Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biomédica
Print version ISSN 0120-4157
Biomédica vol.34 supl.1 Bogotá Apr. 2014
https://doi.org/10.7705/biomedica.v34i0.1648
ARTÍCULO ORIGINAL
doi: http://dx.doi.org/10.7705/biomedica.v34i0.1648
1 Grupo de Inmunología y Epidemiología Molecular, Facultad de Salud, Universidad Industrial de Santander, Bucaramanga, Colombia
2 Grupo PAIDOS, Facultad de Salud, Universidad Industrial de Santander, Bucaramanga, Colombia
Author contributions:
Clara Isabel González and Luis Miguel Sosa: Experiment design, reagents, materials and analysis tools.
Mayra Alejandra Machuca: Performance of experiments.
All authors contributed equally to the data analysis and the drafting of the article.
Recibido: 17/05/13; aceptado: 13/12/13
Introduction: Methicillin-resistant Staphylococcus aureus (MRSA) is a frequent cause of infection in the pediatric population. Initially, MRSA was restricted to hospitals; however, outbreaks in the community among people without health care-related risk factors have been reported worldwide. Currently, MRSA is a frequent cause of both hospital and community-associated infections.
Objective: To describe the relationships between the molecular characteristics of MRSA isolates (staphylococcal cassette chromosome mec (SCCmec) type and Panton-Valentine leukocidin (PVL) carriage) and the characteristics of infection (the origin and localization of infection) in pediatric patients at the Hospital Universitario de Santander in Bucaramanga, Colombia.
Materials and methods: A total of 43 MRSA isolates were obtained from hospitalized pediatric patients. SCCmec typing (I-V), SCCmec IV subtyping and PVL carriage were determined and related to the clinical characteristics.
Results: Among the MRSA isolates studied, SCCmec IVc was present in 77%, followed by 16% for SCCmec I and 2% for SCCmec IVa. Two isolates were not typeable (NT). PVL genes were carried by 88% of the MRSA isolates, including the SCCmec IVc/IVa and SCCmec I isolates. SCCmec IV caused both community-acquired infection (CAI) (47%) and nosocomial infection (HAI) (53%). SCCmec IV, PVL-positive MRSA was associated with both CAI (47%) and HAI (53%) and caused mostly SSTI and osteoarticular infection.
Conclusions: These findings suggest that the presence of community-associated MRSA (CA-MRSA) (SCCmec IV and PVL positive) causes both health care-associated infection (HCAI) and nosocomial infection (HAI) in pediatric patients in Colombia.
Key words: Methicillin-resistant Staphylococcus aureus , patients, child.
doi: http://dx.doi.org/10.7705/biomedica.v34i0.1648
Staphylococcus aureus resistente a meticilina causante de infecciones comunitarias y de infecciones asociadas a la atención en salud en pacientes pediátricos del Hospital Universitario de Santander
Introducción. Staphylococcus aureus resistente a la meticilina (SARM) es un agente frecuente de infección en la población pediátrica. Aunque inicialmente las cepas de SARM estaban restringidas a los hospitales, se han reportado a nivel mundial brotes de infección por SARM en individuos sin factores de riesgo y, actualmente, SARM es una causa frecuente de infecciones hospitalarias y comunitarias.
Objetivo. Describir la relación entre las características moleculares de aislamientos de SARM (casete cromosómico estafilocócico mec SCCmec y leucocidina Panton-Valentine) y el origen de la infección y su presentación clínica en pacientes pediátricos del Hospital Universitario de Santander en Bucaramanga, Colombia.
Materiales y métodos. Se incluyeron 43 aislamientos de SARM obtenidos de niños hospitalizados. La clasificación del SCCmec (I-V) y la subclasificación del SCCmec-IV se realizaron en todos los aislamientos. Además, los genes de la leucocidina Panton-Valentine se detectaron mediante amplificación por PCR. Las características moleculares fueron asociadas con las características clínicas de cada paciente.
Resultados. Entre los 43 SARM tipificados, el SCCmec-IVc fue el más frecuente con 77 %, seguido por el SCCmec-I con 16 % y el SCCmec-IVa con 2 %. Tres aislamientos no pudieron ser tipificados. Los genes de la leucocidina Panton Valentine se detectaron en 88 % de los SARM en aislamientos portadores del SCCmec-IVc/IVa y el SCCmec-I. Los SARM SCCmec-IV positivos para la leucocidina Panton-Valentine se asociaron con infecciones adquiridas en la comunidad (47 %) y en el hospital (53 %) con compromiso de piel y tejidos blandos, y en los casos más graves, con compromiso osteoarticular.
Conclusiones. Estos resultados sugieren la presencia de cepas SARM-CO (SCCmec-IV positiva para PVL) causantes de infecciones adquiridas en la comunidad y en el medio hospitalario en pacientes pediátricos en Colombia.
Palabras clave: Staphylococcus aureus resistente a meticilina, pacientes, niño.
doi: http://dx.doi.org/10.7705/biomedica.v34i0.1648
Staphylococcus aureus is responsible for infections ranging from skin and soft tissue infections to severe diseases, such as endocarditis, bacteremia, necrotizing pneumonia and osteomyelitis (1). Methicillin resistance is encoded by the mecA gene, which is carried in the staphylococcal cassette chromosome mec (SCCmec) (2,3). Based on the class of the mec complex, ccr type and composition of the J regions, eight types of SCCmec, called I-XI, have been described (4,5).
For many years, methicillin-resistant S. aureus (MRSA) was restricted to hospitals, causing infec tions associated with the health care setting (HAI) (6). However, since the early 2000s, outbreaks of community-associated MRSA (CA-MRSA) have been reported worldwide in diverse community populations (7,8). Recently, several reports described the spread of CA-MRSA in the hospital setting, which is beginning to replace typical hospital-associated MRSA (HA-MRSA), especially in the United States and Taiwan, where CA-MRSA prevalence is very high (9). CA-MRSA has been reported to be causing infections in Latin American countries, such as Brazil (10), Uruguay (11), Colombia (12) and Argentina (13). In Colombia, CA-MRSA isolates have recently been reported to be causing HAI in hospitals in Bogotá and Bucaramanga (12,14).
Phenotypic and molecular differences between HA- and CA-MRSA have been described, as follows: i) HA-MRSA strains carry SCCmec types I-III (15), whereas CA-MRSA strains carry SCCmec IV and V (16,17); ii) HA-MRSA tends to be multiresistant, whereas CA-MRSA tends to be susceptible to narrow- spectrum non-beta-lactams, such as clindamycin, trimethoprim-sulfamethoxazole and tetracyclines (18); and iii) a high percentage of CA-MRSA strains carry the genes lukS -PV and lukF -PV, encoding Panton-Valentine leukocidin (PVL) (18), an important virulence factor that has been associated with skin abscesses and necrotizing pneumonia (19-21). The PVL genes are present in CA-MRSA strains with a frequency >75% and are largely absent from HA-MRSA strains (7,22-23). In the present study, we characterized the relationships between the molecular features (SCCmec type and PVL carriage) and the characteristics (the origin and localization of infection) of infection in pediatric patients at the Hospital Universitario de Santander in Bucaramanga, Colombia.
Materials and methods
Clinical isolates
MRSA isolates were obtained from isolated cases of local or systemic infections in a pediatric population (age range: 0-13 years old), who were hospitalized in a third-level university hospital. The isolates were collected in the Clinical Laboratory of the Hospital Universitario de Santander in Bucaramanga, Colombia, during the period from March 2007 to March 2009. The antibiotic susceptibilities of the S. aureus isolates were assessed in accordance with Clinical and Laboratory Standards Institute (CLSI) guidelines (24). The antibiotics tested included erythromycin, clindamycin, ciprofloxacin, gentamicin, tetracycline, oxacillin, rifampicin, vancomycin and trimethoprim-sulfamethoxazole. Clinical and epidemiological information was obtained from the medical records of each patient. Each MRSA infection was classified as a community-associated infection (CAI), nosocomial infection (HAI) or health care-associated infection (HCAI), according to the following definitions:
- CAI: Clinical condition and isolation of MRSA within 48 hours of hospitalization in the absence of risk factors.
- HAI: No obvious clinical disease at admission, isolation of MRSA after 72 hours of hospitalization or presence of risk factors (use of invasive devices, surgery, dialysis and previous hospitalization in the last three months).
- HCAI: Clinical condition and isolation of MRSA at admission or within 48 hours of hospitalization and presence of risk factors (25).
Detection of nuc and mecA genes
All isolates were confirmed as MRSA by PCR amplification of the nuc and mecA genes according to a previously described protocol (26,27).
SCCmec typing and PVL gene detection
The SCCmec type (I-V) was determined based on combinations of the mec complex and ccr type using independent PCRs. SCCmec IV subtypes were identified using a multiplex PCR under reported conditions (28). Amplification of the PVL genes ( lukS / F PV) was performed as previously reported (20). The COL, N315, MW2, E-MRSA-16 and RN4220/pG01 strains were included as reference strains and were supplied by the Network on Antimicrobial Resistance in S. aureus (NARSA).
Ethics statement
The research and informed consent protocols were approved by the Ethics Committee at the Universidad Industrial de Santander in accordance with the ethical standards of the 1964 Declaration of Helsinki (Acta No. 15 27/08/2007). Each child's parents or guardians provided written informed consent for the review of medical information, and all of the information was confidential.
Results
In the period between March 2007 and March 2009, there were 126 S. aureus infections in pediatric patients, of which 42% were caused by MRSA. In total, 8% of the children were aged <1 month; 40%, from 1-24 months; 35%, from 2-10 years old, and 17%, >10 years old.
In this study, 43 non-duplicated MRSA isolates were included. Most of the MRSA isolates were susceptible to the majority of the antibiotics tested; only one isolate presented a multiresistant phenotype (resistant to four antibiotics). In total, 44% (19/43) of the MRSA isolates exhibited resistance to tetracycline; 42% (18/43), to erythromycin; 30% (13/43), to ciprofloxacin; 26% (11/43), to clindamycin; 19% (8/43), to gentamicin, and 5% (2/43), to TMP-SMX. All strains were resistant to oxacillin and susceptible to vancomycin and rifampicin.
SCCmec typing and PVL gene detection
The type SCCmec IVc was the most frequent in MRSA clinical isolates (77%, 33 isolates). Additionally, 2% (1 isolate) was SCCmec type IVa, and 16% (7 isolates) were type I. Two isolates were not typeable (NT) following the search for the five SCCmec types. The lukS/F PV genes were detected in 88% (38/43) of the MRSA isolates and were present in all SCCmec types identified. The lukS/F PV genes were detected in 100% (1/1) and 91% (30/33) of the SCCmec type IVa and SCCmec type IVc isolates, respectively. Most of the SCCmec type I isolates carried the lukF/S PV genes (86%, 6 isolates).
Clinical analysis
Among the infections, 46% were CAIs, 19% were HCAIs, and 35% were HAIs (table 1).

The most frequent clinical manifestation was skin infection, affecting 53% (23/43) of pediatric patients, followed by soft tissue infection in 28% (12/43) and osteoarticular infection in 12% (5/43). More severe infections were present at low frequencies, such as bacteremia in 5% (2/43) and complicated pneumonia in 2% (1/43) (table 2). Classification was performed according to the definitions described before (29).
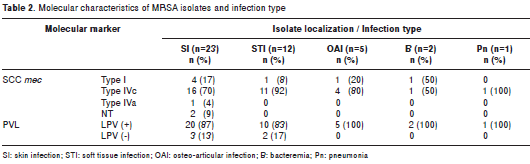
Expression of the molecular markers SCCmec and PVL and the origin of MRSA infection are included in table 1. MRSA carrying SCCmec types IVc and IVa and PVL positivity caused CAI, HCAI and HAI at high frequencies. In total, 80% of CAIs, 88% of HCAIs and 74% of HAIs were caused by isolates with SCCmec type IV, and the isolates carrying SCCmec type I caused 20% of CAIs and 20% of HAIs. SCCmec type IVc and type I isolates that were obtained from all localizations were similarly PVL-positive isolates, but in a large percentage, these isolates were related to skin and soft tissue infections (table 2).
Discussion
HCAI and HAI are generally caused by isolates of MRSA with the molecular characteristics of HA-MRSA strains, such as SCCmec I, the absence of PVL and antimicrobial multiresistance patterns. However, since the emergence of CA-MRSA strains carrying SCCmec IV, the PVL genes and an antimicrobial sensitivity pattern phenotype, CA-MRSA isolation in hospitals has increased significantly in the United States (30,31), in certain European countries, such as France (32) and Italy (33), and in Latin American countries, including Brazil ( 10), Argentina (14), Uruguay (11) and Colombia (12). In Colombia, for the last few years, the presence and circulation of CA-MRSA isolates have been described in hospitals in different regions, and the prevalence of this strain type in the hospital environment has been estimated to be approximately 39% (12,34). In our study, 74% of HAIs and 88% of HCAIs were caused by isolates with the molecular characteristics of CA-MRSA, which were higher percentages than previously reported (12,34) . Therefore, these results confirm that CA -MRSA strains have been introduced into and have become established in hospitals in Colombia . Of the 43 isolates analyzed in our study, only seven carried SCCmec I, similar to what has been reported in certain European countries such as Austria and the United Kingdom, where the frequency of HA-MRSA strains in hospitals has typically decreased (35).
SCCmec type IV has the greatest variability among the SCCmec types (36), and seven subtypes (IVa to IVg) have been identified (28). This subtype identification has been important for understanding the mechanisms of the insertion and acquisition of this mobile genetic element and has also been used to identify new circulating clones (37). The SCCmec subtype IVc was identified for the first time in Japan, and this variant was associated with HAI (2). Although similar results were observed in other countries, such as France (38), in Sweden, this subtype has been associated with CAI (39). Nevertheless, HAI in countries such as the United States and Australia most frequently presents the subtype IVa (27). In our study, 77% of the isolates were classified as SCCmec IVc, and 2% were classified as SCCmec IVa. Both were present in all three types of infection. This result supports the previous reports of studies in our country, which have shown the predominance of the SCCmec IVc subtype among isolates, followed by a smaller proportion for SCCmec IVa (40,41).
As it has been previously described, most CA- MRSA isolates are PVL positive (22). Among the MRSA isolates tested in our study, 88% carried the lukS/F- PV genes, and these genes were detected in all SCCmec types identified (SCCmec IVc, Iva and I). In Colombia, there have been several reports of infections caused by PVL-positive MRSA strains carrying SCCmec IVc belonging to clonal complex 8 (CC8) in hospitals (13,41-43). In a recent previous work, we reported the ST8-MRSA-IVc clone as the main strain causing HAI (13). This finding suggests the possibility that the PVL-positive and SCCmec IVc isolates detected in our study could be related to the CC8. Despite this observation, additional molecular markers should be analyzed to confirm the clonal complex to which our isolates belonged. In contrast, HA-MRSA strains (SCCmec type I) usually do not carry the lukS/F PV genes. However, 80% of HA- MRSA isolates carried these genes in our hospital (13). This finding is interesting because several countries report the absence or a low percentage of these genes in HA-MRSA isolates. Thus, in European countries such as Spain, the United Kingdom, France, Italy and Ireland, the PVL genes are absent among HA-MRSA isolates (44-46), and in a previous study in Colombia, these genes were detected in less than 9% of isolates (40). This result could reflect a different epidemiology of HA- MRSA strains in our country, resulting in a high frequency of the PVL genes.
Although the role of PVL in the severity of S. aureus infection is controversial (47-49), PVL production in MRSA has been associated with severe skin infections, such as abscesses and necrotizing pneumonia. As in a previous report that we published (50), in the present study, all pediatric patients developed moderate to severe infections and had to be treated at a high-complexity hospital. This finding could be related to the observed high frequency of PVL, which was greater than 80%.
Therefore, this descriptive study confirms the presence of strains with the molecular characteristics of CA-MRSA, such as SCCmec IV and PVL positivity, causing both HCAI and HAI in pediatric patients in Colombia. Finally, due to CA-MRSA emergence and establishment in hospitals, it is necessary to implement control measures and appropriate management of infection to prevent CA-MRSA dissemination in both hospitals and the community environment.
The authors thank Martha Jácome and Myriam Fanny Anaya of the Clinical Laboratory of the Hospital Universitario de Santander for their assistance in the collection of the MRSA isolates and the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) for providing the reference strains used in this study.
None to report.
The financial resources for this work were provided by the Departamento Administrativo de Ciencia, Tecnología e Innovación, Colciencias, grant 1102- 40820559; the Universidad Industrial de Santander, and the Hospital Universitario de Santander.
Corresponding author: Luis Miguel Sosa, Departamento de Pediatría, Hospital Universitario de Santander, Universidad Industrial de Santander, Bucaramanga, Colombia Phone: (577) 632 2429 y 634 4000, ext. 3102; fax: (577) 632 2429
lumisosa@gmail.com
1. Lowy FD. Staphylococcus aureus infections. N Engl J Med.1998;339:520-32. http://dx.doi.org/10.1056/NEJM199808203390806 [ Links ]
2. Ito T, Okuma K, Ma XX, Yuzawa H, Hiramatsu K. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: Genomic island SCC. Drug Resist Updat. 2 003;1:41-52. . http://dx.doi.org/10.1016/S1368-7646(03)00003-7 [ Links ]
3. Memmi G, Filipe SR, Pinho MG, Fu Z, Cheung A. Staphylococcus aureus PBP4 is essential for beta-lactam resistance in community-acquired methicillin-resistant strains. Antimicrob Agents Chemother. 2008;52:3955-66. http://dx. doi.org/10.1128/AAC.00049-08 [ Links ]
4. International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements I-S. Classification of Staphylococcal Cassette Chromosome mec (SCCmec): Guidelines for Reporting Novel SCCmec Elements. Antimicrob Agents Chemother. 2009;53:4961-7. http://dx.doi.org/10.1128/AAC.00579-09 [ Links ]
5. Li S, Skov RL, Han X, Larsen AR, Larsen J, Sørum M, et al . Novel types of staphylococcal cassette chromosome mec elements identified in clonal complex 398 methicillin- resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 2001;55:3046-50. http://dx.doi.org/10.1128/AAC.01475-10 [ Links ]
6. Chambers HF. The changing epidemiology of Staphylococcus aureus ? Emerg Infect Dis. 2001;7:178-82. [ Links ]
7. Zetola N, Francis JS, Nuermberger EL, Bishai WR. Community-acquired meticillin-resistant Staphylococcus aureus : An emerging threat. Lancet Infect Dis. 2005;5:275-86. http://dx.doi.org/10.1016/S1473-3099(05)70112-2 [ Links ]
8. Kaplan SL, Hulten KG, González BE, Hammerman WA, Lamberth L, Versalovic J, et al. Three-year surveillance of community-acquired Staphylococcus aureus infections in children. Clin Infect Dis. 2005;40:1785-91. http://dx.doi. org/10.1086/430312 [ Links ]
9. Popovich KJ, Weinstein RA, Hota B. Are community- associated methicillin-resistant Staphylococcus aureus (MRSA) strains replacing traditional nosocomial MRSA strains? Clin Infect Dis. 2008;46:787-94. http://dx.doi.org/10.1086/528716 [ Links ]
10. Ribeiro A, Dias C, Silva-Carvalho MC, Berquo L, Ferreira FA, Santos RN, et al. First report of infection with community-acquired methicillin-resistant Staphylococcus aureus in South America. J Clin Microbiol. 2005;43:1985-8. http://dx.doi.org/10.1128/JCM.43.4.1985-1988.2005 [ Links ]
11. B enoit SR, Estivariz C, Mogdasy C, Pedreira W, Galiana A, Bagnulo H, et al . Community strains of methicillin-resistant Staphylococcus aureus as potential cause of healthcare- associated infections, Uruguay, 2002-2004. Emerg Infect Dis. 2008;14:1216-23. http://dx.doi.org/10.3201/eid1408.071183 [ Links ]
12. Álvarez CA, Yomayusa N, Leal AL, Moreno J, Méndez-Álvarez S, Ibáñez M, et al. Nosocomial infections caused by community-associated methicillin-resistant Staphylococcus aureus in Colombia. Am J Infect Control. 2010;38:315-8. http://dx.doi.org/10.3201/eid1212.060814 [ Links ]
13. Sola C, Paganini H, Egea AL, Moyano AJ, Garnero A, Kevric I, et al . Spread of epidemic MRSA-ST5-IV clone encoding PVL as a major cause of community onset staphylococcal infections in Argentinean children. PLoS One. 2012;7:e30487. http://dx.doi.org/10.1371/journal.pone.0030487 [ Links ]
14. M achuca MA, Sosa LM, González CI. Molecular typing and virulence characteristic of methicillin-resistant Staphylococcus aureus isolates from pediatric patients in Bucaramanga, Colombia. PLoS ONE. 2013:8:e73434. http://dx.doi.org/10.1371/journal.pone.0073434 [ Links ]
15. Hiramatsu K, Katayama Y, Yuzawa H, Ito T. Molecular genetics of methicillin-resistant Staphylococcus aureus . Int J Med Microbiol. 2002;292:67-74. http://dx.doi.org/10.1078/1438-4221-00192 [ Links ]
16. Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;10;290:2976-84. http://dx.doi.org/10.1001/jama.290.22.2976 [ Links ]
17. Naas T, Fortineau N, Spicq C, Robert J, Jarlier V, Nordmann P. Three-year survey of community-acquired methicillin-resistant Staphylococcus aureus producing Panton-Valentine leukocidin in a French university hospital. J Hosp Infect. 2005;61:321-9. http://dx.doi.org/10.1016/j.jhin.2005.01.027 [ Links ]
18. Bukharie HA. A review of community-acquired methicillin- resistant Staphylococcus aureus for primary care physicians. J Family Community Med. 2010;17:117-20. http://dx.doi. org/10.4103/1319-1683.74320 [ Links ]
19. de Bentzmann S, Tristan A, Etienne J, Brousse N, Vandenesch F, Lina G. Staphylococcus aureus isolates associated with necrotizing pneumonia bind to basement membrane type I and IV collagens and laminin. J Infect Dis. 2004;15:190:1506-15. http://dx.doi.org/10.1086/424521 [ Links ]
20. Lina G, Piemont Y, Godail-Gamot F, Bes M, Peter MO, Gauduchon V, et al . Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin Infect Dis. 1999;29:1128-32. http://dx.doi.org/10.1086/313461 [ Links ]
21. Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753-9. http://dx.doi.org/10.1016/S0140-6736(02)07877-7 [ Links ]
22. Takano T, Higuchi W, Otsuka T, Baranovich T, Enany S, Saito K, et al . Novel characteristics of community- acquired methicillin-resistant Staphylococcus aureus strains belonging to multilocus sequence type 59 in Taiwan. Antimicrob Agents Chemother. 2008;52:837-45. http://dx.doi.org/10.1128/AAC.01001-07 [ Links ]
23. Dufour P, Gillet Y, Bes M, Lina G, Vandenesch F, Floret D, et al. Community-acquired methicillin-resistant Staphylococcus aureus infections in France: Emergence of a single clone that produces Panton-Valentine leukocidin. Clin Infect Dis. 2002;35:819-24. http://dx.doi.org/10.1086/342576 [ Links ]
24. Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. Eighteenth informational supplement. Document M100-S18. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. [ Links ]
25. Cohen AL, Calfee D, Fridkin SK, Huang SS, Jernigan JA, Lautenbach E, et al. Recommendations for metrics for multidrug-resistant organisms in healthcare settings: SHEA/ HICPAC position paper. Infect Control Hosp Epidemiol. 2007;10:901-13. http://dx.doi.org/10.1086/591741 [ Links ]
26. Brakstad OG, Aasbakk K, Maeland JA. Detection of Staphylococcus aureus by polymerase chain reaction ampli- fication of the nuc gene. J Clin Microbiol. 1992;7:1654-60. [ Links ]
27. Okuma K, Iwakawa K, Turnidge JD, Grubb WB, Bell JM, O'Brien FG, et al. Dissemination of new methicillin- resistant Staphylococcus aureus clones in the community. J ClinMicrobiol. 2002;40:4289-94. http://dx.doi.org/10.1128/JCM.40.11.4289-4294.2002 [ Links ]
28. Milheirico C, Oliveira DC, de Lencastre H. Multiplex PCR strategy for subtyping the staphylococcal cassette chromosome mec type IV in methicillin-resistant Staphyl- ococcus aureus : 'SCCmec IV multiplex'. J Antimicrob Chemother. 2007;60:42-8 . http://dx.doi.org/ 10.1093/jac/dkm112 [ Links ]
29. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309-32. http://dx.doi.org/10.1016/j.ajic.2008.03.002 [ Links ]
30. Klevens RM, Morrison MA, Fridkin SK, Reingold A, Petit S, GershmanK, et al. Community-associated methicillin-resistant Staphylococcus aureus and health care risk factors. Emerg Infect Dis. 2006;12:1991-3. http://dx.doi.org/10.3201/eid1212.060505 [ Links ]
31. Seybold U, Kourbatova EV, Johnson JG, Halvosa SJ, Wang YF, King MD, et al . Emergence of community- associated methicillin-resistant Staphylococcus aureus USA 300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis. 2006;42:647-56. http://dx.doi.org/10.1086/499815 [ Links ]
32. Do nnio PY, Preney L, Gautier-Lerestif AL, Avril JL, Lafforgue N. Changes in staphylococcal cassette chromosome type and antibiotic resistance profile in methicillin-resistant Staphylococcus aureus isolates from a French hospital over an 11 year period. J Antimicrob Chemother. 2004;53:808-13. http://dx.doi.org/10.1093/jac/dkh185 [ Links ]
33. Campanile F, Bongiorno D, Falcone M, Vailati F, Pasticci MB, Pérez M, et al . Changing Italian nosocomial-community trends and heteroresistance in Staphylococcus aureus from bacteremia and endocarditis. Eur J Clin Microbiol Infect Dis. 2011;7:739-45. http://dx.doi.org/10.1007/s10096-011-1367-y [ Links ]
34. Buitrago CJ, Castillo JS, Leal AL, Sánchez R, Álvarez CA. Staphylococcus aureus . Community-acquired phenotype spread in hospitals in Bogota, Colombia. Clin Microbiol Infect. 2008;14:411. [ Links ]
35. European Centre for Disease Prevention and Control. Antimicrobial resistance surveillance in Europe 2009. Annual report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). Stockholm, Sweden: ECDC; 2010. http://dx.doi.org/10.2900/35994 [ Links ]
36. Shore A, Rossney AS, Keane CT, Enright MC, Coleman DC. Seven novel variants of the staphylococcal chromosomal cassette mec in methicillin-resistant Staphylococcus aureus isolates from Ireland. Antimicrob Agents Chemother. 2005;49:2070-83. http://dx.doi.org/10.1128/AAC.49.5.2070-2083.2005 [ Links ]
37. Ito T, Ma XX, Takeuchi F, Okuma K, Yuzawa H, Hiramatsu K. Novel type V staphylococcal cassette chromosome mec driven by a novel cassette chromosome recombinase, ccrC. Antimicrob Agents Chemother. 2004;48:2637-51. http://dx.doi.org/10.1128/AAC.48.7.2637-2651.2004 [ Links ]
38. Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, et al . Community-acquired methicillin- resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: Worldwide emergence. Emerg Infect Dis. 2003;9:978-84. http://dx.doi.org/10.3201/eid0908.030089 [ Links ]
39. Berglund C, Molling P, Sjoberg L, Soderquist B. Predominance of staphylococcal cassette chromosome mec (SCCmec) type IV among methicillin-resistant Staphyl-ococcus aureus (MRSA) in a Swedish county and presence of unknown SCCmec types with Panton-Valentine leukocidin genes. Clin Microbiol Infect. 2005;11:447-56. http://dx.doi.org/10.1111/j.1469-0691.2005.01150.x [ Links ]
40. Jiménez JN, Ocampo AM, Vanegas JM, Rodríguez EA, Garcés CG, Patiño LA, et al . Characterization of virulence genes in methicillin susceptible and resistant Staphylococcus aureus isolates from a paediatric population in a university hospital of Medellín, Colombia. Mem Inst Oswaldo Cruz. 2011;106:980-5. http://dx.doi.org/10.1590/S0074-02762011000800013 [ Links ]
41. Jiménez JN, Ocampo AM, Vanegas JM, Rodríguez EA, Mediavilla JR, Chen L, et al . CC8 MRSA strains harboring SCCmec type IVc are predominant in Colombian hospitals. PLoS One. 2012;7:e38576. http://dx.doi.org/10.1371/journal.pone.0038576 [ Links ]
42. Álvarez-Olmos MI, Enríquez SP, Pérez-Roth E, Méndez- Álvarez S, Escobar J, Vanegas N, et al . Pediatric cases from Colombia caused by a Panton-Valentine leukocidin-positive community-acquired methicillin-resistant Staphylococcus aureus ST8 -SCCmecIVc clone. Pediatr Infect Dis J. 2009;28:935 . http://dx.doi.org/10.1097/INF.0b013e3181b2102b [ Links ]
43. Jiménez JN, Ocampo AM, Vanegas JM, Rodríguez EA, Mediavilla JR, Chen L, et al. A comparison of methicillin-resistant and methicillin-susceptible Staphylococcus aureus reveals no clinical and epidemiological but molecular differences. Int J Med Microbiol. 2013;303:76-83. http://dx.doi.org/10.1016/j.ijmm.2012.12.003 [ Links ]
44 . Grundmann H, Aanensen DM, van den Wijngaard CC, Spratt BG, Harmsen D, Friedrich AW, et al . Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: A molecular-epidemiological analysis. PLoS Med. 2010;7: e1000215. http://dx.doi.org/10.1371/journal.pmed.1000215 [ Links ]
45. Marimón JM, Villar M, García-Arenzana JM, Caba Ide L, Pérez-Trallero E. Molecular characterization of Staphylococcus aureus carrying the panton-valentine leucocidin genes in northern Spain. J Infect. 2012;64:47-53. http://dx.doi.org/10.1016/j.jinf.2011.10.010 [ Links ]
46. Rossney AS, Shore AC, Morgan PM, Fitzgibbon MM, O'Connell B, Coleman DC. The emergence and importation of diverse genotypes of methicillin-resistant Staphylococcus aureus (MRSA) harboring the Panton-Valentine leukocidin gene (pvl) reveal that pvl is a poor marker for community- acquired MRSA strains in Ireland. J Clin Microbiol. 2007; 45:2554-63. http://dx.doi.org/10.1128/JCM.00245-07 [ Links ]
47. Voyich JM, Otto M, Mathema B, Braughton KR, Whitney AR, Welty D, et al . Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin- resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761-70. http://dx.doi.org/10.1086/509506 [ Links ]
48. Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629-41. http://dx.doi.org/10.1038/nrmicro2200 [ Links ]
49. Otto M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus . Annu Rev Microbiol. 2010;64:143-62. http://dx.doi.org/10.1146/annurev.micro.112408.134309 [ Links ]
50. Sosa LM, Machuca-Pérez MA, Sosa CA, González CI. Infecciones por Staphylococcus aureus meticilino resistente en niños en Bucaramanga, Colombia. Salud UIS. 2010; 42:248-55. [ Links ]













