Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Biomédica
versión impresa ISSN 0120-4157
Biomédica vol.35 no.4 Bogotá oct./dic. 2015
https://doi.org/10.7705/biomedica.v35i4.2690
BRIEF COMMUNICATION
doi: http://dx.doi.org/10.7705/biomedica.v35i4.2690
1 Grupo de Neurociencias de Antioquia, Facultad de Medicina, Universidad de Antioquia, Medellín, Colombia
2 Grupo de Mapeo Genético, Facultad de Medicina, Universidad de Antioquia, Medellín, Colombia
Author´s contributions:
Margarita Giraldo-Chica, Natalia Acosta-Baena, LinaVelilla, Francisco Lopera and Nicolás Pineda: Data collection, manuscript reviewing and preparation Lorena Urbano and Nicolás Pineda: Genetic analysis
Recibido: 03/02/15; aceptado: 17/06/15
Introduction: Cerebrotendinous xanthomatosis is an infrequent cause of dementia. It is an autosomal recessive disorder with clinical and molecular heterogeneity.
Objective: To identify the presence of a possible mutation in a Colombian family with several affected siblings and clinical characteristics compatible with cerebrotendinous xanthomatosis associated to early dementia.
Materials and methods: We studied a series of cases with longitudinal follow-up and genetic analysis.
Results: These individuals had xanthomas, mental retardation, psychiatric disorders, behavioral changes, and multiple domains cognitive impairment with dysexecutive dominance that progressed to early dementia. CYP27A1 gene coding region sequencing revealed a novel mutation ( c.1183_1184insT ).
Conclusion: The mutation found in this family is responsible for the described dementia features. Early identification of familial history with mental retardation, xanthomas and cognitive impairment might prevent the progression to this treatable type of dementia. Even though this mutation lies in the most frequently mutated codon of CYP27A1 gene, it has not been reported previously.
Key words: Xanthomatosis, schizophrenia, dementia, genetics.
doi: http://dx.doi.org/10.7705/biomedica.v35i4.2690
Nueva mutación para xantomatosis cerebrotendinosa causa demencia familiar temprana en Colombia
Introducción. La xantomatosis cerebrotendinosa es una causa poco frecuente de demencia. Es un trastorno autosómico recesivo con heterogeneidad clínica y molecular.
Objetivo. Identificar la presencia de una posible mutación en una familia colombiana con varios hermanos afectados y con características clínicas indicativas de xantomatosis cerebrotendinosa asociada a demencia precoz.
Materiales y métodos. Se estudió una serie de casos, con seguimiento longitudinal y análisis genético.
Resultados. Estos individuos tenían xantomas, retraso mental, trastornos psiquiátricos, cambios de comportamiento y alteraciones cognitivas de múltiples dominios con predominio de la disfunción ejecutiva, que progresaron a demencia temprana. Se identificó una nueva mutación (c.1183_1184insT) en el gen CYP27A1 .
Conclusión. La mutación encontrada en esta familia es responsable de la demencia descrita en los sujetos de estudio. La detección temprana de una historia familiar con retraso mental, xantomas y deterioro cognitivo, podría prevenir la progresión de este tipo de demencia tratable. A pesar de que esta mutación se encuentra en el codón más frecuentemente mutado del gen CYP27A1 , no se había informado anteriormente.
Palabras clave: xantomatosis, esquizofrenia, demencia, genética .
doi: http://dx.doi.org/10.7705/biomedica.v35i4.2690
Cerebrotendinous xanthomatosis is an autosomal recessive disorder of bile acid biosynthesis caused by the abnormal deposition of cholestanol in the body due to a deficiency of the mitochondrial enzyme 27-sterol hydroxylase (CYP27). CYP27A1 gene mutations are the only cause associated with cerebrotendinous xanthomatosisto date (1). The first mutation in the CYP27A1 gene was described Cerebrotendinous xanthomatosis is an autosomal recessive disorder of bile acid biosynthesis caused by the abnormal deposition of cholestanol in the body due to a deficiency of the mitochondrial enzyme 27-sterol hydroxylase (CYP27). CYP27A1 gene mutations are the only cause associated with cerebrotendinous xanthomatosisto date (1). The first mutation in the CYP27A1 gene was described in 1991 (2), but clinical characteristics were first noted by van Bogaert in 1937 (3). The clinical picture depends on the location of the deposits of cholestanol from childhood to adulthood. The brain, tendons and crystalline lens are the tissues primarily affected . Therefore, common clinical findings in cerebrotendinous xanthomatosisinclude chronic diarrhea in childhood, psychomotor retardation, tendon xanthomas, juvenile cataracts, and progressive dementia with cerebello-pyramidal signs, osteoporosis and premature atherosclerosis (4). The diagnosis can be performed based on the determination of serum levels of cholestanol or based on the genetic study by sequencing gene CYP27A1 . The findings of magnetic resonance imaging (MRI) typically include bilateral and symmetric hyperintensity in T2 of the dentate nuclei, periventricular white matter, basal ganglia and brainstem in addition to widespread cortico-subcortical atrophy (5). Clinical improvement of individuals with cerebrotendinous xanthomatosis following chenodeoxycholic acid treatment has been reported (6).
CYP27A1 is located on human chromosomal region 2q35. It has nine exons spanning 18.6 kb of DNA; its coding DNA sequence is 1596 bp long and encodes a 531aa protein (CYP27). At present, 60 mutations have been identified in nearly 317 patients (7-15), and no phenotype-genotype correlation has been observed (14).
Gallus, et al. , described that about half of the mutations have been described in the exon 6-8 tracts of the CYP27A1 gene. From the total of mutations, 45% are missense which together with nonsense mutations add up to about 2/3 of the total reported to date (10).
Here we report a cerebrotendinous xanthomatosis Colombian family with four affected siblings. Three of them presented with cognitive impairment and dementia. Clinical features, and neurological, neuropsychological, imaging and genetic findings in this family were analyzed. This entity was described clinically in another Colombian family in 2003, but without genetic confirmation (Cabrera D, Franco A, Builes C. Características clínicas de una familia antioqueña con xantomatosis cerebrotendinosa. Acta Neurol Colomb. 2003;19. Cartel 68. Memorias, VI Congreso Colombiano de Neurología). This study is the first longitudinal study of cognitive impairment of the cerebro-tendinous xanthomatosis. In addition, mutation reported is the first one identified in Colombia causing this disease.
Materials and methods
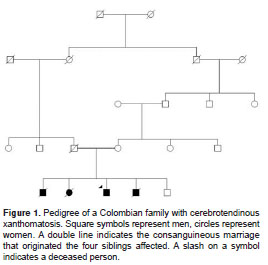
This family is originally from Antioquia, northwest Colombia. Extension of the pedigree from the index case revealed three more affected siblings. The parents were consanguineous and no previous report of the disease in the family was described (figure 1). Four out of five siblings were affected. All affected individuals and their relatives participating in this study signed an informed consent form at the beginning of the study, which was approved by the ethics committee from Universidad de Antioquia . This family has been followed since 2005 in the Grupo de Neurociencias de Antioquia, Facultad de Medicina, Universidad de Antioquia , which is a reference center for research in neurodegenerative diseases and hereditary dementias in Colombia. These participants belonged to an undergoing study of family movement disorders and they were followed up longitudinally during a period of approximately six years starting when they were around 50 years old. Genetic analysis, electroencephalogram, electromyography and four-limb nerve conduction velocity, as well as brain MRI were performed.
The follow-up examination included medical and neuropsychological assessment. The clinical history and the medical and neurological examinations were performed by neurologists and trained general practitioners. Neuropsychological tests were applied by independent neuropsychologists and trained personnel without knowledge of diagnosis or genetic status. The Grupo de Neurociencias de Antioquia Complete Dementia Protocol included the Consortium to Establish a Registry for Alzheimer´s Disease (CERAD) neuropsychological test battery with additional neuropsychological tests, validated for the Colombian population in subjects over 50 years of age, as previously reported in other Grupo de Neurociencias de Antioquia studies (16).
Tests were applied as follows: Minimental state examination, Rey-Osterrieth complex figure (recall), list of words, list of words (intrusions), list of words (recall), list of words (intrusions recall), list of words-recognition "Yes", list of words-recognition "No", recall of line-drawings, naming, phonological verbal fluency "F", constructional praxis, Rey-Osterrieth complex figure (copy), Raven´s test (part A and B), arithmetic test, visual "A" cancellation test (omissions), visual "A" cancellation test (time), trial making test-part A (corrects), trial taking test-part A (time), Wisconsin card sorting test (perseverative errors), Wisconsin card sorting test (correct), Wisconsin card sorting test (error), Wisconsin card sorting test (categories), and Wisconsin card sorting test (conceptual). Clinically significant cognitive decline was considered if neuropsychological test scores were 1.5 standard deviations (SD) away from the mean score (17) in at least one test on any cognitive domain adjusted for age and education level.
Analysis of the CYP27A1 gene
Primers to amplify the nine exons of the coding region of the CYP27A1 gene were designed using the application primer3 available at http://frodo.wi.mit.edu/. These included the exon-intron boundaries. Primer sequences are available upon request. PCR fragments were amplified in a 25 µ l volume using 0.33 µ M of each primer, 0.2 mm each dNPT, 1.5 mm Mg 2+, 1X buffer, 0.02 U of Taq polymerase (Biolase-Bioline), and 30 ng of genomic DNA. DNA samples from index case and case 3 were used to amplify all exons and then underwent nucleotide sequence using an Applied Biosystems genetic analyzer 3130. Restriction analysis was done for mutation c.1183_1184 insT. This insertion destroys the target for the restriction enzyme AciI . Five units were used to digest PCR fragments at 37 o C overnight and according to manufacturer ´ s recommendations (New England Biolabs®). PCR and digestion products were resolved in 2% agar-ose gel electrophoresis and stained with ethidium bromide. Sixty control chromosomes were tested for this mutation.
Results
A family of five siblings, four of them affected with cerebrotendinous xanthomatosis, was identified. The findings in three of these cases (index case, case 2 and case 3) were described. These individuals had cognitive impairment and dementia and it was possible to do follow-up. In case 4, some clinical findings were identified by family interview.
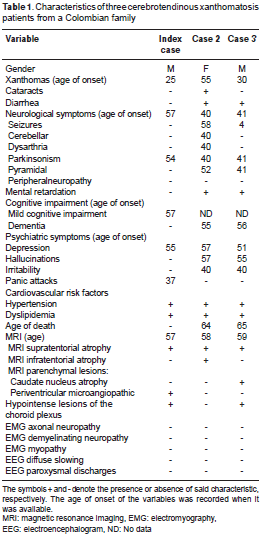
Clinical, radiological, and neurophysiological characteristics of the three cases are summarized in table 1.
Neurological assessment
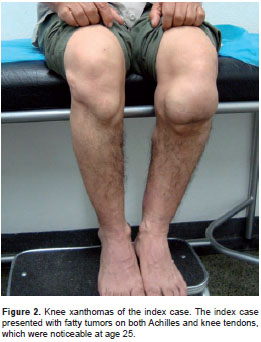
Index case. The index case (male) presented fatty tumors: Xanthoma tuberous in the knee and tendon xanthoma in Achilles tendon, which were noticeable at age 25 (figure 2). Depression, anxiety attacks, akathisia, and behavior changes were evident at age 37. Mild multidomain cognitive impairment was evident at age 57. Examination of the nervous system showed a postural tremor of the right hand with slight rigidity. All deep reflexes were sharp and plantar reflexes were normal. Gait was normal in this patient. He suffered a severe traumatic brain injury when he was 60 years old. Since then, the patient is hemiplegic and inevaluable by neuropsychology, and it was not possible to continue the follow-up.
Case 2. This female patient had bilateral cataracts, which were removed at age 15. She had poor cognitive functioning from childhood and missed several school years. At 40 years, marked rigidity in left hemibody, unsteady gait, dysarthria, depression, irritability and visual hallucinations appeared. She had a hip fracture at 48 years and Achilles tendon xanthomas were found at 55. Clinical examination revealed ataxia, hyperactive reflexes, and extensor plantar response. She presented Parkinsonism at 40. Chronic intermittent diarrhea started at age 59. She also showed a systemic deterioration and cognitive impairment added to her previous cognitive deficit and she died due to aspiration pneumonia at 64.
Case 3 . This male patient presented with episodes of seizures at the age of 4. He also had learning difficulties. At age 30, Achilles and patellar tendon xanthomas were found. At 40, he started to present psychiatric symptoms. A few years later, he had tremor and rigidity of the left hand. Parkinsonism was diagnosed at 41 and the patient underwent a therapeutic test with levodopa (750 mg/day) since age 49. The withdrawal of levodopa worsened the stiffness. Clinical and neurological examination revealed a poorly expressive face, resting tremor, hypereactive tendon reflexes, and extensor plantar response. Gait was slow and rigid with short steps. At 62, he suffered from refractory epilepsy and died at 65 from aspiration pneumonia during a status epilepticus.
Case 4. This was a male patient who died at 47 years.Clinical details of the fourth case were reconstructed by familial interview; very scarce information could be obtained about this patient. He had normal cognition, worked in a textile factory, and presented Achilles tendon xanthomas at age 30.He had a history of arterial hypertension and depressive symptoms after marital separation. He suffered subsequent stroke at 45. He was ran over by a car two years later and died.
Genetic analysis
Data suggested that the four affected individuals in this Colombian family had conditions consistent with the diagnosis of cerebrotendinous xanthomatosis. PCR products ruled out homozygous deletions of CYP27A1 exons. Affected individuals were homozygous for a novel CYP27A1 gene mutation at exon 6, which truncates the heme domain of the protein. Of the 25% expected, 80% of the siblings were affected.
Sequence analysis revealed an insertion of nucleotide T in between positions 1183 and 1184 (from the start codon) in exon 6, c.1183_1184insT (figure 3).
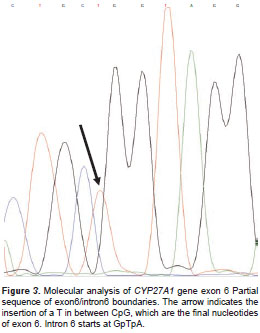
Restriction analysis showed that all three affected available subjects were homozygous for such a mutation. Consistent with autosomal recessive inheritance, the mother was a carrier for this mutation. None of the control chromosomes carried the mutation reported here. The mutation c.1183_1184 insT is predicted to cause the p.R395fsX412 substitution at the protein level. Besides, a premature stop codon is generated 17 codons downstream from codon 395. The new truncated protein is missing the heme binding site. It is worth mentioning that the codon involved in the mutation reported here is the one most frequently mutated in this gene http://grenada.lumc.nl/LOVD2/ctx/home.php?select_db=CYP27A.
Findings of magnetic resonance, electromyography and electroencephalogram
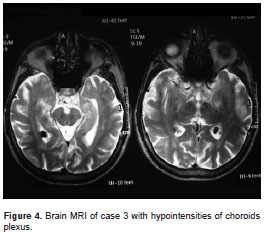
Index case brain MRI showed discrete frontal and parietal atrophy, with periatrial microangiopathy. A T1-weighted sequence showed two hypointense lesions of the choroid plexus. Case 2 showed symmetric atrophy of the frontal and temporal lobes. Additionally, a discrete cerebellar atrophy was observed. Case 3 showed atrophy of the frontal lobes, temporal lobes and caudate nucleus. A T1-weighted sequence showed two hypointense lesions of the choroid plexus compatible with xanthomas (figure 4). Electromyography and nerve four-limb conduction velocity were normal in the three patients tested. The electroencephalogram (EEG) showed a diffuse slowing in all subjects.
Neuropsychological evaluation
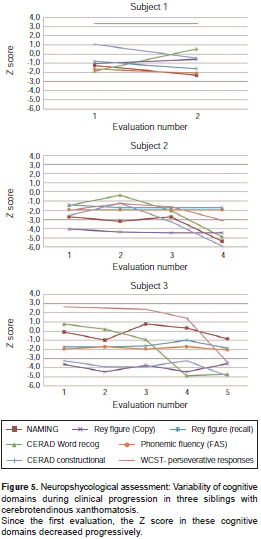
Since the beginning of monitoring cognitive profiles, the three subjects presented cognitive impairment. Cases 2 and 3 had a significantly lower performance. Index case had a better premorbid level, but unlike the cases 2 and 3, he did not develop dementia until the follow-up was possible. The most relevant tests according to each neuropsychological profile can be observed in figure 5.
Discussion
Antioquia in Colombia is famous in the world due to its high prevalence of familial dementia, with the greatest focus of familial Alzheimer´s disease mutation E280A in the presenilin 1. We report a new mutation in a family with a rare hereditary dementia due to cerebrotendinous xanthomatosis in this same region of Colombia.
This is the first study in Colombia aimed at characterizing cerebrotendinous xanthomatosis, from both phenotypic and genetic points of view. Affected individuals presented clinical characteristics compatible with cerebrotendinous xanthomatosis. CYP27A1 gene sequence analysis uncovered a novel mutation associated with cerebrotendinous xanthomatosis. It is predicted that the mutation c.1183_1184insT leads to the change of an Arg residue for a Leu at position 395. A premature stop codon is originated 17 residues downstream. Therefore, the mutant enzyme is predicted to miss the heme binding site (11). Interestingly, codon 395 of CYP27A1 has been previously reported as participating in three other mutations causing cerebrotendinous xanthomatosis (2,18,19).
Our report concerns the fourth mutation in this codon, indicating a hot spot codon for mutational events in CYP27A1 gene. With the mutation reported here, frameshift mutations at this gene account for 20% of the defects associated with cerebrotendinous xanthomatosis. The observation that a decanucleotide is inserted in a cerebro-tendinous xanthomatosis patient (10) and that exactly the same decamer deletion appeared in another patient (20) suggests a possible mechanism of unequal crossover in CYP27A1 gene. This might happen as a result of missalignment of non-allelic sequences (21,22). The mutation of this decamer, in addition to the fourth mutation of codon 395 reported here, points to two CYP27A1 mutational hot spots. Taking into account that the mutation c.1183_1184insT involves the last two nucleotides of exon 6, it was supposed that the splicing site was affected. Prediction of modification of the splice site was carried out with NetGene2 (23,24) server. It was observed that when using the normal sequence, the splice would occur with 80% confidence and when using the mutant sequence it would occur with 70% confidence (data not shown). This analysis is in line with our conclusion about the absence of the heme binding site, either by this mechanism or by the premature stop codon at position 412 of the protein. Experimental data could confirm whether the splicing does occur or not.
One of such experimental approaches could be RNA isolation followed by reverse transcriptase processing to generate a PCR template (cDNA). This PCR should flank exons 5 and 6 and then by gel electrophoresis resolve the amplicon sizes. The patients should present a homozygous larger/shorter PCR fragment compared to controls or unaffected family members. Another approach, which could complement the previous one, could be sequencing the PCR fragment obtained from the cDNA. This would identify where the precise splice did occur in relation to the mutation reported here.
Even though a wide variety of clinical and endo-phenotypes have been observed, qualitative homogeneity has been found for the presence of xanthomas, Parkinsonism, psychiatric symptoms, dysexecutive cognitive impairment and supratentorial atrophy. However, the age of onset for each of these manifestations is heterogenous. Thus, the onset of tendon xanthomas and both neurological and psychiatric symptoms ranged widely, and cataracts were present only in one individual (case 2). Chronic diarrhea from childhood may be the earliest clinical manifestation of cerebrotendinous xanthomatosis, but in this family this symptom was present when individuals were over 50 years old, unlike other studies on cerebrotendinous xanthomatosis (10,15). Parkinsonism was observed in three of the four affected individuals (range of onset: 40-55, average: 45) with poor response to levodopa. Parkinsonism is an uncommon symptom, but cerebrotendinous xanthomatosis patients associated with this extrapyramidal sign have been reported to date (7,8, 22,25-29). Pyramidal symptoms were observed in two individuals (case 2 and 3). Other abnormal movements were not observed. The age of onset of the pyramidal symptoms was very dissimilar.
Mental retardation has been described in cerebro-tendinous xanthomatosis (15). In cases 2 and 3, a worse neuropsychological performance was found since the first evaluation (at 52 and 53 years old, respectively) possibly because the onset of cognitive impairment was earlier and higher in these subjects compared to the index case. Cognitive profiles of these two patients were highly affected by their cognitive impairment at baseline, which made all their performances lower than expected for their age group and school level. Therefore, both patients had probably low intelligence from early infancy and began to exhibit more deterioration. For the index case, previous cognitive level was normal and mild cognitive impairment was observed afterward. We could not detect the onset of dementia in this subject, because of his severe head trauma.
In three individuals analyzed, praxic-mnesic abilities were altered, but the deterioration profile was predominantly dysexecutive, unlike the expected impairment on Alzheimer´s disease, where the deterioration is predominantly amnesic from the beginning.
The psychiatric manifestations in cerebrotendinous xanthomatosis follow a bimodal pattern. They appear during childhood or adolescence with learning impairments, mental retardation, behavioral changes, personality disorders or they appear when the disease has progressed. Dementia and other psychiatric manifestations are the result of the alterations in multiple neuronal networks in the white matter with myelin-lipid substrate and demyelinitization disorders (30-35).
To date, different cerebrotendinous xanthomatosis demential syndrome profiles have been reported. One of these profiles is the frontotemporal dementia and/or cortico-subcortical compromise which are characterized by a poor performance in the executive function (30) and neuropsychiatric features (35).
A previous case report mentions a 53 year-old woman with predominant frontal dysfunction, behavioral alterations and poor performance on executive tasks with preservation of visual-constructive abilities. This patient fulfilled the criteria for frontotemporal lobe dementia (30). Another case has been reported in a woman who, at age 38, began to present changes in personality and apathy, and dementia syndrome with frontal predominance (36). Some other cases were also compatible with a clinical diagnosis of frontotemporal dementia in genetically confirmed cerebrotendinous xanthomatosis (35).
MRI findings were heterogenous. Thus, all three available affected individuals showed supratentorial atrophy; only one subject (case 2) presented infratentorial atrophy. Her clinical examination disclosed ataxia. Case 3 presented caudate nucleus atrophy. He had Parkinson´s symptoms, but had no other abnormal movements. Hypointense lesions of the choroid plexus were observed in index case and case 3 as well. Imaging findings commonly described in cerebrotendinous xanthomatosis are nonspecific supratentorial atrophy and deep white matter changes; more typical hyperintense lesions were seen on T2-weighted images in the dentate nucleus, globus pallidus, substantia nigra, and inferior olive, which extended into adjacent white matter as the disease progressed (37).
Cholestanol could not be measured in any of the patients and none received treatment with chenodeoxycholic acid as neither the test nor the drug are available in Colombia. These patients received antidepressants and antipsychotics with improvement in their symptoms.
This family case is important for being the first genetically identified in our country. All the genetically studied cases were homozygous and there was a high percentage (80%) of subjects affected, despite being an autosomal recessive disorder.
We were able to perform a neurological and neuro-psychological follow-up, a verification of deterioration over time and to describe some characteristics of dysexecutive cognitive performance that differ from the amnestic profile found in Alzheimer´s disease.
We are very thankful to the family members who participated in this study. We also want to thank José Luis Ascencio, Laureano Mestra and Camilo Aguirre, who helped us with the manuscript.
Authors declare no conflict of interests in this study.
The Universidad de Antioquia and its Committee for Research Development funded this research in the framework of the Sustainability Program 2009-2010.
Corresponding author:
Margarita Giraldo-Chica, Grupo de Neurociencias de Antioquia, Facultad de Medicina, Sede de Investigación Universitaria, Universidad de Antioquia, Calle 62 N° 52-59, Medellín, Colombia Telefax: (574) 219 6444 margaritamariagiraldochica@gmail.com
1. Hansson M, Olin M, Floren CH, von Bahr S, van´t Hooft F, Meaney S, et al . Unique patient with cerebrotendinous xanthomatosis. Evidence for presence of a defect in a gene that is not identical to sterol 27-hydroxylase. J Intern Med. 2007;261:504-10. http://dx.doi.org/10.1111/j.1365-2796.2007.01782.x [ Links ]
2. Cali JJ, Russell DW. Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450 that catalyzes multiple oxidation reaction in bile acid biosynthesis. J Biol Chem. 1991;266:7774-8. [ Links ]
3. van Bogaert L, Scherer HJ, Epstein E. Une forme cerebrale de la cholesterinose généralisée. Paris: Masson et Cie; 1937. [ Links ]
4. Moghadasian MH. Cerebrotendinous xanthomatosis: Clinical course, genotypes and metabolic backgrounds. Clin Invest Med. 2004;27:42-50. [ Links ]
5. Barkhof F, Verrips A, Wesseling P, van Der Knaap MS, van Engelen BG, Gabreëls FJ, et al . Cerebrotendinous xanthomatosis: The spectrum of imaging findings and the correlation with neuropathologic findings. Radiology. 2000;217:869-76. http://dx.doi.org/10.1148/radiology.217.3.r00dc03869 [ Links ]
6. Keren Z, Falik-Zaccai TC. Cerebrotendinous xanthomatosis (CTX): A treatable lipid storage disease. Pediatr Endocrinol Rev. 2009;7:6-11. [ Links ]
7. Pilo de la Fuente B, Ruiz I, López de Munain A, Jiménez-Escrig A. Cerebrotendinous xanthomatosis: Neuropathological findings. J Neurol. 2008;255:839-42. http://dx.doi.org/10.1007/s00415-008-0729-6 [ Links ]
8. Su C-S, Chang W-N, Huang S-H, Lui C-C, Pan T-L, Lu C-H, et al . Cerebrotendinous xanthomatosis patients with and without parkinsonism: Clinical characteristics and neuroimaging findings. Mov Disord. 2010;25:452-8. http://dx.doi.org/10.1002/mds.22979 [ Links ]
9. Chen W-C, Wu K-C, Hu C-H, Chern T-C, Jou I-M. A compound heterozygous mutation of CYP27A1 gene in a Taiwanese patient with cerebrotendinous xanthomatosis. J Orthop Sci. 2011;16:825-7. http://dx.doi.org/10.1007/s00776-011-0072-0 [ Links ]
10. Gallus GN, Dotti MT, Federico A. Clinical and molecular diagnosis of cerebrotendinous xanthomatosis with a review of the mutations in the CYP27A1 gene. Neurol Sci. 2006;27:143-9. http://dx.doi.org/10.1007/s10072-006-0618-7 [ Links ]
11. Gallus GN, Dotti MT, Mignarri A, Rufa A, Da Pozzo P, Cardaioli E, et al . Four novel CYP27A1 mutations in seven Italian patients with CTX. Eur J Neurol. 2010;17:1259-62. http://dx.doi.org/10.1111/j.1468-1331.2010.03002 [ Links ]
12. Nozue T, Higashikata T, Inazu A, Kawashiri MA, Nohara A, Kobayashi J, et al . Identification of a novel missense mutation in the sterol 27-hydroxylase gene in two Japanese patients with cerebrotendinous xanthomatosis. Intern Med. 2010;49:1127-31. http://doi.org/10.2169/internalmedicine.49.3277 [ Links ]
13. Schneider H, Lingesleben A, Vogel H-P, Garuti R, Calandra S. A novel mutation in the sterol 27-hydroxylase gene of a woman with autosomal recessive cerebrotendinous xanthomatosis. Orphanet J Rare Dis. 2010;6;5:27. http://dx.doi.org/10.1186/1750-1172-5-27 [ Links ]
14. Verrips A, Hoefsloot LH, Steenbergen GC, Theelen JP, Wevers RA, Gabreels FJ, et al . Clinical and molecular genetic characteristics of patients with cerebrotendinous xanthomatosis. Brain. 2000;123:908-19. http://dx.doi.org/10.1093/brain/123.5.908 [ Links ]
15. Bajaj BK, Singh A, Anand KS, Garg J. Cerebrotendinous xanthomatosis: Report of two cases and a novel genetic mutation in an Indian patient. J Neurosci Rural Pract. 2013;4:S87-90. http://dx.doi.org/10.4103/0976-3147.116420 [ Links ]
16. Ardila A, Lopera F, Rosselli M, Moreno S, Madrigal L, Arango-Lasprilla JC, et al . Neuropsychological profile of a large kindred with familial Alzheimer´s disease caused by the E280A single presenilin-1 mutation. Arch Clin Neuropsychol. 2000;15:515-28. [ Links ]
17. Aguirre-Acevedo DC, Gómez RD, Moreno S, Henao-Arboleda E, Motta M, Muñoz C, et al . Validity and reliability of the CERAD-Col neuropsychological battery. Rev Neurol. 2007;45:655-60. [ Links ]
18. Chen W, Kubota S, Nishimura Y, Nozaki S, Yamashita S, Nakagawa T, et al . Genetic analysis of a Japanese cerebrotendinous xanthomatosis family: Identification of a novel mutation in the adrenodoxin binding region of the CYP 27 gene. Biochim Biophys Acta. 1996;1317:119-26. http://dx.doi.org/10.1016/S0925-4439(96)00043-9 [ Links ]
19. Chen W, Kubota S, Teramoto T, Nishimura Y, Yonemoto K, Seyama Y. Silent nucleotide substitution in the sterol 27-hydroxylase gene (CYP 27) leads to alternative pre-mRNA splicing by activating a cryptic 5 ´ splice site at the mutant codon in cerebrotendinous xanthomatosis patients. Biochemistry. 1998;37:4420-8. [ Links ]
20. Lee MH, Hazard S, Carpten JD, Yi S, Cohen J, Gerhardt GT, et al . Fine-mapping, mutation analyses, and structural mapping of cerebrotendinous xanthomatosis in U.S. pedigrees. J Lipid Res. 2001;42:159-69. [ Links ]
21. Feuk L, Carson AR, Scherer SW. Structural variation in the human genome. Nat Rev Genet. 2006;7:85-97. http://dx.doi.org/10.1038/nrg1767 [ Links ]
22. Stankiewicz P, Lupski JR. Genome architecture, rearrangements and genomic disorders. Trends Genet. 2002;18:74-82. http://dx.doi.org/10.1016/S0168-9525(02)02592-1 [ Links ]
23. Hebsgaard SM, Korning PG, Tolstrup N, Engelbrecht J, Rouzé P, Brunak S. Splice site prediction in Arabidopsis thaliana DNA by combining local and global sequence information. Nucleic Acids Res. 1996;24:3439-52. http://dx.doi.org/10.1093/nar/24.17.3439 [ Links ]
24. Brunak S1, Engelbrecht J, Knudsen S. Prediction of human mRNA donor and acceptor sites from the DNA sequence. J Mol Biol. 1991;220:49-65. http://dx.doi.org/10.1016/0022-2836(91)90380-O [ Links ]
25. Fujiyama J, Kuriyama M, Yoshidome H, Suehara M, Eiraku N, Kashio N, et al . Parkinsonism in cerebrotendi-nous xanthomatosis. Jpn J Med. 1991;30:189-92. http://doi.org/10.2169/internalmedicine1962.30.189 [ Links ]
26. Kuwabara K, Hitoshi S, Nukina N, Ishii K, Momose T, Kubota S, et al . PET analysis of a case of cerebrotendinous xanthomatosis presenting hemiparkinsonism. J Neurol Sci. 1996;138:145-9. [ Links ]
27. Wakamatsu N, Hayashi M, Kawai H, Kondo H, Gotoda Y, Nishida Y, et al . Mutations producing premature termination of translation and an amino acid substitution in the sterol 27-hydroxylase gene cause cerebrotendinous xanthomatosis associated with parkinsonism. J Neurol Neurosurg Psychiatry. 1999;67:195-8. http://doi.org/10.1136/jnnp.67.2.195 [ Links ]
28. Dotti MT, Federico A, Garuti R, Calandra S. Cerebro-tendinous xanthomatosis with predominant parkinsonian syndrome: Further confirmation of the clinical heterogeneity. Mov Disord. 2000;15:1017-9. [ Links ]
29. Grandas F, Martín-Moro M, García-Muñozguren S, Anaya F. Early-onset parkinsonism in cerebrotendinous xanthomatosis. Mov Disord. 2002;17:1396-7. http://doi.org/10.1002/mds.10287 [ Links ]
30. Guyant-Maréchal L, Verrips A, Girard C, Wevers RA, Zijistra F, Sistermans E, et al . Unusual cerebrotendinous xanthomatosis with fronto-temporal dementia phenotype. Am J Med Genet A. 2005;139A:114-7. http://dx.doi.org/10.1002/ajmg.a.30797 [ Links ]
31. Dotti MT, Federico A, Signorini E, Caputo N, Venturi C, Filosomi G, et al . Cerebrotendinous xanthomatosis (van Bogaert-Scherer-Epstein disease): CT and MR findings. Am J Neuroradiol. 1994;15:1721-6. [ Links ]
32. Benarroch EE. Brain cholesterol metabolism and neurologic disease. Neurology. 2008;71:1368-73. http://dx. doi.org/10.1212/01.wnl.0000333215.93440.36 [ Links ]
33. Bhattacharyya AK, Lin DS, Connor WE. Cholestanol metabolism in patients with cerebrotendinous xantho-matosis: Absorption, turnover, and tissue deposition. J Lipid Res. 2007;48:185-92. http://dx.doi.org/10.1194/jlr.M600113-JLR200 [ Links ]
34. Panzenboeck U, Andersson U, Hansson M, Sattler W, Meaney S, Bjorkhem I. On the mechanism of cerebral accumulation of cholestanol in patients with cerebrotendi-nous xanthomatosis. J Lipid Res. 2007;48:1167-74. http://dx.doi.org/10.1194/jlr.M700027-JLR200 [ Links ]
35. Fraidakis MJ. Psychiatric manifestations in cerebrotendinous xanthomatosis. Transl Psychiatry. 2013;3:e302. http://dx.doi.org/10.1038/tp.2013.76 [ Links ]
36. Sugama S, Kimura A, Chen W, Kubota S, Seyama Y, Taira N, et al . Frontal lobe dementia with abnormal cholesterol metabolism and heterozygous mutation in sterol 27-hydroxylase gene (CYP27). J Inherit Metab Dis. 2001;24:379-92. [ Links ]
37. Barkhof F, Verrips A, Wesseling P, van Der Knaap MS, van Engelen BG, Gabreels FJ, et al . Cerebrotendinous xanthomatosis: The spectrum of imaging findings and the correlation with neuropathologic findings. Radiology. 2000;217:869-76. http://dx.doi.org/10.1148/radiology.217.3.r00dc03869 [ Links ]













