Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biomédica
Print version ISSN 0120-4157
Biomédica vol.35 no.spe Bogotá Aug. 2015
https://doi.org/10.7705/biomedica.v35i0.2422
ARTÍCULO ORIGINAL
doi: http://dx.doi.org/10.7705/biomedica.v35i0.2422
1 Línea de Toxicología Ambiental y Toxicogenómica, Grupo de Inmunología y Epidemiología Molecular, Escuela de Microbiología, Facultad de Salud, Universidad Industrial de Santander, Bucaramanga, Colombia
2 Escuela de Medicina, Facultad de Salud, Universidad Industrial de Santander, Bucaramanga, Colombia
Author´s contributions:
Luz Helena Sánchez conceived and designed the study, recruited people, supervised development of the work, performed the experimental assays, helped in data analysis and manuscript evaluation.
Olga Marcela Medina helped to perform the experimental assays.
Guillermo Gómez calculated the sample size, helped to statistical analysis and revised the manuscript.
Clara Isabel González helped with the genotyping assay tool.
Oscar Flórez-Vargas conceived and designed the study, standardised molecular and biochemical assays, analysed results and wrote the manuscript.
Recibido: 26/06/14; aceptado: 20/11/14
Introduction: The determination of cholinesterase (ChE) activity has been commonly applied in the biomonitoring of exposure to organophosphate and carbamate pesticides. However, ChE activity is influenced by genetic factors. Integrating genotype and phenotype information in clinical laboratory tests would increase the accuracy of the reference values in well-defined populations.
Objective: To establish genetic-based reference values for acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activity in a Colombian population.
Materials and methods: A total of 397 healthy adults from Bucaramanga were included in the study. AChE and BChE activities were measured in blood samples by potentiometry and spectrophotometry, respectively. Genotyping for ACHE rs17880573 and BCHE rs1803274 was performed using the TaqMan allelic discrimination assay. The statistical analyses to obtain the reference values were performed with the MedCalc® software.
Results: Allele frequencies were 10.58% for rs17880573 A and 8.82% for rs1803274 A. People with genotypes rs1803274 AA and AG showed a reduction of 20.69% and 10.92% respectively in mean BChE activity compared to people with genotype GG. No significant differences were identified in AChE activity between rs17880573 alleles or genotypes. In the overall sample, the corresponding reference values were as follows: for AChE activity, 0.62–0.98 D pH/h and for BChE activity, 4796.3–10321.1 U/L for people carrying the allele rs1803274A and 5768.2–11180.4 U/L for people carrying the genotype rs1803274 GG.
Conclusion: We strongly recommend using these genetic-based reference values for ChE enzymes in our well-defined population in daily clinical practice.
Key words: Acetylcholinesterase, butyrylcholinesterase , reference values, organophosphorus compounds , carbamates, Colombia.
doi: http://dx.doi.org/10.7705/biomedica.v35i0.2422
Valores de referencia basados en el contexto genético de la actividad enzimática de la colinesterasa en una población colombiana: un paso hacia el diagnóstico personalizado
Introducción. La determinación de la actividad enzimática de la colinesterasa se utiliza comúnmente en la vigilancia biológica de la exposición a pesticidas organofosforados y carbamatos. Sin embargo, los factores genéticos afectan la actividad de la colinesterasa, por lo que la integración de la información sobre genotipos y fenotipos en las pruebas de laboratorio clínico, incrementaría la precisión de los valores de referencia.
Objetivo. Establecer los valores de referencia basados en el contexto genético para la actividad enzimática de la acetilcolinesterasa (AChE) y la butirilcolinesterasa (BChE), en una población colombiana.
Materiales y métodos. Se incluyeron 397 adultos sanos. La actividad de la acetilcolinesterasa y la de la butirilcolinesterasa, se determinaron en muestras de sangre por potenciometría y espectrofotometría, respectivamente. Los genotipos de los ACHE rs17880573 y BCHE rs1803274 se obtuvieron mediante el ensayo TaqMan y los valores de referencia se estimaron con el programa MedCalc®.
Resultados. La frecuencia alélica fue de 10,58 % para rs17880573 A y de 8,82 % para rs1803274 A. Las personas con los genotipos rs1803274 AA y AG, mostraron una reducción en el promedio de la actividad de la butirilcolinesterasa de 20,69 % y de 10,92 %, respectivamente, comparados con aquellas con el genotipo GG. No se encontraron diferencias significativas en la actividad de la acetilcolinesterasa con respecto a los alelos y genotipos del rs17880573. Los valores de referencia determinados para esta población fueron de 0,62-0,98 D pH/h para acetilcolinesterasa y de 4796,3-10321,1 U/L para butirilcolinesterasa, en las personas portadoras del alelo rs1803274 A, y de 5768,2–11180,4 U/L, en las portadoras del genotipo rs1803274 GG.
Conclusión. Se recomienda el uso de estos valores de referencia basados en el contexto genético para las colinesterasas, en la práctica clínica diaria en esta población.
Palabras clave: acetilcolinesterasa, butirilcolinesterasa, valores de referencia, compuestos organofosforados, carbamatos, Colombia.
doi: http://dx.doi.org/10.7705/biomedica.v35i0.2422
Cholinesterases, or ChE, are a family of enzymes that catalyse the hydrolysis of the neurotransmitter acetylcholine (ACh) into choline and acetic acid, an essential reaction necessary to allow a cholinergic neuron to return to the resting state after impulse transmission. This process is important to prevent an overstimulation of muscles due to the build-up of acetylcholine in the neuromuscular junction and thus muscle spasms that can lead to death. Cholinesterases are divided into two classes based on differences in their substrate specificity, susceptibility to various kinds of inhibitors and tissue distribution: acetylcholinesterase (AChE; EC 3.1.1.7) and butyrylcholinesterase (BChE; EC 3.1.1.8) (1).
The determination of ChE activity has been com monly applied in the biomonitoring of exposure to organophosphate and carbamate pesticides (2), especially in high-risk individuals such as farmworkers. The progressive inhibition of AChE or BChE by these two pesticides may produce a cholinergic response, inducing bronchospasm and bradycardia. In Colombia, for instance, it has been found that the most common pesticide poisoning cases were due to organophosphates and car bamates (3). The basic clinical diagnosis due to the poisoning by these two pesticides is based on depression in the activity of serum and red blood cell ChE that, in turn, are based on the knowledge of reference ranges of these enzyme activities in the corresponding general population.
Laboratory reference ranges for ChE in healthy populations that are currently used in Colombia and other Latin American countries are derived mainly from data obtained from North American and European populations. However , the laboratory parameters vary geographically by factors such as ethnic origin and the altitude above sea level of populations, and inter-individually by demographic and genetic factors (4,5). Nevertheless, among them the genetic factors presumably play a major role. Thus, it is important to adjust the laboratory parameters of ChE accurately based on those factors in well-defined populations.
AChE and BChE share approximately 54% amino acid sequence identity. Nevertheless, in comparison with BChE, AChE is a highly conserved molecule across species (6) and only a few naturally occurring genetic polymorphisms have been reported in the human gene (7). The genetic variation of single nucleotide polymorphisms (SNPs) in these genes has been largely studied in relation to the onset of Alzheimer´s disease (8,9). There are no studies that prove SNPs in the ACHE gene affect AChE activity. However, SNPs in the BCHE gene, and especially the polymorphic K-variant (the A allele of the Ala539Thr polymorphism; rs1803274), have been shown to be associated with a substantial reduction in the catalytic activity of BChE (10). In this context, integrating genetic and laboratory information could increase the accuracy of reference intervals by reducing outliers related to genetic variation, which is a prerequisite for correct interpretation of toxicological findings. Therefore, in this study we established comprehensive genetic-based reference values for AChE and BChE activity in a Colombian population.
Materials and methods
Population
The study included 397 healthy adults from Bucaramanga, Colombia, with no history of exposure to ChE inhibitors and without physiological conditions that could affect the ChE levels such as pregnancy or obesity. The population of this region does not have a concentration of ethnical groups and it is not structured (11,12). Health status was evaluated by body mass index (BMI=18 and =30) and blood analysis: haemoglobin, albumin and glutamic-pyruvate transaminase were measured by spectrophotometric methods according to the kit manufacturer´s instructions (Human; Germany). Subjects with abnormal liver function (serum albumin <3.8 g/dL and glutamic-pyruvate trans-aminase at 37°C >32 U/I for women and >42 U/I for men) or haemoglobin levels (<12 g/dL or >18 g/dL for women and >19 g/dL for men) or BMI (< 18 or > 30) were excluded. People were also excluded if they had a history of anaemia, malnutrition, diabetes, hepatitis, thyroid disease or if they were taking a strong analgesic such as codeine.
Determination of cholinesterase activities
ChE activities were measured in human blood samples drawn after a 12-h overnight fast. People should not have consumed alcohol for at least 72 hours prior to blood-sampling. Intravenous blood was collected in 7 mL heparin Vacutainer tubes (Becton-Dickinson; USA). The blood samples were centrifuged at 2000 rpm for 15 min within 2 hours of collection to separate erythrocytes and plasma that were used for AChE and BChE measurement, respectively. All specimens were processed immediately after they were obtained.
Acetylcholinesterase assay. AChE-activity was measured by the potentiometric method described by Michel (13). Briefly, the erythrocytes were washed twice with 0.9% NaCl by repeated resuspension and centrifugation at 2500 rpm for 5 min. Samples were adjusted to a haematocrit of 50% (v/v) in isotonic saline solution as an erythrocyte suspension, then 80 µl of the erythrocyte suspension were lysed with 1.92 mL of 0.01% saponin. One millilitre of the haemolysate was mixed with 1 mL of a buffer solution containing 0.02 M sodium barbital, 0.004 M KH 2 PO 4 and 0.6 M KCl of pH 8.10 at 25°C. Thereafter, the pH of the mixture (pH1) was measured with a glass electrode using a Seven EasyTM pH meter (Mettler-Toledo International Inc.; USA), and then, 200 µl of 0.11 M aqueous solution of acetylcholine chloride were added to the mixture. The reaction mixture was incubated at 25°C for 1 h. At the end of the incubation period, the pH of the reaction mixture (pH2) was measured. The enzyme activity was calculated using the following equation:
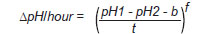 equation (1),
equation (1), where the initial and final pH values correspond to pH 1 and pH 2, respectively; t is the reaction time (1 hour); b is the corrected value due to non-enzymatic hydrolysis and f is the variation value of D pH/h (13).
Butyrylcholinesterase assay. BChE-activity was measured with a spectrophotometer equipped with a thermocontrolled cell-holder using the Selectra JR clinical chemistry analyser (Myco Instrumentation, Inc.; USA), and butyrylthiocholine (60 mmol/L) as a substrate (BioSystems, S.A.; Spain). The first read was done after reaction mixture was incubated at 37°C for 90 s, and then readings were taken every 30 s for 90 s. The enzyme activity was calculated using the following equation:
where the molar extinction coefficient ( e ) of the chromogen at 405 nm is 927; l is the light-path (1 cm); Vt is the total volume of reaction mixture (1.525); Vs is the sample volume (0.025) and 1 U/L is equivalent to 0.0166 µkat/L (BioSystems S.A.; Spain).
Single-nucleotide polymorphism selection and genotyping. Single-nucleotide polymorphisms were selected according to functional considerations and prediction as tag SNPs based on a minor allele frequency (MAF) =10% and a linkage disequilibrium (LD) coefficient r 2 =0.80 using the aggressive tagging algorithm implemented in Haploview 4.2 software (14). The genotype data set of European–American ancestry [CEU] and Mexican–American ancestry [MEX] published in the International HapMap Project (15) phase 3 release 3 was used as the reference population.
Genomic DNA was isolated from 7 mL of EDTA-treated blood samples using the standard salting-out technique (16). DNA concentration was measured by spectrophotometry at A 260 and A 280 using a NanoDrop 2000 (Thermo Fisher Scientific; USA). The final concentration of genomic DNA was adjusted to 100 ng/µL. ACHE rs17880573 and BCHE rs1803274 were genotyped by TaqMan allelic discrimination assay using a StepOnePlus™ Real-Time PCR (Life Technologies; USA). Allele-specific probes were labelled with VIC and FAM fluorescent dyes. The design and validation of TaqMan® SNP genotyping assays were performed by Applied BioSystems (Life Technologies; USA).
Statistical analysis
The sample size was determined based on an estimated population of 165,263 people in Bucaramanga who were between 20 and 64 years of age according to the Colombian Departamento Administrativo Nacional de Estadística (DANE). The minimum sample size was calculated as 390 subjects considering a sampling error of 0.0082 and ChE (total) population values, a significance level of 0.05 and a confidence interval of 95%.
Allele and genotype frequencies were determined by direct counting. Genotype frequency deviation from Hardy-Weinberg equilibrium was estimated using the Markov chain random-walk algorithm implemented in Arlequin 3.11 software (17) . A p -value <0.05 was considered as evidence of deviation from Hardy–Weinberg equilibrium. Statistical comparisons of ChE activities among different alleles, genotypes, sex, and ag e- groups were performed by the use of ANOVA or an unpaired T-test using the software Stata 10.0 (18). The smoothed local regression (loess) curves were used to examine the effect of sex and age on AChE and BChE activity levels. A variance partitioning analysis was performed as an exploratory method to assess the usefulness of partitioning. A difference between subpopulation averages =40% of the subsample standard deviations (Eq. 3) and a proportion of the total variance attributable to the genetic polymorphism =4% (Eq. 4) were the criteria considered for justifying the partitioning (19) using the following equations:
where x 1 and x 2 are the average of the analyte in each subgroup, and a 1 and a 2 are the relative proportions of each subgroup with respect to the total group; s t and s t 2 are the standard deviation and variance of the analyte concentration in the total group, respectively; q represents the minor allele frequency of the polymorphism and p the frequency of the common allele (19).
Reference intervals, defined as the central 95% of the population (2.5 th -97. 5th percentiles), were calculated based on normal distribution following the International Federation of Clinical Chemistry & Laboratory Medicine (IFCC) recommendations reported in the guidelines C28-A2 and C28-A3 for estimating percentiles and their 90% confidence intervals as set by the Clinical and Laboratory Standards Institute (CLSI). The statistical analysis used to obtain the reference values were performed with MedCalc® v13.1.0 software (20).
Ethical considerations
This study complied with the Colombian Medical Code of Ethics and it has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. The Scientific Investigation Committee on Medical Ethics of the Universidad Industrial de Santander approved the research protocol. All subjects were enrolled in the study following informed consent.
Results
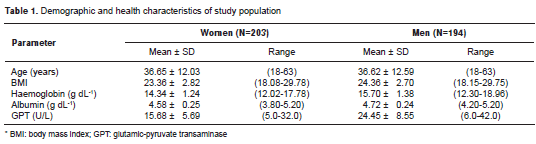
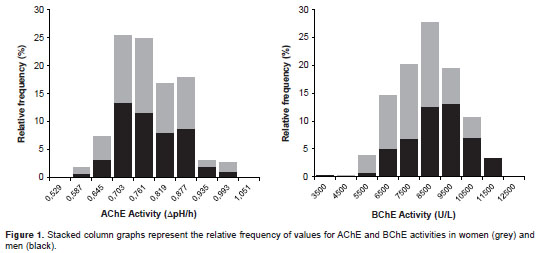
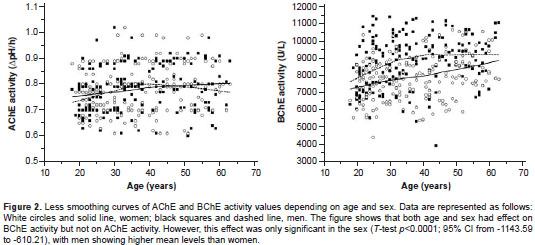
Socio-demographic and health characteristics of the participants are summarized in table 1. A relative frequency distribution shows the proportion of women and men according to ChE activity values in figure 1. In the overall sample, the AChE and BChE activity values follow an approximately normal distribution with a mean ± standard deviation of 0.776 ± 0.083 ? pH/hour (95% CI from 0.767 to 0.784) and 8397.09 ± 1412.88 U/L (95% CI from 8254.46 to 8539.71), respectively. The distributions of AChE and BChE activity levels according to age and sex are illustrated in figure 2. Sex had a significant effect on BChE activity ( T- test p <0.0001; 95% CI from -1143.59 to -610.21) but not on AChE activity ( T- test p =0.84), with men showing higher mean levels than women (figure 2). In addition, the AChE and BChE activity in men tended to rise until age 30-45 where it then leveled off, as can be observed in the smoothing curves (figure 2).
Frequency of genotypes and alleles of the SNPs and ChE activities are presented in table 2. The genotype frequencies for both SNPs were distributed in accordance with the Hardy-Weinberg equilibrium ( ACHE rs17880573: c 2 =0.59; p =0.444, and BCHE rs1803274: c 2 =1.43; p =0.232). No differences in AChE activity between ACHE rs17880573 alleles and among genotypes were identified (T-test p=0.53). However, in BChE activity a statistically significant difference was identified between BCHE rs1803274 alleles (T-test p<0.0001; 95% CI from 621.02 to 1376.36) and among genotypes (ANOVA-test, p<0.001). People with genotype rs1803274 AA and AG showed a reduction of 20.69% and 10.92% respectively in mean BChE activity as compared to people with genotype GG (table 2 and figure 3).
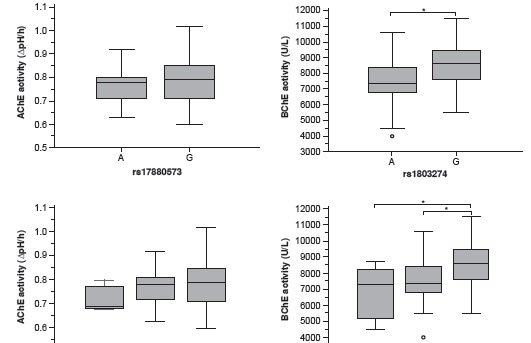
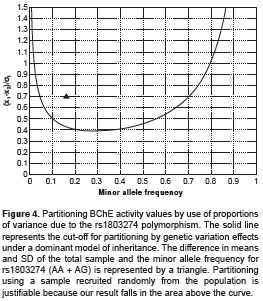
The partitioning analysis by genetic variant showed that the average BChE activity differed between BCHE rs1803274 subpopulations by 70.6% and that this SNP could explain 6.38% of the total variance of the enzymatic activity under a model of dominant inheritance (figure 4). Therefore, the genetic effect of this polymorphism is large enough to justify the partitioning of the population into two subpopulations: BCHE rs1803274 A (AA + AG) and GG. Partitioning by sex and age was justified based on the T -test and ANOVA-test results (table 2). Reference values are presented in table 3. These reference values were determined considering the differences found in the enzyme activity in terms of sex, age and genetic variation.
Discussion
Laboratory reference values based on genetic information would increase the accuracy of correct identification of pathological conditions by eliminat ing results related to genetic variation . The reference values reported in this study are a combination of both individual and population level approaches that can be used to help improve the diagnosis and treatment of poisoning caused by the handling of organophosphate and carbamate pesticides, as well as to evaluate sensitivity to succinylcholine prior to surgical procedure. To our knowledge, this is the first report in the literature performed with the aim of establishing reference values for the AChE and BChE enzyme activities based on genetic variants in a Colombian population.
Prior publications have reported reference values for AChE and BChE enzyme activities in Colombia. However, as was expected, our values were found to be different to the ranges previously published in the department of Antioquia, Colombia (4,21). These differences are related mainly to variation in geographic, demographic and genetic factors, as well as in the laboratory techniques used; hence, the results are difficult to compare. Michel, EQM® and Monotest®, for instance , were the methods used to quantify the enzymatic activity in these studies; each method reports the enzymatic activity using different units of measurement. Moreover, and although only the last two are commercial tests (which in principle means that they are standardised), the Michel method was not adequately described; e.g . there is no information about the substrate itself, i .e., was it acetylcholine chloride or bromide? Or were the samples processed fresh or after freezing? These and others variables do influence the results (22) and, as a consequence, the reference values.
Geographically, Bucaramanga is situated on a plateau in the Cordillera Oriental of the Colombian Andes at an altitude of 959 meters (3,146 feet) above sea level. This, in comparison with the other studies, is more than 500 and up to 1200 meters difference in elevation. Altitude is a well- known non-physiological parameter that affects red blood cell count, the source of AChE in blood. Regarding the demographic and genetic factors, it is important to note that the population from this region does not have a concentration of ethnic groups and it is not structured (11,12), meaning that it has one subpopulation that is the population itself. Thus, in principle, any consideration based on genetic parameters applies to the population level. Therefore, it is reasonable to believe that the results presented in this article are an accurate representation of Bucaramanga´s population.
There is no information about the effect of the ACHE rs17880573 polymorphism on AChE activity. This SNP was chosen because it was the tag SNP with the highest minor allele frequency (15%) in the CEU population according to the HapMap and because it maps closely to the regulatory region of the ACHE gene and, hence, it could be a marker for the regulation of this gene. However, no significant difference in the AChE activity was observed with respect to the SNP rs17880573 (figure 3 and table 2).
In the case of BChE activity, it is well known that the BCHE rs1803274 polymorphism (nucleotide substitution G>A) determines an amino acid change from Ala (GCA) to Thr (ACA) at residue 539 (K variant) located in the C-terminal tetramerization domain of BChE. Contradictory findings have been reported for the effects of this polymorphism on the catalytic properties of the enzyme: Peptides with the C-terminal sequence carrying the K-variant exhibited similar (23) and different (24) enzymatic activity, protein folding and tetramer formation. Thus, more experimental evidence is needed to clear up these biochemical inconsistencies. Be that as it may, it has been reported that individuals with Thr/Thr genotype have a reduced BChE activity of about 30% (10). However, although a significant difference in the BChE activity was observed with the SNP rs1803274, our experimental evidence shows that subjects with genotype rs1803274 AA and AG showed a reduction in mean BChE activity of 20.69% and 10.92%, respectively, compared to subjects with genotype GG (figure 3 and table 2). These differences could be explained, at least in part, by the patterns of linkage disequilibrium with other SNPs such as rs1799807 (10) and rs1126680 (25), which impact the enzymatic activity by affecting the affinity for cholinesterase inhibitors (26) and the level of BChE expression (27), respectively. Regarding this, there is epidemiological evidence from a body mass index study in Brazil that suggests that the lower BChE activity associated with the SNP rs1803274 is strongly influenced by the SNP rs1126680 (27). However, if this were true in our population, it would not have a significant impact on the reference values reported here; on the one hand, because the SNP rs1803274 is globally more frequent according to the NCBI dbSNP (MAF 0.16 vs 0.035), being 0.088 the MAF found in our population (table 2), and on the other hand, because the majority of chromosomes bearing the SNP rs1126680 also carry the SNP rs1803274 due to the strong linkage disequilibrium ( D´ > 90%) between these two SNPs (27).
The results of the BChE enzyme activity by genotype clearly suggest an additive effect of the rs1803274 polymorphism (table 2 and figure 3). Thus, the partitioning strategy should be based on the genotypes. However, due to the low frequency of individuals being homozygous for this polymorphism (table 2) a dominant model of inheritance was assumed, because individuals with the genotype AG and GG represent the vast majority of the total population (19).
In conclusion, for the first time we have established sex and age-related normal ranges for the enzyme activity of AChE and BChE based on genetic polymorphisms. We believe that this approximation is an important step toward personalised medicine. In this way, we strongly recommend using these reference values from our well-defined population in daily clinical practice not only at hospital level, but also at occupational level.
The authors want to thank all people who partici pated in the study for allowing access to the blood samples and for their patience and kindness. The authors are especially grateful to Linda Rocha, Lina Flórez and Julio Flórez for their excellent technical assistance. Thanks also go to Michael Bramhall for his proofreading of the manuscript and suggestions .
The authors declare that they have no conflict of interest.
This work was funded by Universidad Industrial de Santander, Bucaramanga, Colombia, through grant 5670.
Corresponding author:
Óscar Flórez-Vargas, School of Computer Science, University of Manchester, Kilburn building, Oxford Road, Manchester M13 9PL, United Kingdom Telephone: (440) 16 1306 9280, extension: 56132 florezvo@cs.man.ac.uk, o.florezvargas@gmail.como.florezvargas@gmail.com
1. Pohanka M. Cholinesterases, a target of pharmacology and toxicology. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155:219-29. http://dx.doi.org/10.5507/bp.2011.036 [ Links ]
2. Nigg HN, Knaak JB. Blood cholinesterases as human biomarkers of organophosphorus pesticide exposure. Rev Environ Contam Toxicol. 2000;163:29-111. http://dx.doi.org/10.1007/978-1-4757-6429-1_2 [ Links ]
3. Idrovo AJ. Vigilancia de las intoxicaciones con plaguicidas en Colombia. Rev Salud Pública. 2000;2:36-46. [ Links ]
4. Carmona-Fonseca J. Reference values for erythrocyte cholinesterase activity in the working population of Antioquia, Colombia, according to the Michel and EQM techniques. Rev Panam Salud Pública. 2003;14:316-324. [ Links ]
5. Brock A, Brock V. Factors affecting inter-individual variation in human plasma cholinesterase activity: Body weight, height, sex, genetic polymorphism and age. Arch Environ Contam Toxicol. 1993;24:93-9. http://dx.doi.org/10.1007/BF01061095 [ Links ]
6. Johnson G, Moore SW. Why has butyrylcholinesterase been retained? Structural and functional diversification in a duplicated gene. Neurochem Int. 2012;61:783-97. http://dx.doi.org/10.1016/j.neuint.2012.06.016 [ Links ]
7. Hasin Y, Avidan N, Bercovich D, Korczyn AD, Silman I, Beckmann JS, et al . Analysis of genetic polymorphisms in acetylcholinesterase as reflected in different populations. Curr Alzheimer Res. 2005;2:207-18. http://dx.doi.org/10. 2174/1567205053585909 [ Links ]
8. Piccardi M, Congiu D, Squassina A, Manconi F, Putzu PF, Mereu RM, et al . Alzheimer´s disease: Case-control association study of polymorphisms in ACHE , CHAT , and BCHE genes in a Sardinian sample. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:895-9 . http://dx.doi.org/10.1002/ajmg.b.30548 [ Links ]
9. Scacchi R, Gambina G, Moretto G, Corbo RM. Variability of AChE, BChE, and ChAT genes in the late-onset form of Alzheimer´s disease and relationships with response to treatment with Donepezil and Rivastigmine. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:502-7. http://dx.doi.org/10.1002/ajmg.b.30846 [ Links ]
10. Bartels CF, Jensen FS, Lockridge O, van der Spek AF, Rubinstein HM, Lubrano T, et al . DNA mutation associated with the human butyrylcholinesterase K-variant and its linkage to the atypical variant mutation and other polymorphic sites. Am J Hum Genet. 1992;50:1086-1103. [ Links ]
11. Hincapié ML, Gil AM, Pico AL, Gusmao L, Rondón F, Vargas CI, et al . Análisis de la estructura genética en una muestra poblacional de Bucaramanga, departamento de Santander. Colom Med. 2009;40:362-73. [ Links ]
12. León FJ, Rondón F, Vargas CI, Oróstegui M, Bautista L, Serrano NC, et al . Analysis of population genetic structure from Bucaramanga (Colombia) based on gene poly- morphisms associated with regulation of blood pressure. Colomb Med. 2012;43:154-61. [ Links ]
13. Michel HO. An electrometric method for the determination of red blood cell and plasma cholinesterase activity. J Lab Clin Med. 1949;34:1564-8. [ Links ]
14. Barrett JC, Fry B, Maller J, Daly MJ. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263-5. http://dx.doi.org /10.1093/ bioinformatics/ bth457 [ Links ]
15. Thorisson GA, Smith AV, Krishnan L, Stein LD. The International HapMap Project web site. Genome Res. 2005;15:1592-3. http://dx.doi.org/10.1101/gr.4413105 [ Links ]
16. Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. [ Links ]
17. Excoffier L, Laval G, Schneider S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol Bioinform Online. 2005;1:47-50. [ Links ]
18. StataCorp. Stata Statistical Software: Release 11. 2009. [ Links ]
19. Shirts BH, Wilson AR, Jackson BR. Partitioning reference intervals by use of genetic information. Clin Chem. 2011;57:475-81. http://dx.doi.org/10.1373/clinchem.2010.154005 [ Links ]
20. MedCalc. MedCalc Statistical Software version 12.7.8. 2014. [ Links ]
21. Carmona-Fonseca J. Levels of plasma cholinesterase in Colombian working- class populations. Biomédica. 2003;23: 437-55. [ Links ]
22. Tecles F, Gutiérrez C, Cerón JJ. Estabilidad de la actividad colinesterasa en muestras de sangre entera de distintas especies animales sometidas a diferentes condiciones de almacenamiento. Anales de Veterinaria. 2002;18:33-41. [ Links ]
23. Altamirano CV, Bartels CF, Lockridge O. The butyrylcholinesterase K-variant shows similar cellular protein turnover and quaternary interaction to the wild- type enzyme. J Neurochem. 2000;74:869-77. http://dx.doi.org/10.1046/j.1471-4159.2000.740869.x [ Links ]
24. Podoly E, Shalev DE, Shenhar-Tsarfaty S, Bennett ER, Ben Assayag E, Wilgus H, et al . The butyrylcholinesterase K variant confers structurally derived risks for Alzheimer pathology. J Biol Chem. 2009;284:17170-9. http://dx.doi.org/10.1074/jbc.M109.004952 [ Links ]
25. Bartels CF, van der Spek AF, La Du BN. Two poly-morphisms in the non-coding regions of the BCHE gene. Nucleic Acids Res. 1990;18:6171. >http://dx.doi.org/10.1093/ nar/18.20.6171-a [ Links ]
26. Dimov D, Kanev K, Dimova I. Correlation between butyrylcholinesterase variants and sensitivity to soman toxicity. Acta Biochim Pol. 2012;59:313-6. [ Links ]
27. Furtado-Alle L, Andrade FA, Nunes K, Mikami LR, Souza RL, Chautard-Freire-Maia EA. Association of variants of the -116 site of the butyrylcholinesterase BCHE gene to enzyme activity and body mass index. Chem Biol Interact. 2008;175:115-8. http://dx.doi.org/10.1016/j.cbi.2008.04.019 [ Links ]













