.
Ethical aspects
The ethical principles regarding human research contained in the standards for health research in Colombian Ministry of Health Resolution 8430 of 1993 were considered. According to article 11 of that Resolution, this study is a research without risk, as the information used is retrospective, without any intervention or modification involving biological, physiological, psychological, social variables or individuals participating in the study. However, records of the data generated from the different variables were used for the analysis of cost-effectiveness (29).
Results
Table 4 shows the data obtained in the deterministic analysis within the baseline case for each of the four branches with and without discount. The most effective option within the model was the one using nilotinib followed by dasatinib (15.21 and 14.91 PF-LYs, respectively) as opposed to imatinib (12.64 and 14.54 PF-LYs , respectively). The branch treated with imatinib was the least expensive option of the three.
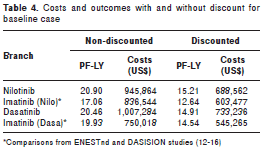
From total costs, 7.56% was attributed to the treatment of this type of patient, 21.76% to the costs of the technologies analyzed, and adverse events represented 12.13% of the total cost in the different branches, while the remaining 58.55% were progression costs.
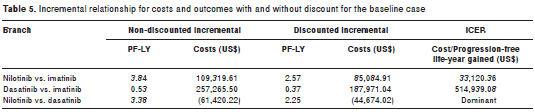
The increments for the data with and without discount and the respective incremental cost-effectiveness ratio (ICER) are presented in table 5, where it is observed that the most cost- effective option is the use of nilotinib, which has an incremental effectiveness of 2.57 PF-LYs and a cost of $ 5,085, in comparison with imatinib. The ICER showed that the cost per PF-LYs was $ 33,120 when comparing nilotinib with imatinib, as opposed to $ 514,939 when comparing dasatinib with imatinib. When performing an indirect comparison between nilotinib and dasatinib, an extended dominance was shown equaling greater effectiveness (2.25 PF-LYs) and a lower incremental cost ($ 44,674) in the conditions of the baseline case.
Sensitivity analysis
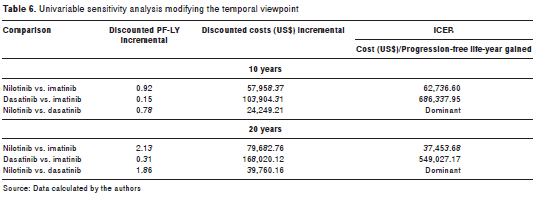
A univariable analysis was performed from a temporary standpoint at 10 and 20 years (table 6), and with changes in the discount rate (5% and 0%), showing that the results favoring nilotinib had been maintained.
The multivariable Montecarlo analysis with 1,000 repetitions is shown with point graphs (Scatter Plot) that demonstrate that nilotinib maintains its cost- effectiveness versus imatinib in more than 99% of the cases with variations of ±20% (figure 3). Likewise, it is shown that dasatinib demonstrates 2.2% cost-effectiveness versus imatinib, keeping the proposed threshold in mind.
Figure 4 shows the comparison between dasatinib and nilotinib,revealing that nilotinib was better in all cases, as it is 10.91% cost-effective and dominant by 70.67% within the outcome analyzed.
Discussion
The results show similarities to the clinical trial reports: the clinical effectiveness of nilotinib versus comparable drugs by virtue of a greater proportion of patients who reach major molecular response and accordingly a lower progression rate (30) than that reported recently in several publications (31-33, Savova A, Petrova G. PCN27 Budget impact analysis for chronic myeloid leukemia therapy in Bulgaria. Value Health. 2011;14:A438. http://dx.doi. org/10.1016/j.jval.2011.08.1128).
A head-to-head trial of the efficacy and safety of these two agents is necessary in order to estimate the different therapeutic effects of these first generation technologies.
There are no similar comparisons to those presented here, but the individual results of nilotinib and dasatinib versus imatinib were similar to other recently-developed studies (34,35), which concluded that nilotinib is cost-effective in patients with newly diagnosed chronic phase Philadelphia chromosome-positive CML initiating therapy with TKI (tyrosine kinase inhibitor) after such outcomes as life-years (LY) saved or quality-adjusted life-years (QALY) saved were analyzed.
In 2011, Signorovitch, et al., performed an indirect adjusted comparison of MMR rates from the results of the random trials of nilotinib versus imatinib (ENESTnd) and dasatinib versus imatinib (DASISION), progression-free survival and overall survival after 12 months of treatment with nilotinib and dasatinib for new diagnoses of CML-CP, during which the individual data of the patients treated with nilotinib and dasatinib were estimated in order for their basic characteristics to coincide, including age, gender, ECOG performance status, and hematological laboratory values. After comparing the efficacy results of patients treated with nilotinib, 300 mg, twice daily versus those treated with dasatinib, 100 mg, once daily, they were compared with patients treated with imatinib in each trial. This was used to evaluate the accuracy of the correlation (negative control). Nilotinib was associated with significantly higher rates of MMR (56.8% as compared to 45.9%, p=0.001) and overall survival (OS) versus dasatinib (99.5% vs . 97.3%, p=0.046) (35).
The National Institute for Health and Clinical Excellence (NICE) performed a review, taking the aforementioned evidence for 12 and 18 months and the indirect comparison studies between dasatinib and nilotinib. They claimed that there is a lack of information to evaluate the long-term results in patients (for example, progression-free survival PFS, overall survival OS, health-related quality of life HRQoL, etc.) and mentioned that the drugs were well tolerated, with discontinuation rates due to adverse events at <10%. Nevertheless, comparing these new technologies with imatinib at a dose of 400 mg, and taking the second-line usage information from nilotinib, they concluded that the use of first-line nilotinib would be cost-effective in comparison with imatinib. The efficacy ratio for dasatinib beyond the €30,000 threshold per quality-adjusted life-years (QALY) saved (ICER for dasatinib versus imatinib was greater than €200,000 per QALY saved) is, therefore, not recommended for funding because it is not considered to be profitable (36). Finally, NICE Committee recommends the use of first-line nilotinib for patients with CML, and its use is considered profitable for the resources available to the NHS (36).
Studies such as those carried out in México (Hernández-Rivera G, Aguayo Á, Cantu-Rodríguez O, Cervera E, Gomez-Almaguer D, Gutiérrez H, et al. PSY24 Cost of care for chronic myeloid leukemia (CML) in patients experiencing resistance and/or intolerance to imatinib from the public health system perspective in Mexico. Value Health. 2010;13:A464), Brasil (Ouissak C, Litalien G, Alves MR. PCN35 Cost-effectiveness analysis of dasatinib for the treatment of imatinib resistant or intolerant CML patients in Brazil. Value Health. 2008;11:A64. http://dx.doi.org/10.1016/S1098-3015(10)70213-8), Colombia and Venezuela (37), and Chile (38), only show second-line results by measuring quality-adjusted life-years (QALY) gained as an outcome when comparing the costs and cost-effectiveness of the use of 100 mg/day and 140 mg/day of dasatinib with the use of 800 mg/day of nilotinib and a dose of imatinib at 800 mg/day in patients who have developed resistance and intolerance to normal doses of imatinib; they are, therefore, not comparable with the results of this study.With the improvement in the technology, there is evidence that treatment results are better in terms of molecular response than of cytogenetic response, as demonstrated in the ENESTnd study, which has established nilotinib as a highly effective agent in the first-line treatment of CML (31).
A complete economic analysis was performed that presented all of the factors related to the treatment and compared the three most common treatment options. A double analysis of imatinib was performed when comparing it independently with nilotinib and dasatinib, with similar results. This allowed for the indirect establishment of the relationship between the two comparable drugs without losing sight of the bias caused by the lack of head-to-head trials data.
Probability ranges were obtained from clinical studies (12-15) and analyzed based on the information backed by qualified clinical studies with an evidence level of 1++ and an A recommendation level according to the SIGN-50 qualification (39). Nevertheless, one important limitation is that the data corresponds to phase III studies with results published after 24 months (13,15), and they are used for the long-term analysis. In this sense, the interpretation would be similar to that reported in the IRIS study for imatinib after the 7-year follow-up (24); upon viewing the IRIS results and their performance, it can be assumed that similar performance will continue.
It is important to recognize that the cost of the medications is included in the principal result factor, and as of the date of this study, dasatinib and imatinib are regulated in Colombia (25) in such a manner that the price of nilotinib corresponds to an estimated price based on the distributor sales prices in that country.
It would be worthwhile to perform an additional quality of life (QoL) analysis of the patients treated with nilotinib, which would allow us to establish whether, from a cost-utility standpoint, the same results are maintained. However, there are no published studies to date on the quality of life in Colombia for CML, and trying to utilize information about preferences in other countries would include a significant bias that would make the obtained results disputable. Therefore, this is another matter that must be researched. Under ideal conditions, QoL evaluations should be carried out for patients treated with imatinib, nilotinib and dasatinib, and they should be compared with each other.
Keeping the proposed threshold in mind to evaluate cost-effectiveness, the ICER obtained from the comparison of nilotinib versus imatinib presented it as cost-effective in the baseline case conditions. The comparison of dasatinib versus imatinib showed an ICER with cost-effectiveness that was greater than the will to pay. The comparison of nilotinib versus dasatinib showed dominance in the majority of cases by costing less and offering better results measured in progression-free life-years (PF-LY). In this manner, from the standpoint of the analyzed outcome, the advantage that the use of nilotinib implies over the two comparable drugs can be established.
In conclusion, nilotinib under baseline case conditions was the most effective option out of the three analyzed. From a cost standpoint, its performance would be one of savings as compared to dasatinib and, although it costs more than imatinib, its cost-effectiveness shows that the cost per progression-free life-year gained would be less than the estimated cost of caring for a patient in progression. Consequently, for the purposes of this analysis, the use of nilotinib at a dose of 300 mg/BID would be the most recommendable option for use in Colombia as first-line treatment for CML.
The Fundación Salutia, Centro de Investigaciones en Economía, Gestión y Tecnologías en Salud (Bogotá, Colombia) received partial funding from Novartis Colombia to carry out this study. Martín Romero, Nelson Álvis and Diana Chávez received payment as researchers from the Fundación Salutia. Magalí De los Ríos acted as an independent physician and external hematological consultant for Novartis in Colombia, from which she receives payment. Nevertheless, the authors´ conclusions were completely independent and reviewed by the Fundación Salutia research committee prior to their release.
Fundación Salutia and Novartis.
Corresponding author:
Martín Romero, Centro de Investigaciones en Economía, Gestión y Tecnologías en Salud, Fundación Salutia, Carrera 71B N° 116A-12, Bogotá, D.C., Colombia. Teléfono: (571) 613 4609; fax (571) 617 9133 martin.romero@salutia.org
1. Espinosa Martínez E, Espinosa Estrada EE, Pavón Morán V, Hernández Padrón C, Ávila CabreraI O, Ramón Rodríguez L, et al . Tratamiento de la leucemia mieloide crónica con mesilato de imatinib en pacientes resistentes o intolerantes al interferón a recombinante: resultados preliminares. Rev Cubana Hematol Inmunol Hemoter. 2010;26:12-26. [ Links ]
2. National Comprehensive Cancer Network. Clinical practice guidelines in oncology: Chronic myelogenous leukemia, version 2.2011. Fort Washington, PA: National Comprehensive Cancer Network; 2011. p. 65. [ Links ]
3. American Cancer Society. Leukemia-chronic myeloid (myelogenous). Atlanta, GA: American Cancer Society; 2009. p. 40 [ Links ]
4. Cortes J. Natural history and staging of chronic myelogenous leukemia. Hematol Oncol Clin North Am. 2004;18:569-84. http://dx.doi.org/10.1016/j.hoc.2004.03.011 [ Links ]
5. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. http://dx.doi.org/10.3322/caac.20073 [ Links ]
6. American Cancer Society. Cancer facts & figures. Atlanta, GA: American Cancer Society; 2010. p. 68. [ Links ]
7. Fausel C. Targeted chronic myeloid leukemia therapy: Seeking a cure. Am J Health Syst Pharm. 2007;64:S9-S15. http://dx.doi.org/10.2146/ajhp070482 [ Links ]
8. Chávez González MA, Ayala Sánchez M, Mayani Viveros H. La leucemia mieloide crónica en el siglo XXI: biología y tratamiento. Rev Invest Clin. 2009;61:221-32. [ Links ]
9. Morales C, Torres V, Valencia J, Ribón G, Manrique R . Leucemia mieloide crónica: diagnóstico y tratamiento. Rev CES Med. 2010;24:97-108. [ Links ]
10. Go ldman JM, Melo JV . Chronic myeloid leukemia - Advances in biology and new approaches to treatment. N Engl J Med. 2003;349:1451-64. http://dx.doi.org/10.1056/NEJMra020777 [ Links ]
11. Rodríguez M, Cardona AF, Grajales MA, Enciso L, Ruiz G, Yepes A, et al . Leucemia mieloide crónica en crisis blástica bases moleculares y diagnóstico. Rev Venez Oncol. 2007;19:287-96. [ Links ]
12. Saglio G, Kim DW, Issaragrisil S, le Coutre P, Etienne G, Lobo C, et al . Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251-9. http://dx.doi.org/10.1056/NEJMoa0912614 [ Links ]
13. Kantarjian HM, Hochhaus A, Saglio G, De Souza C, Flinn IW, Stenke L, et al . Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomized ENESTnd trial. Lancet Oncol. 2011;12:841-51. http://dx.doi.org/10.1016/S1470-2045(11)70201-7 [ Links ]
14. Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, et al . Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260-70. http://dx.doi.org/10.1056/NEJMoa1002315 [ Links ]
15. Kantarjian HM, Shah NP, Cortes JE, Baccarani M, Agarwal MB, Undurraga MS, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION). Blood. 2011;119:1123-9. http://dx.doi.org/10.1182/blood-2011-08-376087 [ Links ]
16. Doggrell SA, Christensen AM. Are there better Bcr-Abl kinase inhibitors for chronic myeloid leukaemia than imatinib? Expert Opin Pharmacother. 2011;12:157-63. http://dx.doi.org/10.1517/14656566.2011.534780 [ Links ]
17. Quintás-Cardama A, Cortes JE, Kantarjian HM. Early cytogenetic and molecular response during first-line treatment of chronic myeloid leukemia in chronic phase. Cancer. 2011;117:5261-70. http://dx.doi.org/10.1002/cncr.26196 [ Links ]
18. O´Brien S, Berman E, Moore JO, Pinilla-Ibarz J, Radich JP, Shami PJ, et al . NCCN task force report: Tyrosine kinase inhibitor therapy selection in the management of patients with chronic myelogenous leukemia. J Natl Compr Canc Netw. 2011;9(Suppl.2):S1-25. [ Links ]
19. Baccarani M, Dreyling M, ESMO Guidelines Working Group. Chronic myelogenous leukemia: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl.4):105-7. http://dx.doi.org/10.1093/annonc/mdp143 [ Links ]
20. Combariza JF, Rodríguez ML, García JS, Acevedo M, Cardona AF, GálvezK, et al . Consenso sobre diagnóstico y tratamiento de leucemia mieloide crónica en Colombia. Rev Colomb Cancerol. 2008;12:126-42. [ Links ]
21. N ational Comprehensive Cancer Network. Clinical practice guidelines in oncology: Chronic myelogenous leukemia, version 2.2012. Fort Washington, PA: National Comprehensive Cancer Network; 2012. p. 75. [ Links ]
22. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683-91. http://dx.doi.org/10.1016/S0895-4356(97)00049-8 [ Links ]
23. Hughes TP. Hochhaus A, Branford S, Müller MC, Kaeda JS, Foroni L, et al . Long-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS). Blood 2010;116:3758-65. . http://dx.doi.org/10.1182/blood-2010-03-273979 [ Links ]
24. Ministerio de la Protección Social. Resolución 2569 de 2012. Bogotá: Ministerio de la Protección Social; 2012. [ Links ]
25. Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F, et al . Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European Leukemia Net. Blood. 2006;108:1809-20. http://dx.doi.org/10.1182/blood-2006-02-005686 [ Links ]
26. Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al . Chronic myeloid leukemia: An update of concepts and management recommendations of European Leukemia Net. J Clin Oncol. 2009;27:6041-51. http://dx.doi.org/10.1200/JCO.2009.25.0779 [ Links ]
27. Departamento Administrativo Nacional de Estadística . Defunciones por grupos de edad y sexo, según departamento, municipio de residencia y grupos de causas de defunción (lista de causas agrupadas 6/67 CIE-10 de OPS). Bogotá: Departamento Administrativo Nacional de Estadística, DANE; 2008. [ Links ]
28. Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. Br Med J. 1996;313:275-283. http://dx.doi.org/10.1136/bmj.313.7052.275 [ Links ]
29. Ministerio de Salud. Resolución 8430 de 1993. Bogotá: Ministerio de Salud; 1993. [ Links ]
30. Giles FJ, Rosti G, Beris P, Clark RE, le Coutre P, Mahon FX, et al . Nilotinib is superior to imatinib as first-line therapy of chronic myeloid leukemia: The ENESTnd study. Expert Rev Hematol. 2010;3:665-73. http://dx.doi.org/10.1586/ehm.10.61 [ Links ]
31. Jabbour EC, Kantarjian JH. Nilotinib for the treatment of chronic myeloid leukemia: An evidence-based review. Core Evid. 2010;4:207-13. [ Links ]
32. Wei G, Rafiyath S, Liu D. First-line treatment for chronic myeloid leukemia: Dasatinib, nilotinib, or imatinib. J Hematol Oncol. 2010;3:47. http://dx.doi.org/10.1186/1756-8722-3-47 [ Links ]
33. Kim TD, Coutre Pl. The expanding role of nilotinib in chronic myeloid leukemia. Expert Opin Drug Saf. 2011;10:97-107. http://dx.doi.org/10.1517/14740338.2011.532486 [ Links ]
34. Signorovitch JE, Wu EQ, Betts KA, Parikh K, Kantor E, Guo A, et al . Comparative efficacy of nilotinib and dasatinib in newly diagnosed chronic myeloid leukemia: A matching-adjusted indirect comparison of randomized trials. Curr Med Res Opin. 2011;27:1263-71. http://dx.doi.org/10.1185/03007995.2011.576238 [ Links ]
35. Hoyle M, Pavey T, Ciani O, Jones-Hughes T, Osipenko L, Venkatachalamet M, et al . Dasatinib, nilotinib, and standard dose imatinib for the first-line treatment of chronic myeloid leukaemia: Systematic reviews and economic analyses. National Institute for Health and Clinical Excellence (NICE): Peninsula Technology Assessment Group (PenTAG). Exeter: University of Exeter; 2011. [ Links ]
36. National Institute for Health and Clinical Excellence. Leukaemia (chronic myeloid, first line) - dasatinib, nilotinib and standard-dose imatinib: Appraisal consultation document. London, UK: National Institute for Health and Clinical Excellence NICE; January 11, 2012. Fecha de consulta: 29 de marzo de 2012. Disponible en: http://guidance.nice.org.uk/TA/Wave24/15/Consultation/DraftGuidance. [ Links ]
37. Valencia JE, Orozco JJ. Adaptación a Colombia y Venezuela del modelo económico dasatinib primera línea del York Health Economics Consortium para el tratamiento de la leucemia mieloide crónica. Medwave. 2012;12:e5348. http://dx.doi.org/10.5867/medwave.2012.04.5348 [ Links ]
38. Orozco-Giraldo JJ, Valencia JE, Aiello E, Caputo M. Evaluación económica del dasatinib en el tratamiento de la leucemia mieloide crónica en pacientes resistentes al imatinib en Chile. Medwave. 2011;11:e5012. http://dx.doi.org/10.5867/medwave.2011.04.5012 [ Links ]
39. Scottish Intercollegiate Guidelines Network. SIGN 50 A guideline developer´s handbook. Edinburg; Scotland: Scottish Intercollegiate Guidelines Network SIGN; 2008. p. 112. [ Links ]













