Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Biomédica
versão impressa ISSN 0120-4157
Biomédica vol.35 no.4 Bogotá out./dez. 2015
https://doi.org/10.7705/biomedica.v35i4.2530
BRIEF COMMUNICATION
doi: http://dx.doi.org/10.7705/biomedica.v35i4.2530
1 Grupo de Investigaciones en Enfermedades Parasitarias e Infecciosas, Universidad de Pamplona, Pamplona, Norte de Santander, Colombia
2 Department of Pathology, Microbiology and Immunology, University of South Carolina, Columbia, SC, USA
3 Hospital Local Los Patios, Norte de Santander, Colombia
4 Pathobiological Sciences, Vector-borne Disease Laboratories, School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA
5 School of Public Health and Tropical Medicine, Tulane University, New Orleans, LA, USA
Author´s contributions:
Berlín Londoño: Experiment design, data analysis, writing of the manuscript
María Paloma Villamizar and Jeniffer Rolón: Mosquito sample collection, experiment design
Carolina Cárdenas: Collection of serum samples in Los Patios and Cúcuta, data analysis, writing of manuscript
Lucio Cárdenas: Mosquito sample collection and organization, data analysis
Daisy Carvajal: Sample collection and manuscript review
Christopher Mores: Data analysis, writing of the manuscript
Jennifer E. Giovanny: Manuscript review, assessment for statistical analysis
Daniel M. Chisenhall: Antigen preparation
Rebecca C. Christofferson: Molecular diagnosis, manuscript review
Omar G. Pérez-Ortiz: Coordination of field activities
Dawn M. Wesson: Coordination and design of entomological work
Recibido: 22/09/14; aceptado: 06/07/15
Introduction: Mosquito salivary proteins are able to induce an antibody response that reflects the level of human-vector contact. IgG antibodies against dengue virus (DENV-IgG) are indicators of previous exposure. The risk of DENV transmission is not only associated to mosquito or dengue factors, but also to socioeconomic factors that may play an important role in the disease epidemiology.
Objective: To determine the effect of the presence of Aedes aegypti mosquitos in different stages in households and the history of dengue exposure on vector-human contact determined by the level of anti-salivary protein antibodies in people living in a Colombian endemic area.
Materials and methods: A pilot study of 58 households and 55 human subjects was conducted in Norte de Santander, Colombia. A questionnaire for socioeconomic factors was administered and houses were examined for the presence of Ae. aegypti specimens in the aquatic stages. The level of DENV-IgG antibodies (DENV-IgG), in addition to IgG and IgM anti- Ae. aegypti salivary gland extract (SGE) antibodies (SGE-IgG, SGE-IgM) were evaluated by ELISA using blood collected in filter paper.
Results: We found a significant higher level of SGE-IgG antibodies in subjects living in houses with Ae. aegypti in aquatic stages. We also found a higher concentration of SGE-IgG antibodies in people exposed to DENV, a positive correlation between IgM-SGE and IgG-DENV and a negative correlation with IgG-SGE.
Conclusion: Anti-salivary proteins antibodies are consistent with the presence of Ae. aegypti aquatic stages inside houses and DENV-IgG antibodies concentrations.
Key words: Aedes aegypti , dengue, dengue virus, disease vectors.
doi: http://dx.doi.org/10.7705/biomedica.v35i4.2530
Concentración de anticuerpos contra proteínas de las glándulas salivales de Aedes aegypti e historia de la exposición al virus del dengue en residentes de una zona endémica colombiana
Introducción. Las proteínas salivales de los mosquitos son capaces de inducir la producción de anticuerpos, lo que a su vez refleja el grado de contacto hombre-vector. Además, los anticuerpos IgG contra el virus del dengue son indicadores de una exposición previa a este virus. El riesgo de transmisión del virus del dengue está asociado no solo con factores relacionados con la biología del mosquito, o factores virales, sino también, con factores socioeconómicos, como la disponibilidad de agua en el hogar, que pueden desempeñar un papel importante durante la temporada epidémica.
Objetivo. Determinar el efecto de la presencia de mosquitos Aedes aegypti en las casas y la exposición previa al virus del dengue, sobre los niveles de anticuerpos contra mosquitos en el contacto humano-vector en habitantes de un área endémica de Colombia.
Materiales y métodos. Se hizo un estudio piloto de 58 casas y 55 participantes en Norte de Santander, Colombia. Se empleó un cuestionario para recopilar la información sobre los factores socioeconómicos y se examinaron las casas para detectar la presencia de sitios de cría de Ae. aegypti . Se recolectó una muestra de sangre humana total en papel de filtro y se estableció el nivel de anticuerpos IgG contra el virus del dengue, además del de los anticuerpos IgG e IgM anti- Ae. aegypti de extracto de glándula salival mediante ELISA.
Resultados. Los resultados revelaron un mayor nivel de anticuerpos IgG de extracto de glándula salival en sujetos que vivían en casas con presencia de mosquitos Ae. aegypti en la fase acuática. Asimismo, se encontró una mayor concentración de anticuerpos IgG de extracto de glándula salival en personas previamente expuestas al virus del dengue. Los resultados evidenciaron una correlación positiva significativa entre los niveles de IgM de extracto de glándula salival y los de IgG anti-virus del dengue de extracto de glándula salival, y una correlación negativa con los de IgG de extracto de glándula salival, aunque esta última no fue significativa.
Conclusión. La concentración de anticuerpos fue mayor en quienes vivían en casas con estadios acuáticos de Ae. aegypti , como también en las personas con anticuerpos IgG anti-virus del dengue.
Palabras clave: Aedes aegypti , dengue, virus del dengue, vectores de enfermedades.
doi: http://dx.doi.org/10.7705/biomedica.v35i4.2530
Dengue virus (DENV) is a systemic viral infection maintained in a human-mosquito transmission cycle by the urban vector Aedes aegypti (1). There are four serotypes (DENV 1-4) with antigenic relatedness, but co- or subsequent infection with a different serotype is thought to enhance disease pathology (2). Serotype-specific antibodies confer lifetime immunity against the infecting serotype, but heterologous protection has limited duration (3,4). In the absence of a vaccine or other preventative therapy, DENV prevention and control efforts focus on vector control, specifically habitat abatement. Containers of standing water are the preferred breeding sites for Ae. aegypti in urban and suburban environments (5). Despite global public health campaigns about DENV risk and water storage, unpredictable access to running water perpetuates the practice of water storage, particularly in smaller towns and rural areas (6).
Previous studies have shown that the type and concentration of antibodies against vector salivary gland extract (SGE) can serve as markers for disease transmission and risk (7-10) and to evaluate the efficacy of vector control efforts (11,12). Additionally, estimating the prevalence of antibodies against all DENV serotypes that have circulated in a specific area gives insight of the population exposure to the virus (13).
In Colombia, the four serotypes of DENV may co-circulate at any given point of time (14). Norte de Santander, a region of national and international commerce, is also an area with a high DENV index within the country, where more than 4,000 cases of DENV were reported, 700 of which were severe cases (15). Our study focused on three locations within Norte de Santander: Cúcuta, Los Patios, and Pamplona. The objective was to measure the prevalence of IgG antibodies specific for DENV 1-4 and Ae. aegypti salivary proteins among residents from the study sites and correlate these antibody concentrations with water storage practices and larval presence in the households.
The study hypothesis is that socioeconomic factors such as household water availability and storage are related to the level of exposure to Ae. aegypti bites measured by the concentration of IgG and IgM (SGE-IgG, SGE-IgM) antibodies against salivary proteins, and that these levels are associated with the risk of dengue exposure and infection. The study showed that there was an association between mosquito bite exposure and history of exposure to DENV also related to water availability for household use. This is the first report of association between history of dengue exposure measured as DENV-IgG antibody levels and mosquito bite exposure.
Materials and methods
Study area
The study was conducted in the cities of Cúcuta, Los Patios, and Pamplona in Norte de Santander, Colombia. The city of Pamplona is the third largest city in this province. It is located in the northeast of the country at 2,342 meters above sea level (m.a.s.l.); due to the high altitude this area is not endemic for Ae. aegypti and dengue cases are sporadic, mainly imported from surrounding DENV-endemic populations like Cucuta (320 m.a.s.l) and Los Patios (410 m.a.s.l.), since at least 49% of the population travel to these areas for commerce or work (Londoño-Rentería B, Londoño-Rentería F, Cárdenas C, Cárdenas L. Main mosquito breeding sites for Aedes aegypti in the Pan-American Highway: Cúcuta-Pamplona Area (Norte de Santander, Colombia) in 2010. Am J Trop Med Hyg. 2011;86(Suppl. 6):376). Contact among these three cities is along a 75 km route on the Pan-American Highway. Los Patios is a suburb of Cúcuta with almost a tenth of its population. This city is a necessary pass through to Pamplona and the center of the country.
Human participants
A group of 58 households located around the hospitals in Cúcuta (n=13), Los Patios (n=25), and Pamplona (n=20) were included in a study of housing characteristics influencing the presence of mosquitoes. These houses were randomly selected according to the distance from the hospitals and after obtaining consent in each of them. In Cúcuta, the houses were selected from an ongoing investigation in Primavera neighborhood, an area with high incidence of dengue cases in 2009. Houses whose dwellers declined to have them inspected, as well as abandoned houses, were excluded from the study.
Trained interviewers administered questionnaires to one adult per household to obtain information on water storage practices and household construction. After written informed consent was obtained, capillary blood was collected from participants by finger prick directly on filter paper (Protein Saver Cards, Whatman ® 903), and stored at -20 o C until analysis. In total, blood samples from 55 individuals were collected (Cúcuta: n=20, Los Patios: n=18 and Pamplona: n=17). In order to determine exposure status to DENV, we also included five filter paper samples from subjects living in New Orleans in 2009 as DENV-negative controls.
Field mosquito sample collection
Mosquito larvae were collected intra- and peri-domiciliary at the time of the visit, and the containers positive for mosquito larvae were recorded. Visits were performed during the beginning of the rainy season (September - October, 2010). Aedes aegypti larvae were identified using an illustrated taxonomic key (16).
Salivary gland extract preparation
Aedes aegypti (Rockefeller strain) mosquito dissection and salivary gland extract (SGE) preparation was performed as published elsewhere (7,8). Protein concentration was determined using the Thermo Scientific NanoDrop™ (Thermo Fisher Scientific, Wilmington, DE). The salivary gland extract (SGE) was stored in PBS at -80 °C until use.
Anti-SGE antibody detection
Detection of anti-SGE antibodies was optimized and tested following the methods published elsewhere (7,8). Samples were tested in duplicate. Three controls were included in each plate: 1) control blank: two wells without SGE to control for nonspecific induction of color for any of the reagents used in the test; 2) negative control: Two wells with SGE but without human serum to control for any nonspecific color induction of the coating antigen, and 3) positive control: two wells with pooled human serum from confirmed Ae. aegypti -exposed individuals from Colombia. Level of antibodies is represented as optical density (OD) measured at 450 nm.
Anti-DENV antibody levels by ELISA
Working conditions were previously standardized to test IgG antibody levels against each DENV serotype (DENV1 strain WestPac-74 [Nauru Island 1974], DENV2 strain 1232 [Indonesia, 1978], DENV3 strain CH5548904500 [Thailand, 1973] and DENV4 strain LN 634441 [Malyasia, 1988]) cultured under conditions described elsewhere (17). ELISA conditions were performed as published elsewhere (7) with minor modifications.
In summary, plates were coated with 1 µ g/ml of Flavivirus group capture antibody 4G2 overnight at 4°C, blocked for 1 h with 5% dry milk in PBS at 37°C, and incubated with one of the DENV serotypes (DENV 1-4) or control media from uninfected cell culture (media control plate) 2 hours at 37°C. A 100 µl/well of each diluted human sample was incubated 2 hours at 37°C on a shaker. Plates
were washed 3 times with wash solution (1× PBS and 0.1% Tween) and incubated with 100 µl/well of goat anti-human IgG diluted 1:1,000 horseradish peroxidase (HRP)-conjugated antibodies (Caltag Laboratories, Burlingame, CA) at 37°C for 1 hour.
Colorimetric development was obtained using 100 µl/well tetra-methyl-benzidine (TMB, one-solution microwell, Gene-Script, Piscataway, NJ) incubated for 10 minutes at room temperature. The reaction was stopped with 100 µl/well of stop solution (1 M phosphoric acid), and absorbance was measured at 450 nm. Each sample was tested in duplicate.
Three controls were included in each plate: 1) control blank: Two wells without 4G2 or DENV to control for nonspecific induction of color for any of the reagents used in the assay; 2) negative control: Two wells with 4G2 and no DENV to control for any nonspecific color induction of the coating antigen, and 3) positive control from a human serum with a known concentration of IgG antibodies.
Data analysis
Antibody concentration for each sample was expressed as adjusted OD calculated for each sample by subtracting the mean OD value of the controls from the mean OD value of the duplicates for each sample. DENV IgG-ELISA cut-off value was calculated to determine virus exposure status (Receiver Operating Characteristic [ROC] curve, area=0.9268, 95% confidence interval [CI]=0.8682 to 0.9854, p=0.0014).
To measure risk by odd ratios (OR), IgG antibody concentration was categorized to determine level of bite exposure. The average OD value was 1.012. All OD values lower than the average OD (<1.012) were classified as low; OD values higher or equal to the average OD (=1.020) were classified as high. After verifying that the values did not meet the normal distribution (p = 0.02; skewness and kurtosis normality test), the difference in antibody concentration between two groups ( Ae. aegypti presence or absence in the households) was tested using the nonparametric Mann-Whitney U test.
Spearman´s rank correlation test was used to measure the strength and significance of an association between two numeric variables. All differences were considered significant with a probability of committing a type 1 error set at p<0.05. All statistical tests were computed using Prism version 6 (GraphPad Software Inc. 2007) and STATA TM, version, 13 (Stata Corporation, College Station, Texas).
Ethics statement
Protocols and research methods were reviewed and approved by Universidad de Pamplona and the Ethics Review Board of Hospital Erasmo Meoz . The investigation was explained to each individual, and a written informed consent was obtained from each participant or their legal guardian before collecting samples. Blood samples were collected in compliance with the regulations on ethics of research in human participants for Colombia and the United States.
Results
Water availability and Aedes aegypti presence in the households
Fifty-eight households were included in the study with an average of 5.4 people (range 1-11) and 3.1 rooms per house. All households had connections installed for running water, but water was not received on daily basis in Los Patios and Cúcuta (n=38). Additionally, in 77.5% (45/58) of the houses water was stored for household activities such as cooking and/or cleaning. In these houses, water was mainly kept in floor tanks, roof tanks, and/or plastic or metal water cans (table 1). Thirty-one percent of houses had more than one type of water storage. In Pamplona, all households received daily water service and a majority of the households did not store water (15/20). However, two households were found using roof tanks for water storage and three more houses using plastic water cans. Furthermore, 60.3% (35/58) of the study households had containers with standing water around the house (front or back patios).
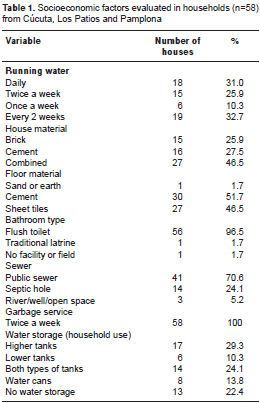
Houses were inspected for the presence of Ae. aegypti larvae in water holding containers. No Ae. aegypti were found in any of the Pamplona households (0/20). All Cúcuta households were positive for Ae. aegypti (13/13), as well as 84% (21/25) of the households in Los Patios. A total of 80 possible mosquito breeding sites among the 58 households were inspected in and around the property. Twenty nine percent (23/80) of all containers were found positive for Ae. aegypti larvae. Out of 35 inspected tanks inside houses, 16 (45.7%) were positive for mosquito water stages. Additionally, 23.8% (5/21) unused containers around the households and 40% of tires were found positive for these stages.
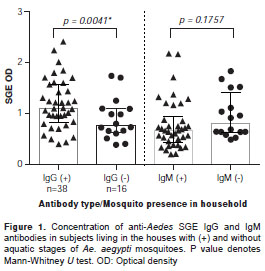
The concentration of IgG-SGE antibodies was significantly higher in participants living in houses positive for mosquito larvae (Mann-Whitney U test p=0.0041). However, the SGE-IgM antibody concentration was similar between the groups (Mann-Whitney U test, p=0.1757) (figure 1). Odd ratios showed that a person living in a household with breeding sites positive for mosquito larvae was 3.6 times more likely to have a higher concentration of IgG antibodies (95% CI: 1.172-11.630, p=0.0229).
Household characteristics, water availability and anti-SGE antibody concentration
The current study included 55 human subjects; all were tested for antibodies against Ae. aegypti SGE. The study population´s mean age was 32.7 years old (range: 2 to 75 years old), and 13% (7/55) of subjects were children under the age of 10. Participants living in Pamplona presented the lowest SGE-IgG antibody concentration (Mann-Whitney U test p>0.05) followed by Los Patios. There were no significant differences in the concentration of SGE-IgG between Cúcuta and Los Patios (Mann-Whitney U test p<0.05), and no significant difference among locations in SGE-IgM antibody concentration. However, this study showed that SGE-IgG antibody concentration correlates with SGE-IgM antibodies (Spearman correlation 0.7225, p=0.0346). Also, age was negatively correlated with both SGE-IgG and SGE-IgM antibody concentrations (Spearman correlation -0.2247 p=0.0285 [IgG], -0.2481 p= 0.0153 [IgM]). There was no significant correlation between the number of people in the household (Spearman correlation -0.254 p=0.8598 [SGE-IgG], -0.011 p=0.9391 [SGE-IgM]) or the number of rooms (Spearman correlation -0.0399 p=0.7810 [SGE-IgG], -0.0816 p=0.5693 [SGE-IgM]). The concentration of antibodies was similar when comparing the house or floor material (Kruskal-Wallis test, p>0.05).
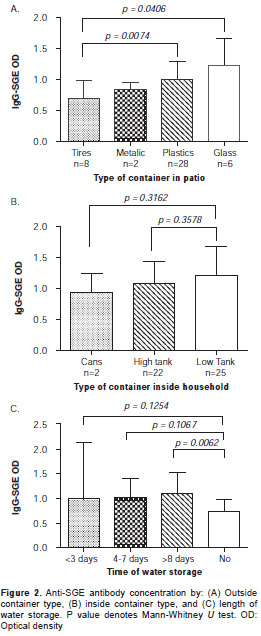
A comparison between SGE-IgG antibody con-centration of subjects living in houses with different types of water storage showed that the concentration of antibodies was higher in people using floor tanks, although we did not find a statistically significant difference among groups. However, we found a significantly higher SGE-IgG antibody concentration in subjects living in houses with unused plastic or glass containers (i.e., beer bottles) in their patios (Kruskal - Wallis test, p>0.05). The analysis also showed that IgG-SGE antibody concentration in households storing water for more than eight days was significantly higher than those in households with no water storage (Mann-Whitney U test, p=0.0062) (figure 2).
SGE antibody concentration and exposure to DENV
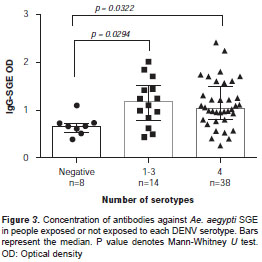
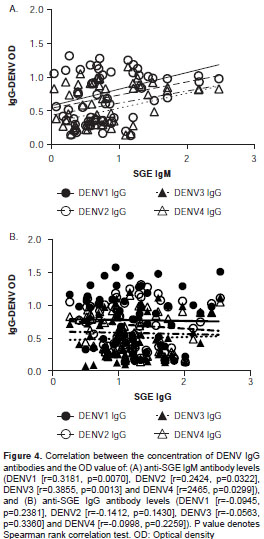
Fifty-five subjects were screened for antibodies against each DENV serotype. Results indicated that 84% (46/55) of the study subjects had been exposed to at least one DENV serotype. Fifty-seven percent of children <10 years old (4/7) had been exposed to at least one DENV serotype. Serum reactivity to each serotype was evaluated on each subject. Among study participants, the majority of antibodies were against DENV1 (45/55), followed by DENV2 (44/55), DENV4 (42/55) and DENV3 (33/55). People with antibodies for at least one DENV serotype had a significantly higher SGE-IgG antibody concentration than people with negative exposure (Mann-Whitney U test, p<0.05) (figure 3). A significant positive correlation was observed between SGE-IgM and DENV-IgG (Spearman Rank Correlation test, p<0.05), but not between SGE-IgG antibodies and IgG-DENV (figure 4).
Discussion
In Colombia, DENV is a major public health concern due to the steady increase in severe cases, the presence of all four serotypes, and the distribution of the primary vector, Ae. aegypti, across more than 90% of the country (18). Barrera, et al., found that the most important factor determining DENV endemicity was the practice of water storage, which provided a source of mosquito breeding sites (19), especially when the water was not frequently replaced (20). In addition, improvements to water distribution infrastructure have been shown to decrease the incidence of vector-transmitted diseases by decreasing the amount of exposure to infected bites (21). Production of Ae. aegypti from urban containers can be instrumental in triggering DENV outbreaks during the dry season (19). In fact, this study found a significant number of houses with tanks holding water for human consumption that were infested with mosquito larvae, and that the concentration of SGE antibodies was significantly higher in participants with previous exposure to DENV.
Water security, or the reliable access to clean, running water, is associated with water storage behavior (22). Lack of daily water supply inside the houses forces residents to build or improvise water storage options, such as cement/brick water tanks or plastic containers. Container type has been shown to influence the development of aquatic stages and adult mosquito presence in the home (23). Aedes aegypti breeding sites are closely associated with human habitation, as the species prefers to breed in human-made containers (24). In this study we found a rather significant number of houses (>40%) with mosquito aquatic stages inside them. Maintaining stored water for longer time periods provides larvae with sufficient time for development to the adult stage. As adults, female mosquitoes are more likely to feed and rest indoors, reducing the requirement to travel when searching for a blood meal (25).
The study showed gradients of antibody concentration, which translates as exposure to mosquito bites, that depended on the type of water storage kept inside the house. A significant difference in the concentration of antibodies in subjects living in houses with different types of containers in the peridomestic space was also found, as well as a significantly higher concentration of SGE-IgG antibodies in people residing in houses with glass and plastic containers around them, which shows that poor breeding site-reducing practices influence mosquito-human contact and lastly, the risk for pathogen transmission (26,27).
In our study, the level of SGE-IgG antibodies was significantly higher among participants living in households with containers positive for mosquito larvae. Similar results were registered in a recent study in Bolivia, where researchers found a higher antibody concentration against Ae. aegypti SGE in people living in areas with a greater abundance of this mosquito (28). They also found an important association between mosquito presence and the level of anti-salivary proteins antibodies. Another important finding s of this study was that people exposed to DENV had a significantly higher SGE-IgG antibody concentration. These results support the assumption that the risk of virus transmission increases in DENV-endemic areas where people may be exposed to several DENV serotypes in a single infection (29,30). A recent retrospective study showed that the IgG response to the Ae. aegypti salivary peptide Nterm-34 kDa was an indicator for risk of dengue virus transmission in urban endemic areas (31). Our results are consistent with those findings; this is the first study showing an association between total salivary proteins and DENV antibody levels with the presence or absence of mosquitoes in houses and water management.
The study evidenced a negative correlation between anti-SGE antibody levels and age, which is consistent with previous studies (8,32) In general, SGE-IgM antibody concentration is usually associated with a recent exposure to mosquito bites due to its short life span (33) while IgG antibodies have been found to have remain in blood for months (7). SGE-IgM antibodies were significantly positively correlated with the level of DENV-IgG antibodies while this association with SGE-IgG antibodies was negative, although not significant. In hyperendemic areas, immunity against DENV virus is developed early in life (2,21). Previous reports of seroprevalence in Colombia have shown that it is possible to find areas where up to 40% of children under five year-old have DENV-IgG antibodies (34). The level of DENV-IgG is known to rise rapidly in the presence of a secondary infection (2). In contrast, SGE-IgG antibodies seem to decrease in the presence of chronic exposure to salivary antigens mainly due to the induction of tolerance (32,35). Consequently, the observed negative correlation between SGE-IgG and DENV-IgG, although not significant, is consistent with an increase in the risk of dengue exposure in the presence of chronic exposure to salivary proteins. Additionally, the increase of DENV-IgG along SGE-IgM antibodies suggests a recent contact with potentially infected mosquito bites. The significance of these findings in dengue epidemiology and transmission are currently under investigation.
This study has several limitations. First, the lack of storage and transport logistics limited blood collection to filter paper. Consequently, it was not possible to test for DENV infection/viremia, which are important to determine both the timing and order of exposure (36). Second, previous studies have shown that entomological parameters, such as the human biting rate, can vary within small geographical areas (37) and that factors that affect individual attractiveness to mosquitoes should be also taken into account (38). We are currently recruiting a larger study population in the same three areas of Norte de Santander to address these issues.
The use of anti-vector saliva antibodies has been used as a marker for exposure after vector control methods have been performed (11,39). As seasonal variation in human-vector contact is a dynamic process that informs DENV transmission potential, developing methods to detect changes in and risk factors for increased contact are critically important. Given the demonstration of the relationship between anti-SGE antibodies and DENV exposure, and the findings that anti-vector saliva antibodies are associated with water insecurity, it is recommended to test for such antibodies in surveillance programs to regularly monitor human-vector contact both during and between outbreaks.
Research endeavors are ongoing to elucidate the role of Ae. aegypti salivary proteins in DENV transmission and disease severity. Here we found that the immune response against salivary proteins of Ae. aegypti was associated with the presence of mosquito larva in or around houses. Our results also suggest that socioeconomic factors are associated with the concentration of antibodies to Ae. aegypti salivary proteins, and, most notably, a higher concentration of anti-DENV antibodies. This finding underscores the importance of addressing water insecurity and housing quality to control vector-borne diseases. The use of anti-vector saliva antibodies may aid in the identification of populations at greatest risk for exposure to the DENV vector, Ae. aegypti .
The authors want to thank the communities of Pamplona, Cúcuta and Los Patios for their collaboration. We are grateful to the microbiology laboratory of the local hospital of Los Patios and microbiologist Marlén Carrillo Hernández for their assistance in the collection of samples, and to Tonya Colpitts for her support in the preparation of this manuscript. We also thank Samuel Jameson at Tulane University who kindly donated the control samples.
Authors declare no conflicts of interest.
This work was supported by Universidad de Pamplona-Colombia (50-year Anniversary Grant), Colciencias-LASPAU International Doctoral Program, and by the National Institutes of Health (USA).
Corresponding author:
Berlín Londoño-Rentería, 6439 Garners Ferry Rd., Bldg 2, Columbia, South Carolina, USA
Telephone: (803) 216-3421; fax: (803) 216-3413 blondono@uscmed.sc.edu; cmores@lsu.edu
1. Gubler DJ, Rosen L. A simple technique for demonstrating transmission of dengue virus by mosquitoes without the use of vertebrate hosts. Am J Trop Med Hyg. 1976;25:146-50. [ Links ]
2. Wahala WM, Silva AM. The human antibody response to dengue virus infection. Viruses. 2011;3:2374-95. http://dx.doi.org/10.3390/v3122374 [ Links ]
3. Russell PK, Udomsakdi S, Halstead SB. Antibody response in dengue and dengue hemorrhagic fever. Jpn J Med Sci Biol. 1967;20(Suppl.1):103-8. [ Links ]
4. Rothman AL. Immunity to dengue virus: A tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol. 2011;11:532-43. http://dx.doi.org/10.1038/nri3014. [ Links ]
5. Tapia-Conyer R, Betancourt-Cravioto M, Méndez-Galván J. Dengue: An escalating public health problem in Latin America. Paediatr Int Child Health. 2012;32(Suppl.1):14-7. http://dx.doi.org/10.1179/2046904712Z.00000000046 [ Links ]
6. Santacoloma L, Chaves B, Brochero HL. Estado de la susceptibilidad de poblaciones naturales del vector del dengue a insecticidas en trece localidades de Colombia. Biomédica. 2012;32:333-43. http://dx.doi.org/10.7705/biomedica.v32i3.680 [ Links ]
7. Londoño-Rentería B, Cárdenas JC, Cárdenas LD, Christofferson RC, Chisenhall DM, Wesson DM, et al . Use of anti- Aedes aegypti salivary extract antibody concentration to correlate risk of vector exposure and dengue transmission risk in Colombia. PloS One. 2013;8:e81211. http://dx.doi.org/10.1371/journal.pone.0081211. [ Links ]
8. Londoño-Rentería BL, Eisele TP, Keating J, James MA, Wesson DM. Antibody response against Anopheles albimanus (Diptera: Culicidae) salivary protein as a meas-ure of mosquito bite exposure in Haiti. J Med Entomol. 2010;47:1156-63. [ Links ]
9. Waitayakul A, Somsri S, Sattabongkot J, Looareesuwan S, Cui L, Udomsangpetch R. Natural human humoral response to salivary gland proteins of Anopheles mosquitoes in Thailand. Acta Trop. 2006;98:66-73. http://dx.doi.org/10.1016/j.actatropica.2006.02.004 [ Links ]
10. Doucoure S, Mouchet F, Cornelie S, Drame PM, D´Ortenzio E, DeHecq JS, et al . Human antibody response to Aedes albopictus salivary proteins: A potential biomarker to evaluate the efficacy of vector control in an area of Chikungunya and dengue virus transmission. Biomed Res Int. 2014;2014:746509. http://dx.doi.org/10.1155/2014/746509 [ Links ]
11. Brosseau L, Drame PM, Besnard P, Toto JC, Foumane V, Le Mire J, et al . Human antibody response to Anopheles saliva for comparing the efficacy of three malaria vector control methods in Balombo, Angola. PloS One. 2012;7: e44189. http://dx.doi.org/10.1371/journal.pone.0044189 [ Links ]
12. Drame PM, Poinsignon A, Besnard P, Cornelie S, Le Mire J, Toto JC, et al . Human antibody responses to the Anopheles salivary gSG6-P1 peptide: A novel tool for evaluating the efficacy of ITNs in malaria vector control. PloS One. 2010;5:e15596. http://dx.doi.org/10.1371/journal.pone.0015596. [ Links ]
13. Yamashiro T, Disla M, Petit A, Taveras D, Castro-Bello M, Lora-Orste M, et al . Seroprevalence of IgG specific for dengue virus among adults and children in Santo Domingo, Dominican Republic. Am J Trop Med Hyg. 2004;71:138-43. [ Links ]
14. Méndez JA, Usme-Ciro JA, Domingo C, Rey GJ, Sánchez JA, Tenorio A, et al . Phylogenetic history demonstrates two different lineages of dengue type 1 virus in Colombia. Virol J. 2010;7:226. http://dx.doi.org/10.1186/1743-422X-7-226. [ Links ]
15. Instituto Nacional de Salud. Eventos por departamento año 2010. Sivigila, 2010. Fecha de consulta: 3 de mayo de 2013. Disponible en: http://www.ins.gov.co/lineas-de-accion/Subdireccion-Vigilancia/sivigila/Paginas/vigilancia-rutinaria.aspx [ Links ]
16. Almirón WR, Brewer ME. Classification of immature stage habitats of Culicidae (Diptera) collected in Córdoba, Argentina. Mem Inst Oswaldo Cruz. 1996;91:1-9. http://dx.doi.org/10.1590/S0074-02761996000100001 [ Links ]
17. Christofferson RC, Mores CN. Estimating the magnitude and direction of altered arbovirus transmission due to viral phenotype. PloS One. 2011;6:e16298. http://dx.doi.org/10.1371/journal.pone.0016298 [ Links ]
18. Castañeda O, Segura O, Ramírez AN. Conocimientos, actitudes y prácticas comunitarias en un brote de dengue en un municipio de Colombia, 2010. Rev Salud Pública (Bogotá). 2011;13:514-27. http://dx.doi.org/10.1590/S0124-00642011000300013 [ Links ]
19. Barrera R, Amador M, MacKay AJ. Population dynamics of Aedes aegypti and dengue as influenced by weather and human behavior in San Juan, Puerto Rico. PLoS Negl Trop Dis. 2011;5:e1378. http://dx.doi.org/10.1371/journal.pntd.0001378 [ Links ]
20. Rojas Y, Brochero H. Hallazgo de Aedes aegypti (Linnaeus 1762), en el casco urbano del corregimiento de La Pedrera, Amazonas, Colombia. Biomédica. 2008;28:587-96. http://dx.doi.org/10.7705/biomedica.v28i4.65 [ Links ]
21. Overgaard HJ, Alexander N, Matiz MI, Jaramillo JF, Olano VA, Vargas S, et al . Diarrhea and dengue control in rural primary schools in Colombia: Study protocol for a randomized controlled trial. Trials. 2012;13:182. http://dx.doi.org/10.1186/1745-6215-13-182 [ Links ]
22. Toledo-Romani ME, Vanlerberghe V, Pérez D, Lefevre P, Ceballos E, Bandera D, et al . Achieving sustainability of community-based dengue control in Santiago de Cuba. Soc Sci Med. 2007;64:976-88. http://dx.doi.org/10.1016/j.socscimed.2006.10.033 [ Links ]
23. Gomes A de C, Gotlieb SL, Marques CC, de Paula MB, Marques GR. Duration of larval and pupal development stages of Aedes albopictus in natural and artificial containers. Rev Saúde Pública. 1995;29:15-9. [ Links ]
24. Harrington LC, Edman JD, Scott TW. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and fre-quently on human blood? J Med Entomol. 2001;38: 411-22. [ Links ]
25. Halstead SB. Dengue virus-mosquito interactions. Annu Rev Entomol. 2008;53:273-91. http://dx.doi.org/10.1146/annurev.ento.53.103106.093326 [ Links ]
26. Saied KG, Al-Taiar A, Altaire A, Alqadsi A, Alariqi EF, Hassaan M. Knowledge, attitude and preventive practices regarding dengue fever in rural areas of Yemen. Int Health. 2015. http://dx.doi.org/10.1093/inthealth/ihv021 [ Links ]
27. Tran HP, Adams J, Jeffery JA, Nguyen YT, Vu NS, Kutcher SC, et al . Householder perspectives and preferences on water storage and use, with reference to dengue, in the Mekong Delta, southern Vietnam. Int Health. 2010;2:136-42. http://dx.doi.org/10.1016/j.inhe.2009.12.007. [ Links ]
28. Doucoure S, Mouchet F, Cournil A, Le Goff G, Cornelie S, Roca Y, et al . Human antibody response to Aedes aegypti saliva in an urban population in Bolivia: A new biomarker of exposure to dengue vector bites. Am J Trop Med Hyg. 2012;87:504-10. http://dx.doi.org/10.4269/ajtmh.2012.11-0477. [ Links ]
29. Lorono-Pino MA, Cropp CB, Farfán JA, Vorndam AV, Rodríguez-Angulo EM, Rosado-Paredes EP, et al . Common occurrence of concurrent infections by multiple dengue virus serotypes. Am J Trop Med Hyg. 1999;61: 725-30. [ Links ]
30. Bharaj P, Chahar HS, Pandey A, Diddi K, Dar L, Guleria R, et al . Concurrent infections by all four dengue virus serotypes during an outbreak of dengue in 2006 in Delhi, India. Virol J. 2008;5:1. http://dx.doi.org/10.1186/1743-422X-5-1 [ Links ]
31. Ndille EE, Dubot-Peres A, Doucoure S, Mouchet F, Cornelie S, Sidavong B, et al . Human IgG antibody response to Aedes aegypti Nterm-34 kDa salivary peptide as an indicator to identify areas at high risk for dengue transmission: A retrospective study in urban settings of Vientiane city, Lao PDR. Trop Med Int Health. 2014;19:576-80. http://dx.doi.org/10.1111/tmi.12280 [ Links ]
32. Rizzo C, Ronca R, Lombardo F, Mangano V, Sirima SB, Nebie I, et al . IgG1 and IgG4 antibody responses to the Anopheles gambiae salivary protein gSG6 in the sympatric ethnic groups Mossi and Fulani in a malaria hyperhendemic area of Burkina Faso. PloS One. 2014;9:e96130. http://dx.doi.org/10.1371/journal.pone.0096130 [ Links ]
33. Orlandi-Pradines E, Almeras L, Denis-de Senneville L, Barbe S, Remoue F, Villard C, et al . Antibody response against saliva antigens of Anopheles gambia e and Aedes aegypti in travellers in tropical Africa. Microbes Infect. 2007;9:1454-62. http://dx.doi.org/10.1016/j.micinf.2007.07.012 [ Links ]
34. Restrepo BN, Arboleda M. Estudio seroepidemiológico de dengue en la region del Urabá antioqueño, Colombia. Infectio. 2004;8:255-62. [ Links ]
35. Reunala T, Brummer-Korvenkontio H, Palosuo K, Miyanij M, Ruiz-Maldonado R, Love A, et al . Frequent occurrence of IgE and IgG4 antibodies against saliva of Aedes communis and Aedes aegypti mosquitoes in children. Int Arch Allergy Immunol. 1994;104:366-71. [ Links ]
36. Prince HE, Yeh C, Lape-Nixon M. Utility of IgM/IgG ratio and IgG avidity for distinguishing primary and secondary dengue virus infections using sera collected more than 30 days after disease onset. Clin Vaccine Immunol. 2011;18:1951-6. http://dx.doi.org/10.1128/CVI.05278-11 [ Links ]
37. Mbogo CM, Mwangangi JM, Nzovu J, Gu W, Yan G, Gunter JT, et al . Spatial and temporal heterogeneity of Anopheles mosquitoes and Plasmodium falciparum transmission along the Kenyan coast. Am J Trop Med Hyg. 2003;68:734-42. [ Links ]
38. Qiu YT, Smallegange RC, van Loon JJ, Ter Braak CJ, Takken W. Interindividual variation in the attractiveness of human odours to the malaria mosquito Anopheles gambiae s. s. Med Vet Entomol. 2006;20:280-7. http://dx.doi.org/10.1111/j.1365-2915.2006.00627.x [ Links ]
39. Drame PM, Poinsignon A, Besnard P, Le Mire J, Dos-Santos MA, Sow CS, et al . Human antibody response to Anopheles gambiae saliva: An immuno-epidemiological biomarker to evaluate the efficacy of insecticide-treated nets in malaria vector control. Am J Trop Med Hyg. 2010;83:115-21. http://dx.doi.org/10.4269/ajtmh.2010.09-0684 [ Links ]













