Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biomédica
Print version ISSN 0120-4157
Biomédica vol.34 supl.1 Bogotá Apr. 2014
https://doi.org/10.7705/biomedica.v34i0.1686
ARTÍCULO ORIGINAL
doi: http://dx.doi.org/10.7705/biomedica.v34i0.1686
Author contributions:
Martha Inírida Guerrero contributed with the idea, carried out the bibliographic search, participated in the design, in data collection, supervision, auditing and analysis, in interpreting the results and in the discussion, writing and approval of the final document.
Claudia Colorado collected and analyzed data, participated in developing laboratory methodologies, in interpreting results, and in reviewing and approving the final document.
José Fernando Torres participated in the bibliographic search, in collecting and analyzing data, developing laboratory methodologies, interpreting results, and in reviewing and approving the final document.
Clara Inés León contributed with the idea, and participated in the bibliographic search, in the design, in data collection, supervision, auditing and analysis, in interpreting the results and in the discussion, writing and approval of the final document.
Recibido: 13/06/13; aceptado: 27/11/13
Introduction: There is no information in Colombia on Mycobacterium leprae primary and secondary drug resistance in regards to the WHO-multidrug therapy regime. On the other hand, public health authorities around the world have issued various recommendations, one of which prompts for the immediate organization of resistance surveillance through simple molecular methods.
Objective: To determine the prevalence of Mycobacterium leprae drug resistance to rifampicin, ofloxacin and dapsone in untreated and previously treated patients at the Centro Dermatológico Federico Lleras Acosta during the 1985-2004 period.
Materials and methods: We conducted a retrospective study which included multibacillary patient biopsies through elective sampling: 381 of them from new patients and 560 from previously treated patients. Using a microtome, we obtained six slides from each skin biopsy preserved in paraffin, and we extracted M. leprae DNA. We amplified three molecular targets through PCR and obtained the patterns of drug resistance to dapsone, rifampicin and ofloxacin by reverse hybridization. Finally, we collected epidemiological, clinical and demographical data for analyses.
Results: From 941 samples under study, 4.14% of them were resistant to one or more drugs, and 5.77 and 3.04% had resistant genotypes in new and previously treated patients, respectively. Total resistance for each drug was 0.43% for dapsone, 3.19% for rifampicin and 1.17% for ofloxacin. We found statistically significant differences for rifampicin and for the total population when comparing the results from untreated versus previously treated patients. Two thirds of the resistant samples were resistant to rifampicin alone or combined.
Conclusions: The standard multidrug therapy schemes continue being effective for leprosy cases; however, it is necessary to guarantee adherence and regularity. Surveillance to drug resistance in new and previously treated leprosy cases should be established.
Key words: Mycobacterium leprae , drug resistance, rifampicin, dapsone, ofloxacin, molecular detection, Colombia, PCR
doi: http://dx.doi.org/10.7705/biomedica.v34i0.1686
¿Es la resistencia de Mycobacterium leprae a los medicamentos un verdadero motivo de preocupación? Primera aproximación a la vigilancia molecular de pacientes colombianos multibacilares con tratamiento previo para lepra y sin él
Introducción. Colombia no dispone de información sobre farmacorresistencia primaria y secundaria de Mycobacterium leprae al esquema de terapia múltiple de la Organización Mundial de la Salud (OMS) y las autoridades de salud pública del mundo han emitido varias recomendaciones, entre las cuales está organizar de inmediato la vigilancia a la resistencia empleando métodos moleculares simples.
Objetivo. Determinar la prevalencia de la resistencia de M. leprae a rifampicina, ofloxacina y dapsona en pacientes del Centro Dermatológico Federico Lleras Acosta con tratamiento previo y sin él durante el período de 1985 a 2004.
Materiales y métodos. Se realizó un estudio retrospectivo. Mediante muestreo electivo se incluyeron biopsias de pacientes multibacilares: 381 de pacientes nuevos y 560 de pacientes previamente tratados. Se obtuvieron con micrótomo seis cortes de cada biopsia de piel incluida en parafina, y se realizó la extracción de ADN de M. leprae. Se llevó a cabo la amplificación de tres blancos moleculares mediante PCR y se obtuvieron los patrones de resistencia a los medicamentos dapsona, rifampicina y ofloxacina por hibridación inversa. Se recolectaron datos epidemiológicos, clínicos y demográficos para llevar a cabo los análisis.
Resultados. De las 941 muestras estudiadas, 4,14 % era resistente a uno o más fármacos, y se detectaron 5,77 y 3,04 % con genotipos resistentes en pacientes nuevos y previamente tratados, respectivamente. La resistencia total para cada fármaco fue de 0,43 % a dapsona, 3,19 % a rifampicina y 1,17 % a ofloxacina. Se encontró una diferencia estadísticamente significativa para rifampicina y para la población total al comparar los resultados de los pacientes no tratados con los de los pacientes tratados previamente. Dos tercios de las muestras resistentes lo fueron a rifampicina sola o combinada.
Conclusiones. Los esquemas de terapia múltiple estándar siguen siendo efectivos para los casos de lepra; sin embargo, es necesario garantizar el cumplimiento y la regularidad y establecer la vigilancia de la farmacorresistencia en pacientes nuevos y previamente tratados.
Palabras clave: Mycobacterium leprae , resistencia a los medicamentos, rifampicina, dapsona, ofloxacina, detección molecular, Colombia, PCR.
doi: http://dx.doi.org/10.7705/biomedica.v34i0.1686
Leprosy control is based on the principle that identifying and treating chronic infectious diseases with an effective combination of antibiotics limits the emergence and expansion of both new and existing drug- resistant pathogens (1). In this frame, WHO multidrug therapy has shown to be effective since its introduction in 1982, but, nevertheless, it is important to monitor drug resistance trends periodically and genotype Mycobacterium leprae strains in order to understand resistant strains transmission patterns and genetic diversity. In contrast to what we know about tuberculosis, the prevalence of primary and secondary resistance is unknown for drugs used in multidrug therapy for leprosy such as dapsone, rifampicin and ofloxacin. Consequently, the risk of resistance cannot be assessed and a regimen for retreatment cannot be appropriately designed.
Mycobacterium leprae resistance to dapsone, rifampicin and ofloxacin has been studied in countries with high leprosy prevalence, like some in Southeast Asia (2). However, complete quantification of the magnitude of the drug resistance problem is crucial to evaluating the efficacy of multidrug therapy and maintaining the efficiency of current leprosy-control strategies around the world. Moreover, knowing the initial M. leprae susceptibility to drugs in patients who are initiating treatment and in those previously treated would be beneficial as a surveillance strategy.
Given its complexity, the classic detection methodology for drug susceptibility of M. leprae using mice footpads cannot be the tool of choice for these purposes (3). In this sense, progress in molecular biology and knowledge of molecular mechanisms of resistance (4) offer a unique opportunity to determine M. leprae in vitro drug susceptibility , which has agreed in multiple studies with the results obtained from mouse footpads, for which these methodologies have been accepted worldwide (5).
Resistance to dapsone, rifampicin and ofloxacin evolves by amino acid substitution at their binding sites. These changes are determined by mutations in the drug resistance-determining region (DRDR) in the folP1 , rpo β, and gyrA genes which have demonstrated to confer resistance to dapsone, rifampicin, and ofloxacin, respectively. By defining resistance mechanisms it is possible to use DNA-based assays, namely PCR-direct DNA sequencing or reverse hybridization to examine susceptibility to these drugs (6-19).
In spite of all this information being available, few molecular studies have been conducted on strains in which drug resistance is suspected (20), and, therefore, the current situation is not known. For this reason, resistance surveillance in M. leprae , as well as the evaluation of treatment regime efficacy and control program impact, is justified (20). Among the few studies of drug resistance in leprosy carried out in the world, we can cite those reported in table 1.
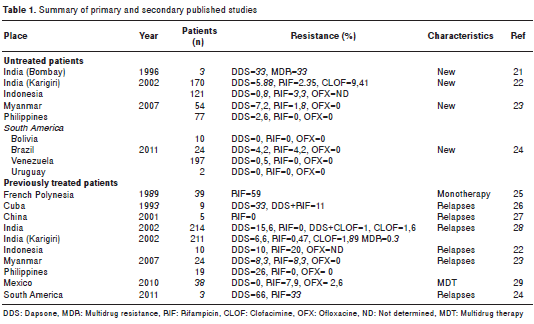
It is alarming to find scientific reports on M. leprae cases with multiresistance to rifampicin, ofloxacin and dapsone, which, albeit few, ring an alarm about the possibility that one of the causes of therapeutic failure may be the presence of multiresistance (17-19).
In this context, both WHO and the Informal Consultation on Leprosy Resistance to Rifampicin, held in 2006, issued several recommendations among which there is one prompting for the immediate organization of surveillance to resist ance through already available simple molecular methods applicable to many types of samples that can differentiate the mutations affecting different drugs (30).
Considering the lack of data on M. leprae primary and secondary resistance to the WHO multidrug therapy in Colombia, we conducted the present study to determine the prevalence of M. leprae drug resistance to rifampicin, ofloxacin and dapsone in untreated and previously treated patients at the Centro Dermatológico Federico Lleras Acosta in Bogotá, during the 1985-2004 period.
Materials and methods
Study population
We conducted a retrospective study of skin biopsies from Colombian patients attending the Centro Dermatológico Federico Lleras Acosta, E.S.E., for leprosy diagnosis or multidrug therapy follow-up during the 1985-2004 period .
Sample
By elective sampling, we obtained skin biopsies from the Centro Dermatológico Federico Lleras Acosta, E.S.E. , biobank. We used 381 biopsies from new patients (samples taken for Hansen's disease diagnosis and classification). To be included, biopsies had to be classified as belonging to multibacillary cases according to any of the criteria used in Colombia for this purpose: positive ZN in histopathology, positive ZN at skin smear examination and/or clinical examination consistent with leprosy. We also included 560 biopsies of patients previously treated for multibacillary leprosy (samples taken to monitor or control the disease), specifically when multidrug therapy failure had been observed or when drug resistance or relapse was suspected.
Obtention of histological slides and DNA extraction
Skin biopsies preserved in paraffin blocks were cut with a Leica® microtome and six slides (4 µm thick) were obtained using a disposable blade for each sample. The slides were placed in 1.5 ml microtubes, and stored at room temperature until processing. We then proceeded to the extraction of M. leprae DNA from the cuts adding 400 µl of TET buffer 1X (Triton X-100 1% TE1X buffer) to each sample and boiling for 15 minutes until it was soluble to remove the paraffin from the tissue. After that, a treatment with proteinase K (10 mg/ml), NaCl 5M, chloroform and isopropanol was performed, and finally the pellet was washed with ethanol, dried and hydrated with TE buffer 0.1X, keeping it at -20°C until use (31).
PCR amplification of molecular targets for the detection of Mycobacterium leprae drug resistance to rifampicin, ofloxacin and dapsone
Using the methodology described previously by Torres ( 31), we amplified a 388 pb fragment from folP1 gene, a 304 pb fragment from rpo b gene and 390 pb fragment from gyrA gene using the primers shown in Annex 1.
Reverse hybridization . To obtain the patterns of drug resistance to dapsone, rifampicin and ofloxacin, reverse hybridization was performed using all the amplified fragments with the three molecular targets (31). The reverse hybridization was performed over a nylon membrane, which had irreversibly joined the wild and mutated oligonucleotides to each gene, as described in Annex 1 (31).
For all procedures, three samples of M. leprae reference DNA, kindly donated by Colorado University (USA), were used as controls: T-53, TM-4923 and TM-4316.
Statistical analysis. Results for drug resistance, as well as the epidemiological, clinical and demographic information of each patient, collected from medical records, were entered into a database created in Excel 5.0 for further analysis of variables using Epi-Info 7.0, which was done by comparing proportions; confidence intervals were set to a significance level of 0.05.
Results
Mutations found in resistant genotypes
Specific mutations for dapsone were detected in folp1 codons 53 and 55.
For rifampicin, mutations were found in rpo β codons 531, 526 and 516, and, besides, a possible insertion was detected between codons 515 and 520. The mutation in codon 531 was the most frequent. Mutations in codons other than 531 were present only in samples taken as of 2001.
As regards ofloxacin, mutations were detected at codons 89 and 91 in gene gyrA ; all samples with double mutation corresponded to previously treated patients.
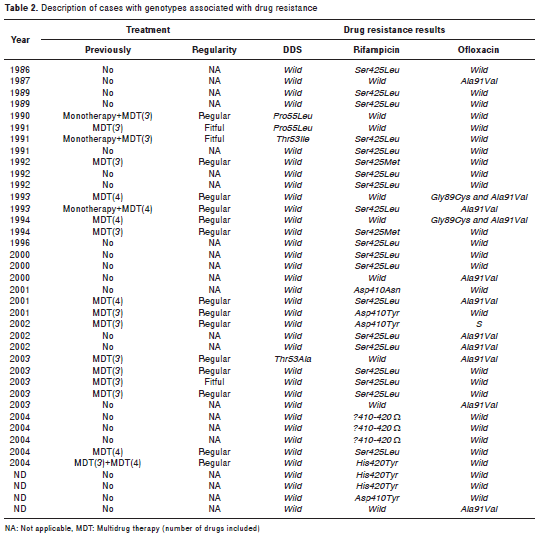
Table 2 shows the characteristics of cases with genotypes associated with dapsone, rifampicin and ofloxacin resistance.
Prevalence of drug resistance
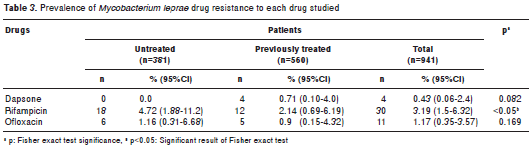
We found primary and secondary drug resistance in both groups of patients (table 3). Using the Mantel-Haenszel chi-square statistic we found statistically significant differences (p<0.05) for rifampicin when comparing results from untreated patients versus previously treated patients.
Description of the genotypes associated with drug resistance
Among the 941 samples under study, 4.14% (n=39) were resistant to one or more drugs; of the 381 samples taken from new patients, 5.77% (n=22) carried mutant genotypes associated with resistance to the drugs under study. Of the remaining 560 biopsies from previously treated patients, 3.04% (n=17) showed mutant genotypes associated with drug resistance (table 4). Statistically significant differences were found for rifampicin (p<0.001) and for the total population (p<0.05) when comparing the results from untreated patients versus those from previously treated patients (table 4).
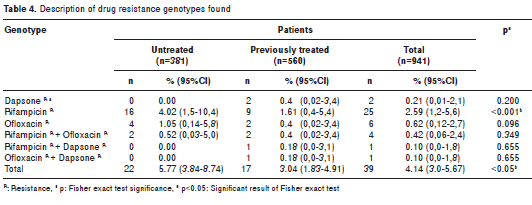
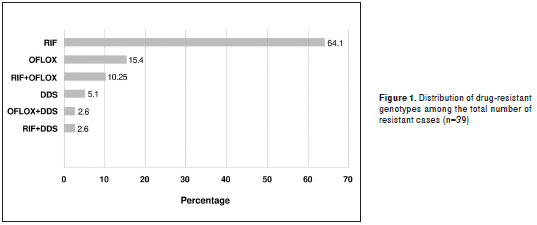
Figure 1 shows the distribution percentage of all samples (n=39) where mutant genotypes associated with resistance to the drugs under study were found.
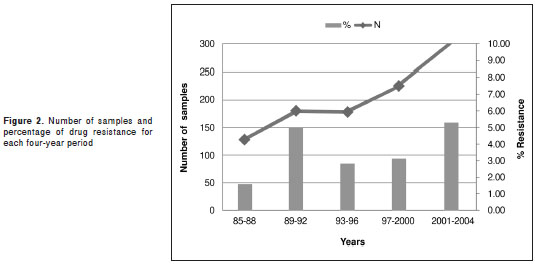
The study time was divided into four-year periods with an increasing number of samples but a stable percentage of mutant genotypes associated with drug resistance: 1.56% (CI95% 0.26-5.06) for the first period, 5.0% (CI95% 2.46-8.97) for the second period, 2.8% (CI95% 1.03-6.11) for the third period, 3.11% (CI95% 1.37-6.05) for the fourth period, and 5.28% (CI95% 3.15-8.25) for the fifth one. This means the difference in the percentages throughout time was not statistically significant in this study (figure 2).
Analysis of the demographic, clinical and epidemiological variables
We analyzed the demographic variables of the study population discriminating by genotypes associated or not with resistance. A statistically significant difference was found between the proportion of patients born in the Santander province who had genotypes associated with drug resistance and those with non-associated genotypes (p<0.01). This same situation arose when we analyzed these patients' municipality of birth, especially in the case of Suaita (p<0.01) and Barrancabermeja (p<0.05) municipalities, both located in the Santander province (table 5). Although in the Cundinamarca province the said difference was not found, one of its municipalities, Agua de Dios, did present a similar situation due to the fact that this is one of the municipalities with the highest prevalence of leprosy cases in the country (table 5).
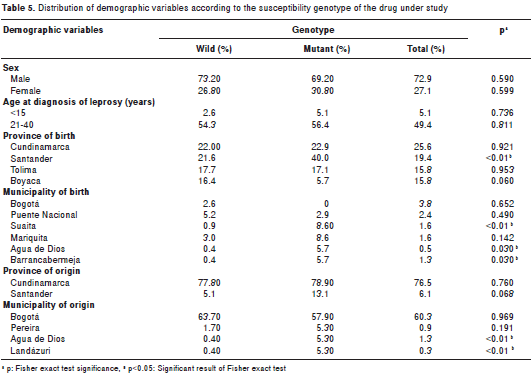
None of the other demographic variables were related to genotypes associated with drug resistance, except for province/municipality of origin, which behaved the same as province/municipality of birth (table 5).
We analyzed the clinical variables related to the total population of the study discriminating by genotypes associated or not with drug resistance. Statistically significant differences were found for patients classified as having polar lepromatous leprosy (LL) (p<0.01) versus no LL, and patients with positive Zielhn-Neelsen (ZN) histopathology (p<0.001) versus negative ZN. The rest of the clinical variables were not associated with the presence of mutant genotypes.
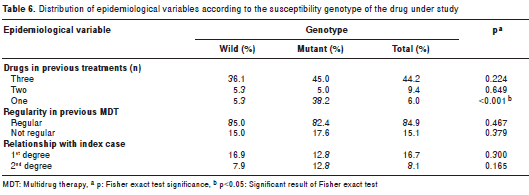
The epidemiological variables were also analyzed according to the presence or absence of genotypes associated with drug resistance. The proportion of patients with previous monotherapy among carriers of mutant genotypes associated with drug resistance was significantly larger (p<0.001) (table 6). The rest of the epidemiological variables under study did not show any differences.
Discussion
The present study offers valuable information since it included a high number of samples from patients with multibacillary leprosy from almost all the Colombian territory examined at the Centro Dermatológico Federico Lleras Acosta, E.S.E., over a twenty-year period. Therefore, these data can be a starting point for the organization of systematic surveillance studies on anti-leprosy drug resistance in Colombia, as has been proposed by international experts in order to monitor the emergence of multidrugresistant bacilli that would hinder the goal of eradicating leprosy (30).
We found that M. leprae circulating between 1985 and 2004 in Colombia showed resistance to dapsone oscillating between 0.06% and 2.4%, and was inexistent in new cases ( 95% CI). This was also the case in the study conducted by Roche in Nepal (32) between 1987 and 1999, and in Mexico (29), demonstrating that multidrug therapy significantly decreases the presence of the dapsone-resistant microorganism thanks to the activity of the other antibiotics in the regime.
It is important to keep in mind that the number of cases studied in this report was much higher than in most studies found in the literature, and that during the period under study, Colombia had already implemented multidrug therapy. Studies in previously untreated patients, such as those conducted in Indonesia (23), Ethiopia (33), the Philippines (34,35) and San Francisco (36), found low frequencies of resistance to dapsone, which is similar to our results where it was zero.
Secondary dapsone resistance ranged between 0.1% and 4.0% (table 1), which suggests that during this period, patients at the Centro Dermatológico Federico Lleras Acosta, E.S.E ., received multidrug therapy regularly on account of the new blister presentations, and that organisms showing resistance to one drug are susceptible to the other drugs in multidrug therapy, as its mechanisms of action are different.
Regarding rifampicin resistance, it oscillated between 1.5% and 6.32% for the total population of the study, similar data to those reported for the French Antilles (25), Indonesia, Myanmar (23) and, recently, for Mexico (29). The fact that rifampicin resistance among new cases was significantly higher than in previously treated cases (p<0.05) ratifies the importance of patients' adherence to multidrug therapy and treatment regularity, as non-adherence and irregularity are responsible for the emergence of resistant strains that will infect new cases.
In general, this result raises concern since it may reflect the indiscriminate use of rifampicin in the treatment of other pathologies, something that could be happening in our setting, thus hindering the effectiveness of multidrug therapy both in paucibacillary and multibacillary patients. Rifampicin, unlike other drugs, is irreplaceable because of its high bactericidal power at the microorganism's intracellular level, and because in a situation where there is high dapsone-resistance and low use of clofazimine, it would result in reducing the effectiveness of multidrug therapy, given that none of the three drugs would act optimally.
We found that resistance to ofloxacin (95% confidence interval) oscillated between 0.35% and 3.57%, which although lower than the 6.8% reported by Maeda (18) in a group of 88 patients from Japan, Haiti, Indonesia, Pakistan and the Philippines, may be considered a realistic picture of our patients' situation regarding ofloxacin resistance. This is even more so when we compared ours to the study by Cambau (17), which used only 49 relapse patients and 34 untreated ones, and where resistance reached 13.3%; this can be explained by the fact that in Colombia this drug is only added to multidrug therapy when patients can buy it with their own money.
In the total study population, we found significant differences between resistance in new patients versus previously treated ones (p<0.05) (table 4), even though the sample size of previously treated patients almost doubled that of new patients and that we did not use a random or representative design for the number of incidents and prevalent patients in Colombia at that time, since we selected samples by convenience from the biopsy bank at the Centro Dermatológico Federico Lleras Acosta, E.S.E.
Notwithstanding that the number of drug-resistant M. leprae samples was low (n=39), it is important to underline that 77% of them showed resistance to rifampicin alone or combined with another drug (figure 1 and table 3). Additionally, 28.3% of these presented resistance to ofloxacin alone or combined with another drug, which, as explained before, reflects the inappropriate use of these drugs in our setting.
In a recent study with samples from three previously- treated Colombian patients, resistance to rifampicin was found in two of them (37), which, even with such a small number of patients, concurs with our results.
Results regarding rifampicin among untreated cases (4.72% CI95%=1.88-11.2 ) confirm the importance of following international experts' recommendations to start surveillance of rifampicin resistance immediately, as it is an irreplaceable drug in multidrug therapy.
After analyzing different variables, we found that, as documented in other studies (25), drug resistance was associated with the place of birth within the Santander province (p<0.01) (table 5), as well as with not finding bacilli in the biopsy (p<0.001), which may be explained by a mistaken classification, and finally, with receiving monotherapy (p=<0.0001).
Resistance to drugs was not associated with previous treatment compliance or with relationship to the index case, since it is known that close contact is more important than the degree of consanguinity (table 6).
On the other hand, it is important to emphasize that drug resistance was not associated with suspicion of relapse (p=0.471), as previously observed by Li (27), Sekar (28) and da Silva, et al. (38), who showed that relapses occur with drug-sensitive strains and correspond more to re-infection events. We were not able to find evidence of multidrug resistance in this study (understood as simultaneous resistance to dapsone, rifampicin and ofloxacin), even though the samples under study were from multibacillary patients with high bacillary populations per gram of tissue, which in principle should have facilitated finding mutations simultaneously resistant to the three drugs, as populations of over 1 x 10 20 microorganisms are needed to obtain one multi-resistant mutant (39).
We were able to corroborate that from a drug- resistance point of view, the standard multidrug therapy regimes continue to be effective for our new and previously treated cases, since the proportion of resistant mutants, at its highest range, did not exceed 11.2% (table 3). Nevertheless, it is necessary to guarantee adherence and regularity in multidrug therapy, since the highest drug resistance was found for rifampicin, which could hinder the effectiveness of this therapy.
We ratify the need to establish immediate drug resistance surveillance for rifampicin, dapsone and ofloxacin in new and previously treated leprosy cases backed by the findings from studies on tuberculosis showing that such surveillance is cost-effective even in groups with moderate drug resistance prevalence. Special attention should be paid to transmission reduction achievable with the help of intervention measures based on surveillance results (40).
According to the results of this study, which included cases until 2004, drug-resistant M. leprae should be of concern for public health authorities in the country since the highest proportion of resistance was found for rifampicin. There was more resistance to rifampicin among new cases than among previously treated patients and they were also resistant to ofloxacin, which is a second-line drug. All of this jeopardizes the effectiveness of multidrug therapy in Colombia for both paucibacillary and multibacillary patients and reveals the need to increase our knowledge on what may have happened regarding this problem after 2004. This could be achieved by implementing national routine surveillance of drug resistance in leprosy.
The authors express that there is no conflict of interest.
The authors want to acknowledge Juan Carlos Salazar at the Centro Dermatológico Federico Lleras Acosta, E.S.E., Clinical History Group for his cooperation in finding the clinical records, and to Nelsy Pineda, histotechnologist at the Centro Dermatológico Federico Lleras Acosta, E.S.E ., for her kind cooperation in obtaining the slide cuts from the paraffin blocks.
The study was completely developed and funded by the Centro Dermatológico Federico Lleras Acosta, E.S.E. , code-project 4000-16.2H.
Corresponding author: Martha Inírida Guerrero, Oficina de Docencia e Investigación, Centro Dermatológico Federico Lleras Acosta, E.S.E., Avenida 1ª N° 13A-61, Bogotá, D.C., Colombia Phone: (571) 242 8160, ext. 145; fax: (571) 242 8160, ext. 142 marthainiridag@yahoo.com
1. World Health Organization. Chemotherapy of leprosy for control programmes. Report of a Study Group. WHO Technical Report Series 675, 1982. [ Links ]
2. Matsuoka M, Kashiwabara Y, Namisato M. A Mycobacterium leprae isolate resistant to dapsone, rifampin,ofloxacin and sparfloxacin. Int J Lepr Other Mycobact Dis. 2000;68:452-5. [ Links ]
3. Shepard CC, Chang YT. Effect of several anti-leprosy drugs on multiplication of human leprosy bacilli in footpads of mice. Proc Soc Exp Biol Med. 1962;109:636-8. [ Links ]
4. Musser J. Antimicrobial agent resistance in mycobacteria: Molecular genetic inslights . Clin Microbiol Rev. 1995;8:496-514. [ Links ]
5. Williams DL, Gillis TP. Drug-resistant leprosy: Monitoring and current status. Review. Lepr Rev. 2012;83:269-81. [ Links ]
6. Kai M, Nguyen Phuc NH, Nguyen HA, Pham TH, Nguyen KH, Miyamoto Y, et al. Analysis of drug-resistant strains of Mycobacterium leprae in an endemic area of Vietnam. Clin Infect Dis. 2011;52:e127-32. [ Links ]
7. Cambau E, Bonnafous P, Perani E, Sougakoff W, Ji B, Jarlier V. Molecular detection of rifampicin and ofloxacin resistance for patients who experience relapse of multibacillary leprosy. Clin Infect Dis. 2001;34:39-45. [ Links ]
8. Matsuoka M, Kashiwabara Y, Liangfen Z, Goto M, Kitajima S. A second case of multidrug resistant Mycobacterium leprae isolated from a Japanese patient with relapsed lepromatous leprosy. Int J Lepr Other Mycobact Dis. 2003;71:240-3. [ Links ]
9. Cambau E, Carthagena L, Chauffour A, Ji B, Jarlier V. Dihydropteroate synthase mutations in the folP1 gene predict dapsone resistance in relapsed cases of leprosy. Clin Infect Dis. 2006;42:238-41. [ Links ]
10. Honore N, Cole ST. Molecular basis of rifampin resistance in Mycobacterium leprae . Antimicrob Agents Chemother. 1993;37:414-8. [ Links ]
11. Honoré N, Perrani E, Telenti A, Grosset J, Cole ST. A simple and rapid technique for the detection of rifampin resistance in Mycobacterium leprae . Int J Lepr Other Mycobact Dis. 1993;61:600-4. [ Links ]
12. Williams DL, Gillis TP. Molecular detection of drug resistance in Mycobacterium leprae. Lepr Rev. 2004;75: 118-30. [ Links ]
13. Williams DL, Waguespack C, Eisenach K, Crawford JT, Portaels F, Salfinger M, et al. Characterization of rifampin-resistance in pathogenic mycobacteria. Antimicrob Agents Chemother. 1994;38:2380-6. [ Links ]
14. Zhang L, Namisato M, Matsuoka M. A mutation at codon 516 in the rpoB gene Mycobacterium leprae confers resistance to rifampin. Int J Lepr Other Mycobact Dis. 2004;72:468-72. [ Links ]
15. Kai M, Matsuoka M, Nakata N, Maeda S, Gidoh M, Maeda Y, et al. Diaminodiphenilsulfone resistance of Mycobacterium leprae due to mutations in the dihydropteroate synthase gene. FEMS Microbio Lett. 1999;177:231-5. [ Links ]
16. Williams DL, Spring L, Harris E, Roche P, Gillis TP. Dihydropteroate synthase of Mycobacterium leprae and dapsone resistance. Antimicrob Agents Chemother. 2000;44: 1530-7. [ Links ]
17. Cambau E, Perani E, Guillemin I, Jamet P, Ji B. Multidrug-resistance to dapsone, rifampicin, and ofloxacin in Mycobacterium leprae . Lancet. 1997;349:103-4. [ Links ]
18. Maeda S, Matsuoka M, Nakata N, Kai M, Maeda Y, Hashimoto K, et al. Multidrug resistant Mycobacterium leprae from patients with leprosy. Antimicrob Agents Chemother. 2001;45:3635-9. [ Links ]
19. You EY, KangTJ, Kim SK, Lee SB, Chae GT. Mutations in genes related to drug resistance in Mycobacterium leprae isolates from leprosy patients in Korea. J Infect. 2005;50:6-11. [ Links ]
20. Ji B. Rifampicin resistant leprosy: A review and a research proposal of a pilot study. Lepr Rev. 2002;73:2-8. [ Links ]
21 . Shetty VP, Uplekar MW, Antia NH. Primary resistance to single and multiple drugs in leprosy--a mouse footpad study. Lep Rev. 1996;67:280-6. [ Links ]
22. Ebenezer GJ, Norman G, Joseph GA, Daniel S, Job CK. Drug resistant- Mycobacterium leprae results of mouse footpad studies from a laboratory in south India. Indian J Lepr. 2002;74:301-12. [ Links ]
23. Matsuoka M, Budiawan T, Aye KS, Kyaw K, Tan EV, Cruz ED, et al . The frequency of drug resistance mutations in Mycobacterium leprae isolates in untreated and relapsed leprosy patients from Myanmar, Indonesia and the Philippines. Lepr Rev. 2007;78:343-52. [ Links ]
24. Singh P, Busso P, Paniz-Mondolfi A, Aranzazu N, Monot M, Honore N, et al . Molecular drug susceptibility testing and genotyping of Mycobacterium leprae strains from South America. Antimicrob Agents Chemother. 2011;55:2971-3. [ Links ]
25. Grosset JH, Guelpa-Lauras CC, Bobin P, Brucker G, Cartel JL, Constant-Desportes M. Study of 39 documented relapses of multibacillary leprosy after treatment with rifampin. Int J Lepr Other Mycobact Dis. 1989;57:607-14. [ Links ]
26. González AB, Maestre JL, Hernández O, Columbié Y, Atrio N, Martín M, et al . Survey for secondary dapsone and rifampicin resistance in Cuba. Lepr Rev. 1993;64:128-35. [ Links ]
27. Li HY, Ran SP, Weng TG, Deng XH, Li FT. Relapses in leprosy patients treated with rifampicin plus dapsone after varying periods of dapsone monotherapy. Indian J Lepr. 2001;73:1-10. [ Links ]
28. Sekar B, Elangeswaran N, Jayarama E, Rajendran M, Kumar S, Vijayaraghavan R, et al . Drug susceptibility of Mycobacterium leprae: A retrospective analysis of mouse footpad inoculation results from 1983 to 1997. Lepr Rev. 2002;73:239-44. [ Links ]
29. Matsuoka M, Suzuki Y, Garcia IE, Fafutis-Morris M, Vargas-González A, Carreño-Martínez C, et al . Possible mode of emergence for drug-resistant leprosy is revealed by an analysis of samples from Mexico. Jpn J Infect Dis. 2010;63:412-6. [ Links ]
30. National JALMA Institute of Leprosy and Other Mycobacterial Diseases. Informal consultation on rifampicin resistance in leprosy. Lepr Rev. 2007;78:295-305. [ Links ]
31. Torres JF. Estudio de farmacorresistencia y genotipificación de M. leprae colombiano período 2000-2004 (tesis). Bogotá: Universidad Nacional de Colombia; 2008. [ Links ]
32. Roche PW, Neupane KD, Failbus SS, Butlin CR. Dapsone drug resistance in the multidrug therapy era. Int J Lepr Other Mycobact Dis. 2000;68:323-5. [ Links ]
33. Warndorff van Diepen T, Aredath SP, Mengistu G. Dapsone-resistant leprosy in Addis Ababa: A progress report. Lepr Rev. 1984;55:149-57. [ Links ]
34. Guinto RS, Cellona RV, Fajardo TT, de la Cruz EC. Primary dapsone-resistant leprosy in Cebu, Philippines. Int J Lepr Other Mycobact Dis. 1981;49:427-30. [ Links ]
35. de la Cruz E, Cellona RV, Balagon MV, Villahermosa LG, Fajardo TT Jr, Abalos RM, et al . Primary dapsone resistance in Cebu, The Philippines; cause for concern. Int J Lepr Other Mycobact Dis. 1996;64:253-6. [ Links ]
36. Gelber RH, Rea TH, Murray LP, Siu P, Tsang M, Byrd SR. Primary dapsone-resistant Hansen's disease in California. Experience with over 100 Mycobacterium leprae isolates. Arch Dermatol. 1990;126:1584-6. [ Links ]
37. Hernández E, Cardona-Castro N, Rodríguez G, Villegas S, Beltrán C, Kimura M, et al . Study of rifampin and dapsone resistance in three patients with recurring leprosy. Rev Panam Salud Publica. 2008;23:73-7. [ Links ]
38. da Silva Rocha A, Cunha MD, Diniz LM, Salgado C, Aires MA, Nery JA, et al . Drug and multidrug resistance among Mycobacterium leprae isolates from Brazilian relapsed leprosy patients. J Clin Microbiol. 2012;50:1912-7. [ Links ]
39. Williams DL, Gillis TP. Molecular detection of drug resistance in Mycobacterium leprae. Review . Lepr Rev. 2004;75:118-30. [ Links ]
40. Acuña-Villaorduna C, Vassall A, Henostroza G, Seas C, Guerra H, Vásquez L, et al . Cost-effectiveness analysis of introduction of rapid, alternative methods to identify multidrug-resistant tuberculosis in middle-income countries. Clin Infect Dis. 2008;47:487-95. [ Links ]














