Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biomédica
Print version ISSN 0120-4157
Biomédica vol.35 no.3 Bogotá July/Sept 2015
https://doi.org/10.7705/biomedica.v35i3.2601
ARTÍCULO ORIGINAL
Martha C. Domínguez 1 , Mercedes Salcedo 2 , Felipe García-Vallejo 1
1 Laboratorio de Biología Molecular y Patogénesis, Departamento de Ciencias Fisiológicas, Escuela de Ciencias
Básicas, Facultad de Salud, Universidad del Valle, Cali, Colombia
2 Escuela de Bacteriología y Laboratorio Clínico, Facultad de Salud, Universidad del Valle, Cali, Colombia
Author´s contributions:
Martha C. Domínguez planned and carried out the experiments, and participated in the discussion, drafting and correction of the manuscript.
Mercedes Salcedo contributed intellectually to the project and participated in the discussion, drafting and correction of the manuscript.
Felipe García-Vallejo contributed with the main idea of the project, raised the funds for developing the research, and participated in the experimental design, the discussion, drafting and correction of the manuscript.
Recibido: 14/11/14; aceptado: 25/03/15
Introduction: To date there has been no statistical evaluation of the profiles of immunoglobulin classes and viral replication as variables in the study of HTLV-1 infection and circulation among families in virus-endemic areas of Colombia.
Objective: To evaluate the correlation of several immunological and molecular characteristics with the transmission and circulation of HTLV-1 among families in the town of Tumaco.
Materials and methods: Plasma levels of HTLV-1 specific immunoglobulin classes IgG, IgM and IgA1, as well as IgG and sIgA in oral fluids, were calculated for 32 members of 10 family groups from Tumaco in which the mother and at least one child were infected with the virus. Levels of the different immunoglobulin classes were correlated with viral RNA circulating in plasma or oral fluids and the proviral burden as detected by RT-PCR.
Results: Significant differences were determined between mothers and carrier children for immunoglobulin levels (p=0.037) and proviral burden (p=0.002). The overall estimate of IgG in plasma and sIgA in oral fluids could be correlated with the circulation of free viral RNA in both fluids and high proviral burden, and associated with HAM/TSP mothers. The detection of anti- tax IgG in plasma revealed differences between HAM/TSP mothers and their offspring.
Conclusion: The study of immunological and molecular variables permitted the analysis of HTLV-1 circulation among families of Tumaco. The strong correlation between levels of IgM specific for the virus and viral RNA circulating in fluids indirectly confirmed the transmission of HTLV-1 among families.
Key words: Human T-lymphotropic virus, molecular epidemiology, infection, virological analysis, immunoglobulins.
doi: http://dx.doi.org/10.7705/biomedica.v35i3.2601
Evaluación serológica y virológica de la infección por el virus linfotrópico de células T humanas de tipo 1 en grupos familiares de Tumaco, Colombia
Introducción. Todavía no hay una evaluación estadística de los perfiles de las clases de inmuno- globulina s y la replicación viral, como variables para estudiar la infección y la circulació n del HTLV-1 en familias de zonas endémicas en Colombia.
Objetivo. Evaluar la correlación de varias características inmunológicas y moleculares, con la transmisión y circulación del virus en familias del municipio de Tumaco.
Materiales y métodos. Se calcularon los niveles de IgG, IgM e IgA1 en plasma, e IgG y IgA secretoria en fluido oral, de 32 miembros de 10 grupos familiares de Tumaco, en los que la madre y, al menos, un hijo estaban infectados con el virus. La concentración de las diferentes clases de inmunoglobulinas se pudo correlacionar con la circulación de ARN viral libre en plasma y fluido oral, y la carga proviral, según su detección mediante reacción en cadena de la polimerasa de transcripción inversa.
Resultados. Se encontraron diferencias significativas en los niveles de inmunoglobulinas (p=0,037) y en la carga proviral (p=0,002) entre madres e hijos portadores. La estimación total de IgG en plasma e IgA secretoria en fluido oral, se pudo correlacionar con la circulación de ARN viral libre en ambos fluidos y una alta carga proviral, y se asoció con las madres paraparesia espástica tropical o mielopatía asociada con el HTLV-1. La detección en plasma de IgG anti-Tax reveló diferencias entre ellas y sus hijos.
Conclusión. El estudio de las variables inmunológicas y moleculares permitió analizar la circulación del HTLV-1 en familias de Tumaco. La fuerte asociación entre los niveles de IgM específica para el virus y el ARN viral circulante en los fluidos y la carga proviral, confirmó indirectamente la transmisión intrafamiliar del virus.
Palabras clave: virus 1 linfotrópico T humano, epidemiología molecular, infección, análisis virológico, inmunoglobulinas.
doi: http://dx.doi.org/10.7705/biomedica.v35i3.2601
Human T-cell lymphotropic virus (HTLV) type 1 was the first human retrovirus to be associated with T-cell malignancies (1,2). Infection with HTLV-1 is a growing worldwide health problem, with over 20 million people estimated to be infected (3). HTLV-1 infection is associated with a variety of human diseases, including an aggressive neoplastic disease known as adult T-cell leukemia (ATL) (4) and HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP), a chronic progressive inflammatory neurological disease (5). HTLV-1 causes disease in only a small fraction of infected subjects, the cumulative incidence of ATL among HTLV-1-infected carriers being estimated as 1-5% for both sexes in endemic areas (6), a rate similar to that of HAM/TSP (7).
An epidemiologically wide spectrum of HTLV-1-associated diseases, including uveitis (8) and infective dermatitis in children (9) has been reported. Moreover, inflammatory and immune-mediated conditions such as polymyositis, arthropathy, Sjögren´s syndrome and facial nerve palsy have also been associated with HTLV-1 infection, although no clear etiological relationship has been established to date (10,11).
F oci of HTLV-1 infection are found in geographical clusters affecting several million individuals world-wide (12). The infection is endemic to the Caribbean (13), southern Japan (14), several countries of Africa, the Middle East, Melanesia, Papua New Guinea (15) and South America (16). In Colombia, as in many other regions of the world, HTLV-1 appears to be geographically concentrated on the Pacific coast, as well as in the Andean and Caribbean regions (17,18). Tumaco, a town located on the Pacific coast of SW Colombia, was the first area characterized as being endemic for HTLV-1 infection, featuring elevated rates of HAM/TSP (19-21).
HTLV-1 is currently spread by sexual contact with an infected person, needle-sharing among injecting drug users or transfusions with infected blood. However, this last type of transmission route is less common in countries where blood is screened for HTLV-1 antibodies (22,23) or even blood-clotting factors (24). Mother-to-child transmission of HTLV-1 is one of the most important routes of viral transmission in endemic areas (25), based on previous reports showing infection to be more prevalent among breast-fed than bottle-fed children. Moreover, the incidence of seroconversion in children who were breast- fed over 12 months was higher than among those given infant formula (26,27 ). Although some studies have established the risk of transmission from infected mothers to offspring in family groups (19,28), to date there has been no statistical evaluation of immunoglobulin class profiles and viral replication among family groups in endemic areas of Colombia as variables to correlate HTLV-1 infection with circulation of virus.
The main objective of the present study was therefore to analyze the correlation between levels of immunoglobulins specific for HTLV-1 in infected family members with detection by RT-PCR of free genomic RNA circulating in plasma and oral fluids, as well as proviral loads (PVL) in peripheral blood mononuclear cells (PBMC) among 10 family groups from Tumac o.
Materials and methods
The sample
We conducted a cross-sectional study of HTLV-1 class-specific antibody, proviral load and free RNA in plasma and oral fluids among family groups positive for HTLV-1 in Tumaco (1°48 ¢ 24 ² N, 78°45 ¢ 53 ² W), a town of approximately 58,000 inhabitants on the Pacific Coast of SW Colombia. The optimal sample size was calculated using the Epi Info , version 3.5.1. Based on the above population Trujillo , et al. (20) , previously reported the overall prevalence rate of HTLV-I antibodies in Tumaco as being 2.8%. The minimum representative sample size for the study was therefore 188 subjects with a = 5 %.
For the current study, a total of 212 individuals belonging to several family groups in Tumaco were voluntarily enrolled. Peripheral blood samples were obtained by venal puncture. Plasma and PBMC were obtained using the Histopaque-1077 ® gradient (Sigma-Aldrich, St. Louis, MO). In addition, oral fluid samples were taken using the FDA- approved commercial device Orasure (Epitope Inc., Beaverton, OR) according to the manufacturer´s instructions.
All plasma samples were screened for HTLV infection by ELISA (Murex Biotech Limited, Dartford, UK) and were further confirmed as HTLV-1 positive by Western Blot (Genelabs Diagnostics Pte Ltd., Singapore Science Park, Singapore). Stringent Western Blot confirmatory criteria were applied and a sample was considered to be HTLV-1 positive only if it exhibited antibodies against one Gag (p19 with or without p24) and two Env (GD21 and rgp46-I). Additionally, a PCR diagnosis was carried out with the pol primers SK110 (5´-CCCTACAATCCAACCAGCTCAG-3´ (nucleotide 4758-4779 (GenBank accession No.J02029), and SK111-1 (5´-GTGGTGAAGCTGCCATCGGG TTTT-3´, nucleotide 4947-4925) to amplify a 159 bp fragment DNA from PMBC DNA of the individuals included in the study. Only 10 of the 14 family groups with HTLV-1 infected members were included in the analysis, each of them with a mother and at least one offspring infected. The mothers had each breastfed their offspring for at least six months, but were not doing so at the time of sample collection .
Immunoglobulin class detection
The ELISA Murex HTLV I + II (Murex Biotech Limited, Dartford , UK) diagnostic test was used for screening with ratios of 1:40 for plasma and 1:5 for oral fluids used to test every paired sample. Samples were incubated in 1% bovine serum albumin, phosphate-buffered saline (PBS) (pH7.4) at 42°C for 1h. Microplates were washed twice and incubated with goat IgG-anti IgG-, IgM (Sigma Chemical Co., St. Louis, MO) and sIgA (Chapel, Organon Teknika, West Chester, PA) at peroxidise-labelled conjugate dilutions of 1:15,000, 1:1,000 and 1:2,000, respectively. The procedure was carried out following the manufacturer´s instructions. ELISA OD measurements were performed in a Spectra II microtiter reader (Molecular Devices, Sunnyvale, CA); cut-offs were calculated as recommended by the manufacturers. The results of ELISAs with oral fluids samples were expressed as OD ratios (OD sample/OD cut-off) and normalized for salivary protein content. The determination of protein con centration in oral fluids was performed using the Bradford colorimetric method (Bio-Rad, Hercules, CA) (18). An OD ratio = 1.1 was considered to be positive.
A tax recombinant protein (29) was used to detect anti- tax antibodies in plasma by Western blotting. Screening of IgG subclass for the recombinant tax protein was also performed with the plasma of all infected family members recruited for the study. Western blot procedures were similar to those previously described by Okayama, et al. (29).
DNA extraction and PCR protocols
High molecular weight DNA was extracted from PBMCs by a classical phenol-chloroform technique (30). DNA from subjects enrolled in the study was used to amplify a 1033 bp pol-env fragment by PCR. Each PCR reaction mixture contained 1.0 µg of DNA, 0.2 mM deoxynucleoside triphosphate mix (Boehringer Mannheim, Ridgefield, CT), 10 µl of a 10x reaction buffer, 25 mM of each oligonucleo- tide primer (pol SK110-1 (4773) CCATACAACCCA CCAGCTCAG (4794) and reverse env3 (5827) CCATACAACCCCACAGCTCAG (5806) and 2.5 U of Taq DNA polymerase (Perkin - Elmer Cetus) in a total volume of 100 µl. Cycling conditions were: one cycle of 5 min at 94°C, followed by 30 cycles at 94°C for 2 min, 55°C for 2 min, 72°C for 2 min and a final cycle of 72°C for 10 min. The amplification products were visualized by electrophoresis in a 1.5% agarose gel (ultrapure agarose; Bethesda Research Laboratories). All PCR products were Southern blot hybridized with pMT2 DNA as an HTLV-1 probe.
Extraction of RNA from plasma and oral fluids
RNA from 200 µl of plasma and 600 µl of oral fluids were extracted using the QIAamp viral RNA mini kit (Qiagen Co., Valencia, CA) following the manufacturer´s instructions. The total RNA was suspended in 50 µl of bidestilated-diethyl pyrocarbonate-treated water and stored at -20°C.
Reverse transcriptase-PCR procedures
Amplification of HTLV-1 free genomic RNAs from plasma and oral fluids samples was performed using a thermostable DNA polymerase from Thermu s thermophilus (Tth-DNA polymerase, Boerhinger Mannheim, Ridgefield, CT). cDNA synthesis was carried out for 30 min at 70°C in a reaction mix containing 10 mM Tris-HCl (pH 8.9), 90 mM KCl (1X RT buffer), 0.9 mM MnCl, 375 µM of each deoxyribonucleoside triphosphate (dNTP) and 750 nM of primer specific for HTLV-1 pol (SK111 GTGGTGGATTTGC-CATCGGGTTTT ) for envelope (env3 -CAAAAAATTGACCTGTTCCCAGT-CCTC) and tax (SK44 GAGCCGATAACGGCGTCCATCG), 100 µg/ml of total plasma and salivary RNA and 4 U of Tth DNA polymerase. The synthesized specific HTLV-1 cDNA was subjected to PCR in 10 mM Tris-HCl (pH 8.9), 100 mM KCl, (1X PCR buffer), 1.25 mM MgCl2, 0.75 mM EDTA, and 150 nM HTLV-1 forward primer. Sk110 primer (CCCTACAATCCAACCAGCTCAG positions 4757- 4778) was used to amplify a 159 bp pol fragment. Annealing conditions were 65°C for 45 s followed by an extension reaction at 72°C for 1 min. The pTU1 primer ( CTTCGTGGACCCTCGACC positions 5344-5366) was used to amplify a 486 bp fragment from the env surface domain. Annealing was per- formed at 55°C for 45 s followed by an extension reaction at 72°C for 1 min. The SK 43 primer (GTACCGGTGGCGGTCTGG-CTT positions 7358- 7377) was used to amplify a 138 bp fragment from the tax gene. Annealing and extension conditions were the same as described for the amplification of 486 bp envelope fragments. All amplification procedures were repeated for 40 cycles. The amplified products were resolved by agarose gel e lectrophoresis and the respective HTLV-1 amplified fragments determined by Southern blot hybridizatio n using specific 32P-labeled oligonu-cleotides Pol-001 (TTACTGACAAACCCGACCTA CCC) (4811-4824) for pol; PTU5 (GAAGTTTCA GCACGATGTCAATT) (5581-5603) for env, and PTAX5 (GACTATGTTCGGCCCGCCTACAT (7400-7423) for tax, as probes.
Many control experiments were performed to adjust not only RT-PCR conditions but the specificity of amplification . RNA from MT-2 cells was treated with RNAse or RNAse free/DNAse. No amplification of specific HTLV-1 gene fragments was observed with RNAse-treated RNA. Similar results were obtained with RNA from supernatants of MT-2 cell cultures that were submitted to the same procedure. The minimum amount of RNA detected by RT-PCR was 10 pg/ml calculated from a semiquantitative RT-PCR of RNA using the pol primers; this is equiva-lent to approximately 100 copies of pol fragment per mL of sample.
Quantification of HTLV-I proviral load
Two sequence-specific primers that detect HTLV-I env region were used to amplify a 185 bp fragment by real-time assay. The sequences of HTLV-I env primers were: 5´-ATCCACTTGGCACGTCCTATA-3´ (5890–5910, GenBank accession Nº NC 001436) and 5´-GCAGGATGAGGGAGTTATGTC-3´ (6054– 6074). The dual-labeled fluorescent probe was FAM -5´-CTTTACCCATCGTTAGCGCTTCCAGCCCCC- 3´-BHQ1 (5954–5983). In order to determine the amount of cellular human beta-globin DNA as internal amplification control of differences in PBMC count and DNA extraction, all samples were run in parallel. A 187 bp fragment of beta-globin gene was amplified by forward primer 5´-GGTATCCTTTTTACAGCA CAA C-3´ and reverse primer 5´-CAGGTCCCCAAAGGACTC G-3´ in a real-time PCR assay. The fluorogenic probe used to detect the b -globin gene was 5´- CCTGGGCTGTTTTCATTTTCTCAGG-3, BHO-2 (471-495) (Biosearch Technologies Inc., Novato, CA). To determine the sensitivity for this assay, scalar dilutions of pMT2 clone DNA ranging from 1 to 10 9 copies were analyzed. The results indicated that a positive signal was consistently detected at HTLV-1 DNA concentrations above 1 copy per ng of DNA. Based on these results, the limitation of this assay was considered to be 1 copy of HTLV-1 proviral DNA per nanogram of genomic DNA.
HTLV-1 env and beta-globin gene fragments were amplified separately in a Mx3000P Real-Time PCR System (Stratagene, La Jolla, CA) in 50 µl reaction mix of 10 µl of DNA sample, 25 µl of Brilliant QPCR Master Mix (containing PCR buffer, SureStart Taq DNA polymerase) (Stratagene, La Jolla, CA), 10 pmol of each primer, and 5 pmol of TaqMan probe. Thermal cycling conditions were as follows: 95°C for 10 min followed by 45 cycles of 95°C for 30 s, 55°C for 1 min and 72°C for 30 s. Each sample was analyzed in duplicate. The HTLV-1 proviral load (PVL) was calculated as the copy number of each per ng of genomic DNA.
Statistical analysis
Data analyses were performed with the Stata- graphics package (Centurion XVI Inc.). A significant sample size was calculated with Epi Info, version 3.5.1 software. The Fisher exact test was applied to estimate statistical differences among HAM/ TSP, AC, sex, age and immunoglobulin class and subclass values. A non-parametric Mann-Whitney test was used to evaluate the average levels of immunoglobulin class and proviral load between mothers and their offspring. Linear regression was applied to calculate the correlation coefficient values between immunoglobulin class levels in plasma and oral fluids with proviral load.
The following expression was used to calculate the General Vertical Transmission (GVT) rate:
GVT= [Number of seropositive offspring / Total of offspring of the sample] x [Serum prevalence]
Based on previous data from Trujillo, et al. (20), the HTLV-1 serum prevalence among inhabitants of Tumaco is 2.8%.
Ethical considerations
The study protocol was approved by the Ethics Committee of the Universidad del Valle in agreement with the guidelines of World Medical Association Declaration of Helsinki and Resolution 008430/1993 of the Colombian Ministry of Health. All adult participants (or, in the case of children, their parents) were informed about the purpose of the study and gave their consent to use the collected samples only for research under a guarantee of remaining anonymous.
Results
Characteristics of the family groups
All 46 (21.7%) of the 212 participants in the study were confirmed as being HTLV-1 seropositive with 16 of them (34.8%) clinically diagnosed as HAM/TSP patients. The mean number of offspring per family was 3.4 ± 2.2. Fourteen families had at least one member infected with HTLV-I and 19/69 offspring were HTLV-1 seropositive, corresponding to a GVT rate of 0.66% [0.55-0.77].
Ten (71.4%) families that met the criteria previously cited (an infected mother and at least one HTLV-1 seropositive offspring) were studied. Family relation-ships, age and ethnic background are documented in table 1. The mean age for HTLV-1 infected mothers was 45.4 ± 7.02 years old and that of offspring was 12.4 ± 5.05 years. The mean age of infected members per family corresponded to 3.26 ± 1.2 and the ratio of offspring per infected mother was 1.7. Four of the families had HAM/TSP mothers (mean age, 47.7 ± 4.9 years old); only one young man aged 15 in family 2 had a preliminary diagnosis of HAM/TSP; the remaining offspring were clinically classified as asymptomatic (table 1).
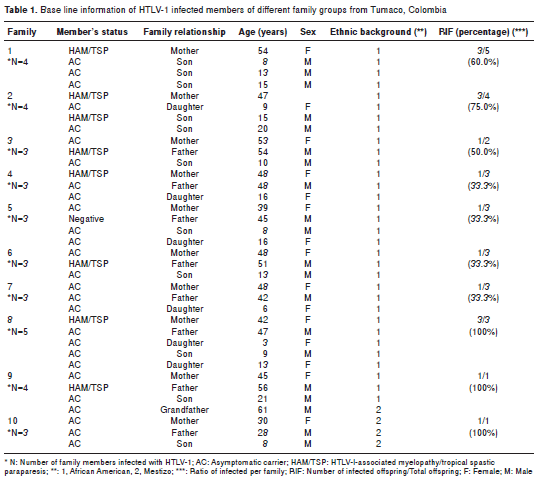
Immunoglobulin class and subclass
As it is shown in figure 1A, only the HAM/TSP mothers showed higher levels of IgG (p<0.001), IgM (p<0.001) and IgA1 (p<0.005) in plasma in comparison with asymptomatic mothers and offspring. Only one asymptomatic young male of family 1 exhibited increased levels of IgM and IgA1 in plasma. These levels contrasted with the findings for the 15 year-old male of family 2 who had a preliminary diagnosis of HAM/TSP but no IgM and IgA1 in his plasma.
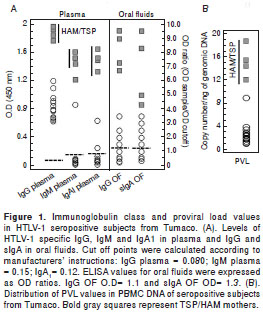
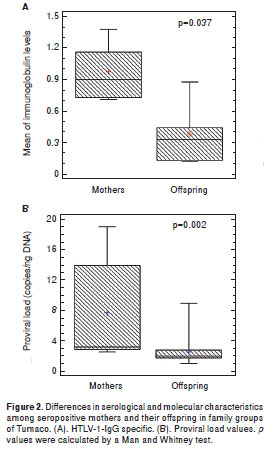
When the immunoglobulin class levels in oral fluids were studied it was possible to observe significant differences for IgG (p<0.001) and sIgA (p<0.001) between HAM/TSP mothers and their asympto matic offspring (figure 1A). It was interesting to note that the asymptomatic mothers of families 5 and 9 showed HTLV-1 specific IgG in oral fluids. Anti- tax antibodies were detected in the plasma of 100% of the infected mothers but only in 12/17 (70.5%) of descendants ( p <0.05). The mean age of anti- tax reactive offspring was 11.1 ± 3.6 years. Anti- tax IgG1 was recorded in all tax -reactive plasma samples; however, an extra reaction of anti- tax IgG3 antibodies could only be detected in HAM/TSP mothers. Statistically significant differences in the median levels of IgG in plasma were recorded between mothers and their offspring (Mann-Whitney test; p=0.037) (figure 2A).
Free HTLV-1 RNAs templates in plasma and oral fluids
Free HTLV-1 RNA templates for pol, env and tax were detected in plasma samples of all HAM/ TSP mothers; these results contrasted with those for most asymptomatic HTLV-1 positive mothers except for the one of family 5 (table 2). The situation in asymptomatic offspring was similar to that of asymptomatic mothers, with the exception of a 15 year-old youth with a preliminary diagnosis of HAM/TSP who had a similar pattern to those of HAM/TSP mothers (table 2).
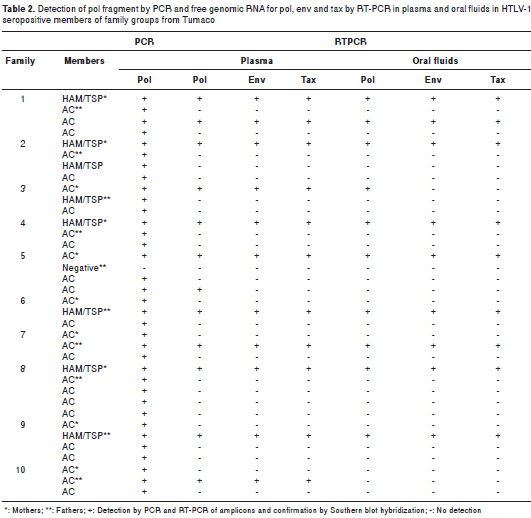
The oral fluids of all HAM/TSP mothers and the young male from family 2 amplified free HTLV-1 RNAs templates for pol, env and tax ; in contrast, the asymptomatic mothers and their offspring did not reveal any genomic RNA circulating in their oral fluids (table 2).
Proviral load correlation with IgG and IgM levels in plasma
The PVL values for all individuals included in this study are shown in figure 1B (median of 4.56), illustrating the wide range of PVL values, with the highest corresponding to HAM/TSP mothers. The mean PVL calculated in PBMC for the mothers was 7.78 ± 6.45 copies/ng DNA vs. 2.63 ± 1.84 for their offspring (p<0.001). Only the 15 year-old of family 2 with the preliminary diagnosis of HAM/TSP had a PVL value of 9.0 copies/ng DNA. The differences between the proviral loads of mothers and their offspring were statistically significant (Mann-Whitney test, p=0.002) (figure 2B).
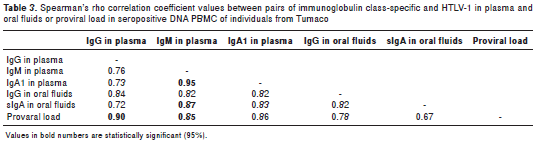
Several correlation analyses were performed between pairs of variables in the study. As shown in table 3 the highest values were recorded for IgG vs. PVL and IgM vs. IgA1 in plasma. A positive regression model between IgG plasma levels and PVL provided the linear equations IgG= 0.6461 + 0.0873 x PVL (r=0.90; p<0.001) (table 3). The linear equation for IgM vs. IgA1 in plasma was IgM Plasma=-0.0245122 + 0.964651 x IgA1 Plasma (r=0.95; p<0.001).
Discussion
We studied the associations between several serological and molecular biological characteristics during HTLV-1 transmission from mother to child. Our results support the existence of a significant correlation between detectable levels of HTLV-1 specific IgM in plasma and sIgA in oral fluids with PVL in PBMC from HAM/TSP mothers included in the study. Moreover, differences were also recorded between sIgA levels in oral fluids, as well as for RT-PCR data between HAM/TSP patients and AC. These results provided strong evidence that HTLV-1 infection in the oral cavity was not restricted to the oral mucosa cells of HAM/TSP mothers and their asymptomatic offspring. Our data also revealed vertical transmission via oral mucosa from mothers to their offspring in a sample of seropositive family members from Tumaco.
Previous reports showed an association of the presence in HAM/TSP patients of free genomic HTLV-1 RNAs with an active production of sIgA in oral fluids and viral mRNA transcription in epithelial cells of oral mucosae (31,32). Thus the differences recorded betw een HAM/TSP mothers and asympto matic carriers in the mucosal associated response suggest that both immunological and cellular changes occur during progression to disease.
The immune status of HAM/TSP mothers included in the current study, with anti-HTLV-1 IgG in plasma and sIgA in oral fluids, as well as free viral RNA templates in saliva, suggest that HTLV-1 infection of epithelial targets could possibly occur by cell-to-cell contact with HTLV-1 infected oral fluids lymphocytes or during breastfeeding. However, it remains to be determined whether those lymphocytes circulate for longer in saliva or are newly deposited in saliva and milk by leaking from microcirculation as has been reported previously (32-35). There is some indirect evidence, both from our own study and previous work for HTLV-1 infection of oral mucosa by cell-to-cell contact with circulating T cells, primarily through breast-feeding (32,36-38).
Although our results indirectly showed active infection of oral mucosa to be correlated with an active immune process via specific anti- tax HTLV-1 sIgA, there still remains the question of whether oral fluids and/or saliva really are infective. There is some evidence to indicate that inoculation of salivary gland cells and cell-free saliva from healthy carriers and patients with HAM/TSP into WKA and F344 female rats does produce a successful infection, as confirmed by nested PCR from inoculated rat DNA, HTLV-1 proviruses in PBMC, spleen, thymus, salivary glands, spinal cord, kidney and brain (39).
Anti- tax IgG1 was recorded in all tax -reactive plasma samples; however, an extra reaction of anti- tax IgG3 antibodies could only be detected in HAM/TSP mothers. In general, anti-viral IgG antibodies are highly restricted to IgG1 and IgG3; however, IgG3 antibodies appear first in the course of infection and are associated with inflammation.
The proviral load with tax may affect cellular genes such as cytokines and oncogenes, besides being involved in pathogenicity. A significant increase of tax expression was observed in PBMCs of HAM/TSP patients compared to healthy carriers, this being associated with PVL in patients (40). Although proviral load has been considered as a valuable parameter for monitoring HTLV-1 infected subjects, previous studies have demonstrated that tax expression in activated PBMCs and proviral load assessment in HAM/TSP patients were more reliable in determining prognoses as well as monitoring healthy carriers and HAM/TSP patients (40).
Transmission of HTLV-1 within families has been documented in different epidemiological studies carried out in several endemic areas around the world (41-43). However, the study reported here is the first in Colombia to follow up HTLV-1 infection dynamics in family clusters using immunological, virological and molecular markers. The results are important given that Colombia has many HTLV-1 endemic areas. Our data suggest that viral transmission may occur when mothers have active infections while breastfeeding their children. Although none of the mothers in the study were breastfeeding at the time samples were collected, the detection of virus in plasma and oral fluids and the high proviral load in lymphocytes pro vided strong evidence for a correlation with viral gene expression in cells, particularly those of the oral epithelium.
Many HTLV-1 infected individuals in Colombia live below the poverty line in areas with poor health facilities. Since measurement of proviral load for HTLV-1 is an expensive and time-consuming process, affordable laboratory tests need to be developed and applied to evaluate not only immunoglobulin class disturbance but also to follow up virus transmission among family groups. The results of the present study lead us to the conclusion that quantificationS by ELISA of HTLV-1 IgG and IgM in plasma are procedures that allow prognoses to be made of the replication status of the infected individuals instead of PVL. The evaluation of those serological variables is crucial to performing public health surveillance of virus circulation in families, particularly in areas that are highly endemic for HTLV-1 .
The authors declare they have no conflict of interest regarding the content of this article.
This work was supported by funds from the Departamento Administrativo de Ciencia, Tecno-logía e Innovación , Colciencias (grant number 1106-04-199-96) and the internal budget of the Vicerrectoría de Investigación , Universidad del Valle (contract number 1644-2011).
Corresponding author: Felipe García-Vallejo, Departamento de Ciencias Fisiológicas, Escuela de Ciencias Básicas, Facultad de Salud, Universidad del Valle, P.O. Box 25360, Cali, Colombia Telephone: (572) 518 5601; fax: (572) 518 5617 labiomol@gmail.com
1. Poiesz BJ, Ruscetti FW, Gazdar AF, Bunn PA, Minna JD, Gallo RC. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc Natl Acad Sci USA. 1980;77:7415-9. [ Links ]
2. Poiesz BJ, Ruscetti FW, Reitz MS, Kalyanaraman VS, Gallo RC. Isolation of a new type C retrovirus (HTLV) in primary uncultured cells of a patient with Sezary T-cell leukemia. Nature. 1981;294:268-71. [ Links ]
3. Hlela C, Shepperd S, Khumalo NP, Taylor GP. The prevalence of human T-cell lymphotropic virus type 1 in the general population is unknown. AIDS Rev. 2009;11:205-14. [ Links ]
4. Yoshida M, Miyoshi I, Hinuma Y. A retrovirus from human leukemia cell lines: Its isolation, characterization, and implication in human adult T-cell leukemia (ATL). Princess Takamatsu Symp. 1982;12:285-9. [ Links ]
5. Osame M, Usuku K, Izumo S, Ijichi N, Amitani H, Igata A, et al . HTLV-I associated myelopathy, a new clinical entity. Lancet. 1986;1:1031-2. [ Links ]
6. Murphy EL, Hanchard B, Figueroa JP, Gibbs WN, Lofters WS, Campbell M , et al . Modelling the risk of adult T-cell leukemia/lymphoma in persons infected with human T-lymphotropic virus type I. Int J Cancer. 1989;43:250-3. [ Links ]
7. Orland JR, Engstrom J, Fridey J, Sacher RA, Smith JW, Nass C, et al. Prevalence and clinical features of HTLV neurologic disease in the HTLV Outcomes Study. Neurology. 2003;61:1588-94. [ Links ]
8. Mochizuki M, Watanabe T, Yamaguchi K, Tajima K, Yoshimura K, Nakashima S, et al . Uveitis associated with human T lymphotropic virus type I: Seroepidemiologic, clinical, and virologic studies. J Infect Dis. 1992;166:943-4. [ Links ]
9. La Grenade L, Hanchard B, Fletcher V, Cranston B, Blattner W. Infective dermatitis of Jamaican children: A marker for HTLV-1infection. Lancet. 1990;336:1345-7. [ Links ]
10. Terada K, Katamine S, Eguchi K, Moriuchi R, Kita M, Shimada H, et al . Prevalence of serum and salivary antibodies to HTLV-1 in Sjogren´s syndrome. Lancet. 1994;344:1116-9. [ Links ]
11. Bartholomew C, Cleghorn F, Jack N, Edwards J, Blattner W. Human T-cell lymphotropic virus type I-associated facial nerve palsy in Trinidad and Tobago. Ann Neurol. 1997;41:806-9. [ Links ]
12. Proietti FA, Carneiro-Proietti AB, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-1infection and associated diseases. Oncogene. 2005;24:6058-68. [ Links ]
13. Murphy EL, Figueroa JP, Gibbs WN, Holding-Cobham M, Cranston B, Malley K, et al . Human T-lymphotropic virus type I (HTLV-I) seroprevalence in Jamaica. I: Demographic determinants. Am J Epidemiol. 1991;133:1114-24. [ Links ]
14. Mueller N, Okayama A, Stuver S, Tachibana N. Findings from the Miyazaki cohort study. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13:S2-7. [ Links ]
15. Watanabe T. Current status of HTLV-1 infection. Int J Hematol. 2011;94:430-4. [ Links ]
16. Carneiro-Proietti AB, Catalan-Soares BC, Castro-Costa CM, Murphy EL, Sabino EC, Hisada M, et al . HTLV in the Americas: Challenges and perspectives. Rev Panam Salud Pública. 2006;19:44-53. [ Links ]
17. Chávez M, Domínguez MC, Blank A, Quintana M, Eizuru Y, García F. Molecular evolution and geographic origins of type 1 human lymphotrophic virus in Colombia detected by RFLP polymorphism. Biomédica. 2004;24:20-32. [ Links ]
18. Quintana M, Villalobos J, Domínguez MC, Tamayo O, García-Vallejo F. Estudio de la seroprevalencia de la infección por los virus linfotrópicos humanos (HTLV) I y II en poblaciones del departamento de Córdoba, Colombia. Colomb Méd. 2004;35:22-30. [ Links ]
19. Arango C, Concha M, Zaninovic V, Borrero I. Epidemiology of tropical spastic paraparesis in Colombia and associated HTLV-I infection. Ann Neurol. 1988;23:s161-5. [ Links ]
20. Trujillo CM, Concha M, Muñoz A, Bergonzoli G, Mora C, Borrero I, et al . Seroprevalence and cofactors of HTLV-I infection in Tumaco, Colombia. AIDS Res Hum Retroviruses. 1992;8:651-57. [ Links ]
21. Balcázar N, Sánchez GI, García-Vallejo F. Sequence and phylogenetic analysis of human T cell lymphotropic virus type 1 from Tumaco, Colombia. Mem Inst Oswaldo Cruz. 2003;98:641-8. [ Links ]
22. Okochi K, Sato H, Hinuma Y. A retrospective study on transmission of adult T cell leukemia virus by blood transfusion: Seroconversion in recipients. Vox Sang. 1984;46:245-53. [ Links ]
23. Gonçalves DU, Proietti FA, Ribas JG, Araújo MG, Pinheiro SR, Guedes AC, et al . Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin Microbiol Rev. 2010;23:577-89. [ Links ]
24. Manns A, Murphy EL, Wilks R, Haynes G, Figueroa JP, Hanchard B, et al . Detection of early human T-cell lympho-trophic virus type I antibody patterns during seroconversion among transfusion recipients. Blood. 1991;77: 896-905. [ Links ]
25. Kawase K, Katamine S, Moriuchi R, Miyamoto T, Kubota K, Igarashi H, et al . Maternal transmission of HTLV-I other than breast milk: Discrepancy between the polymerase chain reaction positivity of cord blood samples for HTLV-I and the subsequent seropositivity of individuals. Jpn J Cancer Res. 1992;83:968-77. [ Links ]
26. Moriuchi H, Masuzaki H, Doi H, Katamine S. Mother-to-child transmission of human T-cell lymphotropic virus type 1. Pediatr Infect Dis J. 2013;32:175-7. [ Links ]
27. Hino S, Katamine S, Miyata H, Tsuji Y, Yamabe T, Miyamoto T. Primary prevention of HTLV-I in Japan. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;13(Suppl.1):S199-203. [ Links ]
28. Houinato D, Verdier M, Preux PM, Josse R, Letenneur L, Ayed Z, et al . Intrafamilial clustering and 4-year follow-up of asymptomatic human T-cell leukaemia virus type I (HTLV-I) infection in Benin (West Africa). Int J Epidemiol. 1998;27:146-52. [ Links ]
29. Okayama A, Cehn YM, Tachibana N, Shiori S, Lee T H, Tsuda K, et al . High incidence of antibodies to HTLV-I-tax in blood relatives of adult T cell leukemia patients. J Infect Dis. 1991;163:47-52. [ Links ]
30. Ramírez-Solis R, Rivera-Pérez J, Wallace JD, Wims M, Zheng H, Bradley A. Genomic DNA microextraction: A method to screen numerous samples. Anal Biochem. 1992;201:331-5. [ Links ]
31. Soto-Ramírez LE, García-Vallejo F, Renjifo B, Vergara, A, Borrero I, Marlink R, et al . Human T-Lymphotropic virus type 1 (HTLV-1)-specific antibodies and cell-free RNA in crevicular fluid rich saliva from patients with tropical spastic paraparesis/HTLV-1-associated myelopathy. Viral Immunol. 1995;8:141-50. [ Links ]
32. Domínguez MC, González N, Sánchez A, García F. Human T-Lymphotropic Virus (HTLV) type I in vivo integration in oral keratinocytes. Braz J Microbiol. 2011;42:310-20. [ Links ]
33. Balestrieri E, Ascolani A, Igarashi Y, Oki, T, Mastino A, Balzarini J, et al. Inhibition of cell-to-cell transmission of human Tcell lymphotropic virus type 1 in vitro by carbohydrate-binding agents. Antimicrob Agents Chemother. 2008;52:2771-9. [ Links ]
34. Yamamoto T, Terada K, Nishida N, Moriuchi R, Shirabe S, Nakamura T, et al . Inhibitory activity in saliva of cell-to-cell transmission of human T-cell Lymphotropic virus type 1 in vitro: Evaluation of saliva as an alternative source of transmission. J. Clin. Microbiol. 1995; 33:1510-15. [ Links ]
35. Li HC, Biggar RJ, Miley WJ, Maloney EM, Cranston B, Hanchard B, Hisada M. Provirus load in breast milk and risk of mother-to-child transmission of human T lymphotropic virus type I. J Infect Dis. 2004;190:1275-8. [ Links ]
36. Carles G, Tortevoye P, Tuppin P, Ureta-Vidal A, Peneau C, El Guindi W, et al . HTLV1 infection and pregnancy. J Gynecol Obstet Biol Reprod (Paris). 2004;33:14-20. [ Links ]
37. Hisada M, Maloney EM, Sawada T, Miley WJ, Palmer P, Hanchard B, et al. Virus markers associated with vertical transmission of human T lymphotropic virus type 1 in Jamaica. Clin Infect Dis. 2002;34:1551-7. [ Links ]
38. Ureta-Vidal A, Angelin-Duclos C, Tortevoye P, Murphy E, Lepère JF, Buigues RP, et al. Mother-to-child transmission of human T-cell-leukemia/lymphoma virus type I: Implication of high antiviral antibody titer and high proviral load in carrier mothers. Int J Cancer. 1999;82:832-6. [ Links ]
39. Shohat M, Shohat, B, Achiron A. Human T-lymphotropic virus type-1 (HTLV-1) in Israeli patients and their family relatives and its transmission to rats. Med Microbiol Immunol. 2006;195:93-9. [ Links ]
40. Yari A, Rezaee SA, Valizadeh N, Rajaee T, Jazayeri SM, Soltani M, et al . Evaluation of HTLV-1 activity in HAM/TSP patients using proviral load and Tax mRNA expression after in vitro lymphocyte activation. Iranian J Basic Med Sci. 2014;17:531-6. [ Links ]
41. Iwahara Y, Takehara N, Kataoka R, Sawada T, Ohtsuki Y, Nakachi H, et al . Transmission of HTLV-I to rabbits via semen and breast milk from seropositive healthy persons. Int J Cancer. 1990;45:980-3. [ Links ]
42. Gastaldello R, Otsuki K, Barbas MG, Vicente AC, Gallego S. Molecular evidence of HTLV-1 intrafamilial transmission in a non-endemic area in Argentina. J Med Virol. 2005;76:386- 90. [ Links ]
43. Cooper CM, James K, Wilks RJ. HTLV-1 related knowledge, attitude and behavior patterns among mothers who participated in the Jamaica Breastfeeding Intervention Study (1996-2000). West Indian Med J. 2010;59:35-40. [ Links ]













