Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biomédica
Print version ISSN 0120-4157
Biomédica vol.34 no.3 Bogotá July/Sept. 2014
https://doi.org/10.7705/biomedica.v34i3.2264
PRESENTACIÓN DE CASO
doi: http://dx.doi.org/10.7705/biomedica.v34i3.2264
1 Oficina de Docencia e Investigación, Centro Dermatológico Federico Lleras Acosta, E.S.E., Bogotá, D.C., Colombia
2 Facultad de Medicina, Fundación Universitaria Sanitas, Bogotá, D.C., Colombia
3 Facultad de Medicina, Universidad de La Sabana, Chía, Colombia
4 División de Apoyo Diagnóstico, Centro Dermatológico Federico Lleras Acosta, E.S.E., Bogotá, D.C., Colombia
Institution in which the research was undertaken: Centro Dermatológico Federico Lleras Acosta, E.S.E.
Author contributions:
Camilo Andrés Morales and Juliana Palacio participated in providing the clinical information of these cases and wrote the first draft.
Gerzaín Rodríguez performed the histological evaluation.
Yenny Carolina Camargo performed the microbiological studies.
All authors participated in the design, literature search, writing, review and approval of the final version of this manuscript.
Recibido: 06/02/14; aceptado: 18/05/14In Colombia, zosteriform leishmaniasis is a little-known and infrequent clinical variant of cutaneous leishmaniasis. Its clinical features include one or more plaques made up of papules and pseudo-vesicles, which conform to a lineal pattern, as well as satellite lesions that affect one or more dermatomes, without crossing the median line.
We present three zosteriform cutaneous leishmaniasis cases in which Leishmania panamensis and Leishmania braziliensis were identified as the infective species. In light of the fact that the disease occurs infrequently, diagnosis was reached by taking into account epidemiological and clinical suspicion.
Key words: Leishmania, Leishmania braziliensis; leishmaniasis, cutaneous; herpes zoster, diagnosis, epidemiology.
http://dx.doi.org/10.7705/biomedica.v34i3.2264
Leishmaniasis cutánea zosteriforme causada por Leishmania ( Viannia ) panamensis y Leishmania ( Viannia ) braziliensis : reporte de tres casos
La leishmaniasis zosteriforme es una variante clínica de la leishmaniasis cutánea, infrecuente y poco conocida en Colombia. Clínicamente se caracteriza por una o varias placas conformadas por pápulas y pseudovesículas que siguen un patrón lineal, y por lesiones satelitales que comprometen uno o varios dermatomas sin sobrepasar la línea media.
Se presentan tres casos de leishmaniasis cutánea zosteriforme en los que se identificaron Leishmania panamensis y Leishmania braziliensis como especies infectantes. La sospecha epidemiológica derivada de la procedencia de los pacientes, así como la sospecha clínica a partir del reconocimiento de una presentación infrecuente de la enfermedad, permitieron hacer el diagnóstico.
Palabras clave: Leishmania, Leishmania braziliensis , leishmaniasis cutánea, herpes zóster, diagnóstico, epidemiología.
http://dx.doi.org/10.7705/biomedica.v34i3.2264
Leishmaniasis is an endemic disease in Colombia. A striking number of new cases has been reported in the past decade: up from 4,130 cases in the year 2001 to 9,684 cases in the year 2011. It accounts for 20% of all leishmaniasis cases registered in the Americas over the period 2001-2011, and places the country second in incidence, surpassed only by Brazil (1). In Colombia, 98% of all cases are classified as cutaneous leishmaniasis (2), generally presenting as a papule or nodule on an exposed area that grows progressively and turns into a painless ulcer with infiltrated edges (3,4). Other clinical manifestations designated as atypical because they are infrequent and little known may also occur. These are given different names according to the lesion´s clinical characteristics (annular, chancriform, cicatricial, keloid, tumoral, acneiform, panniculitis, verrucous), its similarity to other diseases (lupoid, acneiform, eczematous, erysipeloid, sporotrichoid, psoriasiform, rhinophymatous, mycetomatous, zosteriform) and the anatomical localization (palmoplantar, genital, conjunctival, whitlow, paronichial) (4-9).
Among the atypical cutaneous leishmaniasis manifestations, zosteriform is one of the least frequent, accounting for a scant number of reported cases in the Americas (10). Its global prevalence varies between 0.17% and 2.5%, according to some case series from the Middle East (6,11). It is clinically discernible by one or more plaques composed of papules or pseudo-vesicles, located on the head (10,12), back (8,9), abdomen (13) or lower limbs (7), forming a lineal pattern and satellite lesions that involve one or more dermatomes that do not cross the median line (6,8,12). The appearance and distribution of lesions can cause the disease to be confused with herpes zoster, thus complicating initial diagnosis. A biopsy may be ordered which, in turn, may make histological pattern identification difficult since it differs from that observed in typical lesions of cutaneous leishmaniasis.
In the three cases described below cutaneous leishmaniasis diagnosis was confirmed using either microbiological or direct methods. In two cases, the etiological agent was identified using monoclonal antibodies, whereas in the case with biopsy sample (case 2), the species was identified using molecular methods.
Clinical cases
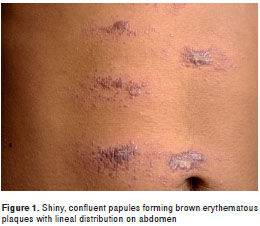
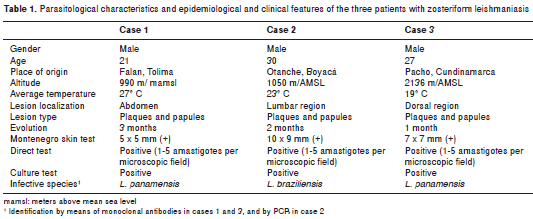
Case 1. A 21-year old male farmer, born in and resident of Falan (Department of Tolima), presented 3 month-old slightly itchy lesions on the abdomen that had spread progressively, and for which he had received several cycles of oral antibiotics with no signs of improvement. Physical examination revealed on the abdomen numerous small infiltrated confluent papules with shiny surface, that formed five erythematous plaques distributed over dermatomes T8, T9 and T10 (figure 1). Direct smear exam and lesion aspirate culture tested positive for leishmaniasis. Leishmania panamensis was cultured and identified by means of species specific B4 and B11 monoclonal antibodies. This species is compatible with the reference strain MHOM/PA/71/LS94. Treatment was begun with meglumine antimoniate (Glucantime®) at a dosage of 20 mg/Kg per weight/day for 20 days, with subsequent lesion involution and healing.
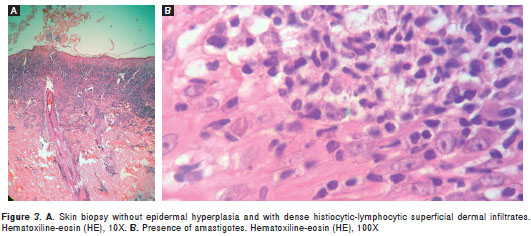
Case 2. A 30-year old baker, born in and resident of Bogotá, presented asymptomatic lesions on the dorso-lumbar region with 2-month evolution, for which he had received various topical and oral acyclovir treatments with no signs of improvement. Physical examination revealed a violet erythematous plaque on the right lumbar region over de rmatomes L2, L3 and L4 that was composed of pseudo-vesicles and yellowish erythematous papules. Some of these were shiny and infiltrated, with slight scaling and a small central scab (figure 2). Under the diagnostic impression of sarcoidosis, a skin biopsy was performed. Histologically, the epidermis showed discrete acanthosis with dense histiocytic-lymphocytic infiltrates throughout the dermis, suggesting the presence of a live agent (figure 3A). The patient was interviewed once again and he mentioned that the lesions had appeared 2 weeks after visiting Otanche (Department of Boyacá). Direct smear exam and lesion aspirate culture were ordered, the results of which resulted positive for leishmaniasis. In a later review of histological slides, numerous amastigotes were observed (figure 3B), and by means of molecular methods L. braziliensis was identified as the etiologic agent. Montenegro skin test results were also positive. Treatment began with meglumine antimoniate (Glucantime®) at a dosage of 20 mg/Kg per weight/day for 20 days, with subsequent lesion involution and healing.

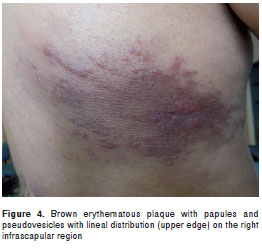
Case 3. A 27-year old male miner, born in and resident of Pacho (Department of Cundinamarca) underwent physical examination with a 1-month history of asymptomatic lesions on his back. Examination of the right infra-scapular region revealed a brown erythematous plaque, over dermatomes T5 and T6 made up of papules and pseudo-vesicles, with a lineal distribution on its upper border (figure 4). Direct smear exam and the culture of the lesion aspirate tested positive for leishmaniasis. Leishmania panamensis was identified by means of species specific B4 and B11 monoclonal antibodies. This species is compatible with the reference strain MHOM/PA/71/LS94. The patient was treated with meglumine antimoniate (Glucantime®) at a dosage of 20 mg/Kg per weight/day for 20 days, with subsequent lesion involution and healing.
Discussion
The clinical manifestations of cutaneous, mucosal or mucocutaneous and visceral leishmaniasis, depend upon the host´s immunological response and the virulence of the infecting strain. Therefore, the spectrum of the disease varies from asymptomatic cutaneous infection to visceral involvement, which is potentially mortal (14). In the Americas, 95.7% of the cases for which clinical information is available are classified as cutaneous leishmaniasis (1). In Colombia, 98% of leishmaniasis cases are cutaneous (2), and lesions are generally caused by species belonging to the subgenus Viannia : L. panamensis; L. braziliensis and L. guyanensis (15,16).
At the National Institute of Dermatology of Colombia, a referral dermatology center receiving patients from almost all regions of the country, most cutaneous leishmaniasis cases (92%), including those with atypical symptoms, are caused by L. panamensis (74.5%) (16). This species had been reported previously as the etiological agent of diffuse cutaneous leishmaniasis in an AIDS patient (16), and in three patients with disseminated, erysipeloid and leishmaniasis recidivans cutis (4). This contrasts with numerous reports on atypical cases from the Mediterranean basin (12) and from other countries, including Pakistan (5-7,11,13,17), India (18), Iran (8,9) and Lebanon (19), where the major infective species is L. major. In these publications, the clinical polymorphism of the disease has been related to parasite dependent factors, such as virulence of the species (5,6,8,9,18), the vector (12), and geographical region where infection took place (11,12,18), and to the host, such as lesion localization (5,8) and immune response (5-8,11,12,18).
In the three cases we present, lesions were localized on the trunk, on covered areas (table 1), which could suggest a relation between infection site and atypical clinical presentation due to anatomical and physiological variations related to aspects such as local immune response, skin temperature and microcirculation of the dermis where the parasite develops (20). The influence of anatomical site on the host´s clinical and immunological response has been studied in experimental animal models with L. panamensis, in which clear differences have been established for time of evolution, quantity of inflammatory infiltrate and tissue necrosis, parasite load, antibody produc- tion and treatment response, depending on the site where inoculation took place (20).
It has also been suggested that in other atypical forms, such as erysipeloid leishmaniasis, parasite dissemination in the papillary dermis hinders the ability of the immune system to control the disease (12,21). Since the three patients in this series had numerous amastigotes, it is possible that the host´s deficient immune response, when faced with certain Leishmania species ( L. panamensis and L. braziliensis in the New World and L. major in the Old World), allows for parasite replication and dissemination with a clinical pattern different from the typical one.
The difficulty of orienting clinical diagnosis in patients with atypical forms of cutaneous leishmaniasis usually calls for a biopsy, which can also be confusing for the pathologist when it means dealing with non-ulcerated lesions due to the lack of epidermal hyperplasia. However, the presence of a diffuse dermal infiltrate rich in plasmocytes and vacuolated macrophages allows for diagnostic orientation and the careful search for the parasite in lesion biopsies of less than 2-to-3-month evolution. In these, it is possible to observe the amastigotes in more than 70% of cases (22). Another scenario where epidermal hyperplasia is seen is in the diffuse form of the disease where the epidermis can be atrophic but the dermal infiltrate has abundant vacuolated macrophages that phagocyte the large quantities of amastigotes present (23).
In addition to herpes zoster, the differential diagnosis in zosteriform leishmaniasis includes other infectious skin diseases such as lupus vulgar and late secondary syphilis; inflammatory ones such as tumid lupus erythematosus, granuloma annulare and cutaneous sarcoidosis; and tumoral ones such as B-cell cutaneous lymphoma, among other diseases. In one of the three cases (figure 2), lesions were confused with another granulomatous disease, and diagnosis was deferred until a history of visiting an endemic area of leishmaniasis was known (24). In the other cases, the epidemiological criteria and lesion recognition by an expert dermatologist allowed for disease suspicion, which was later confirmed by microbiological tests that also provided identification of the infective species (table 1).
In conclusion, zosteriform leishmaniasis in Colombia is an infrequent and little known variant of cutaneous leishmaniasis, which, like other atypical manifestations, could be related to the infective species and to the host´s immune response, among other factors, and can be confused with different dermatological diseases.
The authors declare no existing conflict of interest.
This work was supported by the Centro Dermatológico Federico Lleras Acosta.
Corresponding author: Camilo Andrés Morales, Centro Dermatológico Federico Lleras Acosta, Avenida 1ª N° 13A-61, Bogotá, D.C., Colombia Teléfono: (571) 242 8160, extensión 145; fax: (571) 337 3597 camiderm@yahoo.com
1. Organización Panamericana de la Salud. Leishmaniasis. Informe epidemiológico de las Américas Nº 1 - Abril 2013. Fecha de consulta: 15 de octubre de 2013. Disponible en: http://www.paho.org/hq/index.php?option=com_docman&task=doc_view&gid=21609&Itemid=. [ Links ]
2. Vera M, Galindo F, Zambrano P, Méndez J, Bello B, Olano V. Informe de enfermedades transmitidas por vectores (ETV), 2004. Inf Quinc Epidemiol Nac. 2005;10:34-48. [ Links ]
3. Ministerio de la Protección Social. Guía para la atención clínica integral del paciente con leishmaniasis. Fecha de consulta: 15 de octubre de 2013. Disponible en: http://www.idsn.gov.co/site/images/vectores/02clileishma.pdf. [ Links ]
4. Calvopina M, Gómez EA, Uezato H, Kato H, Nonaka S, Hashiguchi Y. Atypical clinical variants in New World cutaneous leishmaniasis: Disseminated, erysipeloid, and recidiva cutis due to Leishmania (V.) panamensis . Am J Trop Med Hyg. 2005;73:281-4. [ Links ]
5. Iftikhar N, Bari I, Ejaz J. Rare variants of cutaneous leishmaniasis: Whitlow, paronychia, and sporotrichoid . Int J Dermatol. 2003;42:807-9. http://dx.doi.org/10.1046/j.1365-4362.2003.02015.x [ Links ]
6. Raja KM, Khan AA, Hameed A, Rahman SB. Unusual clinical variants of cutaneous leishmaniasis in Pakistan . Br J Dermatol. 1998;139:111-3. http://dx.doi.org/10.1046/j.1365-2133.1998.02325.x [ Links ]
7. Bari AU. Clinical spectrum of cutaneous leishmaniasis: An overview from Pakistan. Dermatol Online J. 2012;18:4. [ Links ]
8. Omidian M, Mapar MA. Chronic zosteriform cutaneous leishmaniasis . Indian J Dermatol Venereol Leprol. 2006;72: 41-2. http://dx.doi.org/10.4103/0378-6323.19717 [ Links ]
9. Momeni AZ, Aminjavaheri M. Clinical picture of cutaneous leishmaniasis in Isfahan, Iran. Int J Dermatol. 1994;33:260-5. [ Links ]
10. Giglio P, Bravo F, del Solar M, Salomón M, Puell L, Feria K, et al . Leishmaniasis cutánea zosteriforme: reporte de un caso . Folia Dermatol Peru. 2011;22:101-5. [ Links ]
11. Bari AU, Rahman SB. Many faces of cutaneous leish-maniasis . Indian J Dermatol Venereol Leprol. 2008;74:23-7. http://dx.doi.org/10.4103/0378-6323.38402 [ Links ]
12. Bongiorno M, Pistone G, Aricò M. Unusual clinical variants of cutaneous leishmaniasis in Sicily. Int J Dermatol. 2009;3:286-9. http://dx.doi.org/10.1111/j.1365-4632.2009.03940.x [ Links ]
13. Kafaie P, Akaberi AA, Amini S, Noorbala MT, Moghimi M. Multidermatomal zosteriform lupoid cutaneous leishmaniasis: A case report. J Pak Assoc Dermatol. 2010;20:243-5. [ Links ]
14. Grimaldi G, Tesh RB. Leishmaniases of the New World: Current concepts and implications for future research . Clin Microbiol Rev. 1993;6:230-50. http://dx.doi.org/10.1128/CMR.6.3.230 [ Links ]
15. Corredor A, Kreutzer RD, Tesh RB, Boshell J, Palau MT, Caceres E, et al. Distribution and etiology of leishmaniasis in Colombia . Am J Trop Med Hyg. 1990;42:206-14. [ Links ]
16. Ovalle CE, Porras L, Rey M, Ríos M, Camargo YC. Distribución geográfica de especies de Leishmania aisladas de pacientes consultantes al Instituto Nacional de Dermatología Federico Lleras Acosta, ESE, 1995-2005 . Biomédica. 2006;26:145-51. [ Links ]
17. Shamsuddin S, Mengal JA, Gazozai S, Mandokhail ZK, Kasi M, Muhammad N, et al . Atypical presentation of cutaneous leishmaniasis in native population of Baluchistan . J Pak Assoc Dermatol. 2006;16:196-200. [ Links ]
18. Sindhu PS, Ramesh V. Unusual presentation of cutaneous leishmaniasis. Indian J Dermatol. 2012;1:55-7. http://dx.doi.org/10.4103/0019-5154.92682 [ Links ]
19. Douba MD, Abbas O, Wali A, Nassany J, Aouf A, Tibbi MS, et al . Chronic cutaneous leishmaniasis, a great mimicker with various clinical presentations: 12 years experience from Aleppo . J Eur Acad Dermatol Venereol. 2012;26:1224-9. http://dx.doi.org/10.1111/j.1468-3083.2011.04266 [ Links ]
20. Osorio Y, Melby PC, Pirmez C, Chandrasekar B, Guarín N, Travi BL. The site of cutaneous infection influences the immunological response and clinical outcome of hamsters infected with Leishmania panamensis. Parasite Immunol. 2003;25:139-48. http://dx.doi.org/10.1046/j.1365-3024.2003.00615.x [ Links ]
21. Salmanpour R, Handjani F, Zerehsaz F, Ardehali S, Panjehshahin MR. Erysipeloid leishmaniasis: An unusual clinical presentation . Eur J Dermatol . 1999;9:458-9. [ Links ]
22. Rodríguez G, Sarmiento L, Hernández CA. Leishmaniasis mucosa y otras lesiones destructivas centrofaciales . Biomédica. 1994;14:215-29. [ Links ]
23. Pérez C, Solías Y, Rodríguez G. Diffuse cutaneous leishmaniasis in a patient with AIDS. Biomédica. 2006;26: 485-97. [ Links ]
24. Santamaría E, Ponce N, Zipa Y, Ferro C. Presencia en el peridomicilio de vectores infectados con Leishmania (Viannia) panamensis en dos focos endémicos en el occidente de Boyacá, piedemonte del valle del Magdalena medio, Colombia . Biomédica. 2006;26:82-94. [ Links ]













