Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Biomédica
Print version ISSN 0120-4157
Biomédica vol.36 supl.2 Bogotá Aug. 2016
https://doi.org/10.7705/biomedica.v36i0.2976
ORIGINAL ARTICLE
doi: http://dx.doi.org/10.7705/biomedica.v36i0.2976
1 Grupo de Gastrohepatología, Facultad de Medicina, Universidad de Antioquia, Medellín, Colombia
2 Facultad de Ciencias Exactas, Instituto Tecnológico Metropolitano, Medellín, Colombia
3 Laboratorio Departamental de Salud Pública, Secretaría Seccional de Salud y Protección Social de Antioquia, Medellín, Colombia
Author´s contributions:
Julio C. Rendón: conception, design and performance of experiments, data analysis and drafting of the manuscript
Fabián Cortés-Mancera: conception and design of experiments, data analysis
Marta C. Ospina: serological markers
María Cristina Navas: conception and design of experiments, data analysis and drafting of the manuscript
Alejandra Duque-Jaramillo: data analysis and drafting of the manuscript
All authors read and approved the final manuscript.
Recibido: 15/07/15; aceptado: 03/12/15
Introduction: Ten viral genotypes (A-J) distributed in all continents have been described for hepatitis B virus (HBV). One of the methodologies for determining the viral genotype is the restriction fragment length polymorphism (RFLP) technique, a simple and relatively inexpensive method, albeit with some limitations.
Objective: The initial objective of the project was to identify the HBV genotypes by RFLP in serum samples obtained from patients and blood donors. However, due to the discrepancies of RFLP patterns it was also necessary to perform phylogenetic genotyping and in silico analysis of HBV sequences.
Materials and methods: We obtained 56 serum samples. DNA extraction was followed by PCR amplification of a fragment of HBV ORF S. We analyzed PCR products by RFLP with Alw I, Bsr I, Cfr I, Hpa II and Sty I, and we sequenced some. We compared the patterns obtained with those in previous reports. We also performed RFLP analysis in silico since we found differences between the patterns expected and those obtained
Results: We identified genotypes A and F, subgenotype F3, in the samples. This result is in agreement with those of previous studies carried out in Colombia; indeed, subgenotype F3 is the most frequent in the Andean region of the country, while genotype A is the most frequent HBV genotype in the western region (department of Chocó). Based on the in silico analysis of 229 HBV sequences from GenBank and 11 sequences of this study, we identified the RLFP pattern for genotype F, subgenotype F3, and we described some modifications of genotype A RFLP patterns.
Conclusions: We identified the single nucleotide polymorphism pattern for genotype F, subgenotype F3, by in silico analysis and sequencing. Further robust in silico analyses are necessary to validate the RFLP patterns of HBV genotype and subgenotypes.
Key words: Hepatitis B virus; polymorphism, restriction fragment length; genotype.
doi: http://dx.doi.org/10.7705/biomedica.v36i0.2976
Análisis de genotipos del virus de la hepatitis B mediante el análisis de polimorfismos de longitud de fragmentos de restricción
Introducción. Se han descrito diez genotipos (A-J) del virus de la hepatitis B (HBV) que están distribuidos en todos los continentes. Una de las técnicas utilizadas para determinar el genotipo viral es el análisis del polimorfismo de longitud de los fragmentos de restricción, un método simple y económico, pero con algunas limitaciones.
Objetivo. El objetivo inicial del estudio fue identificar el genotipo del HBV mediante RFLP en muestras de suero obtenidas de pacientes y donantes de sangre. Sin embargo, por las discrepancias observadas en los patrones de RFLP fue necesario realizar análisis filogenéticos y un análisis in silico de secuencias del HBV.
Materiales y métodos. Se obtuvieron 56 muestras de suero. Tras la extracción de ADN, se amplificó un fragmento del ORF S del HBV mediante reacción en cadena de la polimerasa, cuyos productos se analizaron por RFLP con las enzimas Alw I, Bsr I, Cfr I, Hpa II y Sty I, y algunos se secuenciaron. Los patrones obtenidos se compararon con los reportados previamente. Se efectuó un análisis in silico de RFLP en consideración de las diferencias entre los patrones esperados y los observados.
Resultados. Se identificaron los genotipos A y F, subgenotipo F3, en las muestras. Este resultado coincide con lo descrito en estudios previos en los que se ha demostrado que el genotipo F, subgenotipo F3, es prevalente en la población de la región andina del país, en tanto que el genotipo A predomina en el occidente (departamento del Chocó). Con base en el análisis in silico de 229 secuencias virales obtenidas del GenBank y las 11 secuencias de este estudio, se caracterizó un nuevo patrón de RFLP específico para el genotipo F, subgenotipo F3, y se describieron algunas modificaciones en el patrón de RFLP del genotipo A, subgenotipo A1.
Conclusiones. Se caracterizó el patrón de genotipificación del genotipo F, subgenotipo F3, del HBV mediante RFLP, análisis in silico y secuenciación. Se requieren nuevos análisis in silico con un número mayor de secuencias para validar los patrones de RFLP de los genotipos y subgenotipos del VHB.
Palabras clave: virus de la hepatitis B, polimorfismo de longitud del fragmento de restricción, genotipo.
doi: http://dx.doi.org/10.7705/biomedica.v36i0.2976
Hepatitis B virus (HBV) infection is a worldwide public health problem (1). Hepatitis B virus belongs to the Hepadnaviridae family, genus Orthohepadnavirus and has a partially double-stranded circular DNA genome of 3.2 kb, with four overlapped open reading frames (ORF): S, precore/core, polymerase and X (2-4).
Hepatitis B virus has been classified as having 10 genotypes (A-J), and subgenotypes have been reported for four of these, i.e., A-D, F and I (5-10). The geographical distribution of HBV genotypes is as follows: A (subgenotypes A1-A6) in Asia, Africa, Europe and America; B (subgenotypes B1-B9) in Asia, Oceania and Canada; C (subgenotypes C1-C16), prevalent in Asia and Oceania. Genotype D (subgenotypes D1-D9) has a global distribution, and so does genotype A; however, it circulates mostly in Europe, the Middle East, North Asia, Australia and the USA. Genotype E is found in West and Central Africa, although a few cases have also been reported in Europe (11) and Colombia (12). Genotype G is found in France, Germany, Japan, USA and Africa. The two newly identified and putative genotypes I (I1-I2) and J were described in samples obtained from patients from Laos (13) and Japan (14), respectively. Genotypes F and H are exclusive to America: Genotype F (subgenotypes F1-F4) is found throughout the Americas, from Alaska to Argentina, while genotype H is found in Central America and southern US (7-9,15-18).
Colombia is a country of low-intermediate prevalence for hepatitis B infection (19). According to the Instituto Nacional de Salud , 2,258 cases of hepatitis B were reported in 2014 (incidence: 4.73/100,000); however, the departments of Amazonas, Norte de Santander, Guainía, Guaviare and Chocó showed higher incidence rates of HBV infection (20).
The gold standard technique for HBV genotyping is phylogenetic analysis (21); however, this technique is expensive and time-consuming, it requires technology resources and trained personnel that are not widely available in public health laboratories. Alternative techniques that have been used for HBV genotyping include microarrays (DNA-chips) (22), restriction fragment length polymorphism (RFLP) (23), multiplex PCR (24) and hybridization with genotype-specific probes (INNO-LiPA) (25,26).
Various RFLP protocols for HBV genotyping have been described, most of them based on the amplification and restriction of ORF S sequences (23,27-29). The RFLP method is used in genotyping studies because it is a simple and relatively inexpensive method to determine the HBV genotype, particularly for large-scale analyses (30-32).
In the present study, we used RFLP to characterize HBV strains obtained from Colombian patients and blood donors. As we found inconsistencies with previously published RFLP patterns for A and F genotypes, we carried out an in silico RLFP analysis to clarify these results. We report here the new restriction pattern for genotype F, subgenotype F3, and some modifications of genotype A, sub-genotype A1, pattern.
Materials and methods
Samples
We obtained 56 serum samples from patients, blood donors and asymptomatic individuals with risk factors. We included 45 samples from blood donors positive for hepatitis B surface antigen (HBsAg) and anti-HBV core antibodies (anti-HBc). The samples were sent in 2007 by four different blood banks to the Laboratorio de Salud Pública de Antioquia for confirmatory tests. Nine samples from patients with clinical diagnosis of viral hepatitis (HBsAg+ and anti-HBc+) were obtained during 2008 and 2009 from primary health units in Medellín. We also included two serum samples obtained in 2009 from asymptomatic patients with risk factors for HBV infection from the cities of Quibdó and Apartadó (western Colombia), positive for HBsAg by rapid test (One Step HBsAg Rapid Test Kit, Intec, China) and by ELISA (HBsAg microparticle enzyme immunoassay, Abbot, USA).
Samples were stored at -70°C prior to DNA extraction, which was done no later than six months after serum collection.
All participating patients and blood donors signed the informed consent and donation forms. The ethics committees of Universidad de Antioquia-SIU and the Fundacion Antioqueña de Infectología approved the studies.
HBV DNA detection
We extracted total DNA from 175 µ l of each serum sample using TRIzol Reagent (Invitrogen, USA). We amplified a fragment of the HBV ORF S (585 nt) by hemi-nested or nested polymerase chain reaction (PCR), using primers PresS2, S1R, Ys1 and YS2 (29).
For the nested PCR, we performed both amplification rounds using two units of Taq polymerase (Fermentas, USA), 2.5 mM of MgCl 2 , 5 µ M of dNTP (Promega, USA) and 0.5 mM of primers S1R and PrsS2 for the first PCR, and of YS1 and YS2 for the second one. The thermal cycling conditions were as follows: An initial 3 min step at 95°C followed by 40 cycles of amplification at 94°C for 45 sec, 53°C for 1 min, 72°C for 1 min, and a final step at 72°C for 5 min.
Both amplification rounds of the seminested PCR were carried out using two units of Taq polymerase (Fermentas, USA), 2mM MgCl 2 , 10 µ M dNTPs (Promega, USA), and 0.5 mM primers YS1 and SR1 for the first PCR and YS1 and YS2 for the second PCR. The thermal cycling conditions were: an initial 3 min step at 95°C, followed by 40 cycles of 94°C for 1 min, 53°C for 40 sec and 72°C for 1 min, and a last step at 72°C for 5 min. We visualized PCR products in a 2% agarose gel stained with ethidium bromide.
Hepatitis B virus genotyping
We purified PCR products using standard Exo Sap-IT (USB, Staufen, Germany). We analyzed the nucleotide sequences of PCR products in both senses by automated dideoxy-sequencing (Macrogen Inc. Seoul, Rep. of Korea).
The sequences obtained were aligned with 118 HBV sequences for ORF S available in GenBank using the Clustal W Multiple Alignment application contained in BioEdit 7.0.5.3 (33). Phylogenetic analysis was conducted using MEGA, version 5.0 (34) applying the neighbor-joining method with genetic distances evaluated with Kimura 2 parameters corrections, maximum parsimony and maximum likelihood. We statistically evaluated the reliability of the trees by bootstrap analysis with 1,000 replicates.
PCR products digestion was done in independent reactions with each of these restriction enzymes: Alw I, Bsr I, Hpa II, Sty I (Biolabs, USA) or Cfr I (Fermentas, USA). Enzyme Alw I was used in this analysis instead of Dpn I or Sau3 AI enzymes used in previous studies (33). The Alw I restriction site is GGATC while the restriction site for Dpn I and Sau3 AI is GATC. However, for the definition of the new restriction patterns described in the present study we did not consider the differences between previous reports and our findings using this enzyme.
We visualized digested products in 3% agarose gels stained with ethidium bromide.
The viral genotype was determined by comparing the obtained restriction pattern with the patterns previously reported by Zeng, et al. (29) and Venegas, et al. (30).
In silico RFLP and pairwise sequence analysis
We conducted an in silico RLFP analysis after we found that the patterns obtained did not match those in previous reports. For this analysis, we obtained sequences of genotypes A-H from the GenBank database based on the geographical origin. We selected two hundred and twenty-nine sequences of HBV ORF S for this analysis. The selection criterion was the availability of complete HBV genome sequences at GenBank. Furthermore, we selected sequences from studies carried out with samples from different locations or at the same location but in a different period. Where complete HBV genome sequences were not available, then those of ORF S were included in the analysis.
The region between 203 nt and 767 nt (with sequence NC003977 as reference) was aligned and analyzed to determine the expected pattern obtained after digestion with Alw I, Bsr I, Cfr I, Hpa II and Sty I restriction enzymes using the software BioEdit 7.0.9.0 (Ibis biosciences, Canada).
Results
HBV molecular detection and genotyping
We amplified a fragment of the HBV ORF S by PCR in 17/56 serum samples: 6/45 (13.3%) from blood donors, 9/9 from patients with clinical diagnosis of viral hepatitis, and 2/2 from asymptomatic individuals with risk factors.
The low proportion of samples from blood donors with HBV DNA amplification (13.3%) could be due to the DNA extraction method (Trizol) and its efficiency for low viral load samples.
We conducted RFLP analysis for these 17 samples. These analyses showed three different restriction patterns (figure 1, patterns 1-3); nine samples showed pattern 1, one showed pattern 2, and seven, showed pattern 3. None of them was similar to the previously reported patterns (29,30).
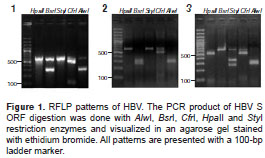
Patterns 1 and 2 as reported by Zeng, et al. and Venegas, et al. (29,30) were quite similar to that of genotype A (figure 2, panel 1); however, restriction with Alw I generated only one fragment instead of three in the study samples (figure 1).
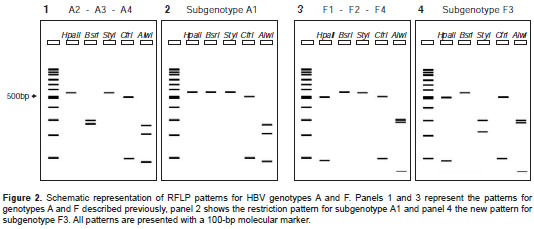
On the other hand, pattern 3 was comparable with the expected result for genotype F (figure 2, panel 3), except for the restriction with S ty I enzyme resulting in two fragments in the samples rather than none, as seen in the pattern previously described.
Sequencing and phylogenetic analysis
We obtained the ORF S sequence from 11 serum samples previously analyzed by RFLP. No serum samples from blood donors remained for the phylogenetic analysis. We aligned the 11 sequences with 118 sequences of HBV selected from Gen Bank . The phylogenetic relationships of sequences were similar using different methodologies. Figure 3 shows a representative phylogenetic tree.
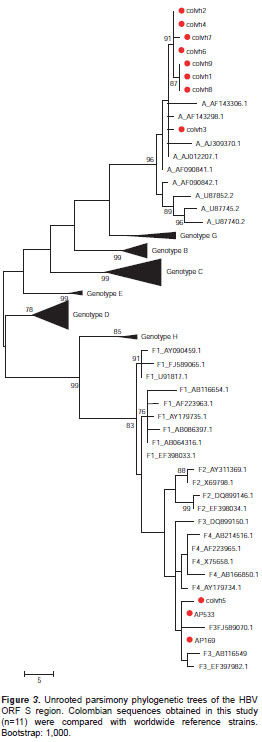
The phylogenetic analysis showed that 8/11 samples (72.7%) clustered with genotype A sequences, while the remaining three sequences (27.3%) clustered with genotype F (figure 3).
In silico analysis
In order to detect polymorphisms that could modify the RFLP patterns, we analyzed 229 HBV sequences available from GenBank in silico. Table 1 summarizes the variations in the sites recognized by the restriction enzymes used on the RFLP protocol.
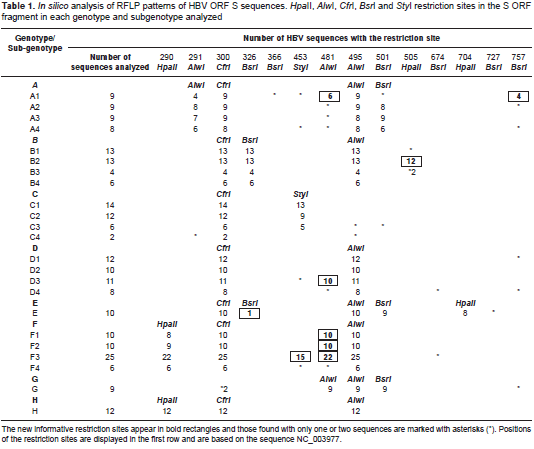
The in silico analysis demonstrated that sequences corresponding to HBV genotype A (subgenotypes A2, A3, A4), genotype B (subgenotype B1), genotype D (subgenotypes D1, D2 and D4), and genotypes C, G and H showed identical patterns to those previously described by Zeng, et al. (29). On the other hand, the sequences corresponding to genotype A, subgenotype A1, previously described as pattern A3 by Zeng, et al ., and to genotype B (subgenotypes B2 to B4), genotype D (subgenotypes D1 to D3), genotype F (subgenotypes F1 to F4), and genotype E exhibited variations on the restriction patterns (table 1).
Two restriction sites in the sequences belonging to subgenotype A1 were found to be absent in 5/9 sequences for Alw I (nt 291) and in 6/9 for Bsr I (nt 501). Moreover, we identified four additional restriction sites for subgenotype A1: Alw I (nt 481) in 6/9 sequences, Bsr I (nt 757) in 4/9 sequences, Sty I (nt 757) in 2/9 sequences and Bsr I (nt 366) in 1/9 sequences. We found variations in subgenotypes A2, A3 and A4, but only in 1-2 of the analyzed sequences, so we did not consider them significant (table 1, marked with asterisks).
Regarding subgenotype F3, 15 of the 25 sequences exhibited an additional restriction site for Sty I (nt 453), with two bands (334 and 251 bp) instead of one (585 bp). This new restriction site could explain the results of the RFLP assays (figure 1, pattern 3) and would be useful in differentiating F3 from the other F subgenotypes.
Discussion
This study describes the HBV genotypes of serum samples obtained from Colombian patients and blood donors. We also report modifications of previously established RFLP patterns of HBV genotypes based on results obtained with serum samples and the in silico analysis of 229 HBV ORF S sequences.
We report a specific RFLP pattern for the F3 subgenotype confirmed by sequencing and in silico analysis. Additionally, we characterized the pattern of genotype A sequences, subgenotype A1, previously reported by Zeng, et al., as pattern A3 (29) in a study conducted in 2004 where the authors described four patterns for genotype A; however, these are not strictly correlated with the subgenotypes A1, A2, A3, A4, considering the limited number of HBV sequences available before 2004. Moreover, the patterns A3 and A4 were identified using one sequence in each case (29).
RFLP is a good technique for viral genotyping because of its simplicity and low cost; however, there are some disadvantages such as the fact that it has to be done on a highly conserved sequence of 6-8 nucleotides and that mutations at the recognition site of the restriction enzymes could alter genotype characterization. It is important to take into account the genetic variability of HBV when identifying the polymorphisms that modify RFLP patterns present at each restriction enzyme recognition site. Nevertheless, RFLP continues to be used for HBV genotyping (32,35,36).
In our study, RFLP genotyping was based on the method described by Zeng, et al. (29). Since then, two new genotypes (13,37), and several new subgenotypes for genotypes A-D and F (38-42) have been described. The current classification by RFLP can now be re-evaluated taking into account this new data.
In our case, we analyzed each sample with the five restriction enzymes previously described in independent reactions. This differs from the methodology used in other studies, where a flow-chart for restriction is followed for RFLP genotyping (29,30). The patterns we obtained were different to those previously reported, which led us to propose an in silico RFLP analysis of a large number of sequences for genotypes A to H. We identified a new specific RFLP pattern for the F3 subgeno-type confirmed by sequencing and phylogenetic analysis, as well as some modifications of the subgenotype A1 pattern.
We identified eight of the ten samples analyzed by RFLP that showed restriction patterns 1 or 2 (figure 1) as genotype A by phylogenetic analysis (figure 3). On the other hand, we sequenced and identified three of seven samples showing restriction pattern 3 as genotype F. The new Sty I restriction site at nt453 was present in these three sequences, as shown in figure 1; given that this site was found in a representative number of F3 sequences in the in silico analysis (15/25), but only one of the six F4 sequences and none of the ten sequences analyzed for F1 and F2, these results suggest that the samples under study belong to subgenotype F3.
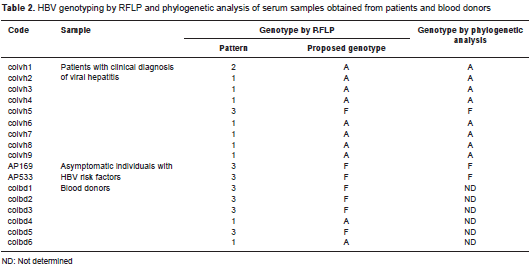
Given the results for the restriction pattern and the phylogenetic analysis, the genotypes of six samples analyzed by RFLP but not sequenced were determined based solely on the restriction pattern: Four of these samples showed pattern 3 and were classified as genotype F3, while the remaining two showed pattern 1 and were classified as genotype A (table 2, figure 2).
Genotype F, considered to be restricted to the Americas (8), has been described mainly in Colombian populations from the Andean region and Amerindians in the southeast of the country; indeed, subgenotype F3 is the most frequent in the former and F1b in the latter (Amazonas department). Meanwhile, genotype A has been very frequently found in western Colombia, where most of the population is of African descent (43-51). Additionally, an F3/A1 recombinant strain (44) and some strains of genotype E have been reported in an Afro-Colombian community (12).
HBV genotype distribution has a complex pattern in Colombia, which has not been described adequately because of the limited amount of HBV genotyping data available. In this sense, it is especially important to conduct studies in northern and eastern Colombia.
The phylogenetic analysis of the sequences included in this study showed that 72.7% (8/11) were genotype A, which represents a high proportion compared to previous reports of HBV genotypes in the country. It is important to note that we had no information about the ethnic background of the participants in this study. Genotype A has also been reported in a high proportion in studies conducted in the cities of Quibdó and Apartadó, where the population is predominantly of African descent (44,49). According to the general census of 2005, around 11% of the population in the department of Antioquia identify themselves as black, mulato (mixed black and white ancestry), Afro-Colombian or otherwise of African descent, while in Chocó this proportion is 82.12% (52). Genotype A might have been introduced by slaves brought to the country during the Spanish colonial period, as has been reported for other South American countries (12,53,54).
A limitation of the present study was the low proportion of positive serum samples from HBsAg and anti-HBc positive blood donors (13.3%). The efficiency of DNA extraction and the detection limit of the in-house semi-nested and nested PCRs could have had a negative impact on HBV DNA detection. HBV DNA amplification in samples from HBsAg+ blood donors was probably influenced by the low viral load, as described previously; these cases could correspond to inactive carriers (IC) of HBV infection, characterized by normal alanine aminotransferase level, HBeAg negativity and viral load =2,000 UI/ml (55). Additionally, Gupta, et al. demonstrated a significant correlation between HBsAg levels and DNA HBV in samples of >2,000 UI/ml but not in those of =2,000 UI/ml using real-time PCR (56). In another study, the authors evaluated in-house semi-nested PCR for HBV DNA detection performance in serum samples reactive for HBsAg comparing it with the performance of a commercial method (Cobas Amplicor HBV Monitor Assay). The HBV DNA detection by in-house semi-nested PCR showed a concordance of 67.8% with the commercial technique. The authors thus concluded that the former technique showed an adequate concordance with some limitations, being a good method in low-resource settings (57).
In conclusion, we identified a new restriction pattern specific for subgenotype F3, and provided an in silico analysis of informative restriction sites for genotypes A-H that complements work which has been published before. Genotypes F and A were identified in the samples by RFLP and phylogenetic analysis according to previous genotyping studies in Colombia. A more robust in silico analysis (i.e., one including more sequences) of the restriction patterns for HBV genotypes and subgenotypes could validate our findings, making HBV genotyping by RFLP more reliable.
The authors thank Dr. Juan Camilo Olarte, from the Banco de Sangre de la Cruz Roja Colombiana , Seccional Antioquia .
The authors declare no conflicts of interest.
This study was funded by Vicerrectoría de Investigación , Universidad de Antioquia (Grant 2547 Mediana Cuantía y Proyecto de Sostenibilidad ), and the Instituto Tecnológico Metropolitano .
Corresponding author:
María Cristina Navas, Grupo de Gastrohepatología, Facultad de Medicina, Universidad de Antioquia, calle 70 N° 52-21, Medellín, Colombia
Telephone: (574) 219 6573; fax: (574) 219 6565
1. World Health Organization. Hepatitis B. Date of entry: April 15, 2015. Available from: http://www.who.int/mediacentre/factsheets/fs204/en/ [ Links ]
2. Lee WM . Hepatitis B virus infection. N Engl J Med. 1997;337: 1733-45. http://dx.doi.org/10.1056/NEJM199712113372406 [ Links ]
3. Milich D, Liang TJ. Exploring the biological basis of hepatitis B antigen in hepatitis B virus infection. Hepatol Baltim Md. 2003;38:1075-86. http://dx.doi.org/10.1053/jhep.2003.50453 [ Links ]
4. Heise T, Sommer G, Reumann K, Meyer I, Will H, Schaal H. The hepatitis B virus PRE contains a splicing regulatory element. Nucleic Acids Res. 2006;34:353-63. http://dx.doi.org/10.1093/nar/gkj440 [ Links ]
5. Norder H, Couroucé A-M, Coursaget P, Echevarría JM, Lee S-D, Mushahwar IK, et al . Genetic diversity of hepatitis B virus strains derived worldwide: Genotypes, subgenotypes, and HBsAg subtypes. Intervirology. 2004;47:289-309. http://dx.doi.org/10.1159/000080872 [ Links ]
6. Campos RH, Mbayed VA, Piñeiro Y Leone FG. Molecular epidemiology of hepatitis B virus in Latin America. J Clin Virol. 2005;34(Suppl.2):S8-13. http://dx.doi.org/10.1016/S1386-6532(05)80028-9 [ Links ]
7. Panduro A, Maldonado-González M, Fierro NA, Román S. Distribution of HBV genotypes F and H in México and Central America. Antivir Ther. 2013;18:475-84. http://dx.doi.org/10.3851/IMP2605 [ Links ]
8. Alvarado-Mora MV, Pinho JR. Distribution of HBV geno-types in Latin America. Antivir Ther. 2013;18:459-65. http://dx.doi.org/10.3851/IMP2599 [ Links ]
9. Sunbul M. Hepatitis B virus genotypes: Global distribution and clinical importance. World J Gastroenterol. 2014;20: 5427-34. http://dx.doi.org/10.3748/wjg.v20.i18.5427 [ Links ]
10. Lin CL, Kao JH. Hepatitis B virus genotypes and variants. Cold Spring Harb Perspect Med. 2015;5:a021436. http://dx.doi.org/10.1101/cshperspect.a021436 [ Links ]
11. Norder H, Couroucé AM, Magnius LO. Complete genomes, phylogenetic relatedness, and structural proteins of six strains of the hepatitis B virus, four of which represent two new genotypes. Virology. 1994;198:489-503. http://dx.doi.org/10.1006/viro.1994.1060 [ Links ]
12. Alvarado-Mora MV, Romano CM, Gomes-Gouvêa MS, Gutiérrez MF, Carrilho FJ, Pinho JR. Molecular epidemiology and genetic diversity of hepatitis B virus genotype E in an isolated Afro-Colombian community. J Gen Virol. 2010;91:501-8. http://dx.doi.org/10.1099/vir.0.015958-0 [ Links ]
13. Olinger CM, Jutavijittum P, Hübschen JM, Yousukh A, Samountry B, Thammavong T, et al . Possible new hepatitis B virus genotype, southeast Asia. Emerg Infect Dis. 2008;14:1777–80. http://dx.doi.org/10.3201/eid1411.080437 [ Links ]
14. Tatematsu K, Tanaka Y, Kurbanov F, Sugauchi F, Mano S, Maeshiro T, et al . A genetic variant of hepatitis B virus divergent from known human and ape genotypes isolated from a Japanese patient and provisionally assigned to new genotype J. J Virol. 2009;83:10538-47. http://dx.doi.org/10.1128/JVI.00462-09 [ Links ]
15. Gandhi MJ, Yang GG, McMahon BJ, Vyas GN. Hepatitis B virions isolated with antibodies to the pre-S1 domain reveal occult viremia by PCR in Alaska Native HBV carriers who have seroconverted. Transfusion. 2000;40:910-6. http://dx.doi.org/10.1046/j.1537-2995.2000.40080910.x [ Links ]
16. Arauz-Ruiz P, Norder H, Robertson BH, Magnius LO. Genotype H: A new Amerindian genotype of hepatitis B virus revealed in Central America. J Gen Virol. 2002;83:2059-73. http://dx.doi.org/10.1099/0022-1317-83-8-2059 [ Links ]
17. Devesa M, Pujol FH. Hepatitis B virus genetic diversity in Latin America. Virus Res. 2007;127:177-84. http://dx.doi.org/10.1016/j.virusres.2007.01.004 [ Links ]
18. Zehender G, Ebranati E, Gabanelli E, Sorrentino C, Lo Presti A, Tanzi E, et al . Enigmatic origin of hepatitis B virus: An ancient travelling companion or a recent encounter? World J Gastroenterol. 2014;20:7622-34. http://dx.doi.org/10.3748/wjg.v20.i24.7622 [ Links ]
19. Alvarado-Mora MV, Gutiérrez-Fernández MF, Gomes-Gouvêa MS, de Azevedo-Neto RS, Carrilho FJ, Pinho JR. Hepatitis B (HBV), hepatitis C (HCV) and hepatitis delta (HDV) viruses in the Colombian population –How Is the epidemiological situation? PLoS One. 2011;6:e18888. http://dx.doi.org/10.1371/journal.pone.0018888 [ Links ]
20. Instituto Nacional de Salud . Boletín Epidemiológico semanal: semana 53 de 2014. Date of entry: June 2, 2015. Available from: http://www.ins.gov.co/boletin-epidemiologico/Boletn%20Epidemiolgico/2014%20Boletin%20epidemiologico%20semana%2053.pdf [ Links ]
21. Okamoto H, Tsuda F, Sakugawa H, Sastrosoewignjo RI, Imai M, Miyakawa Y, et al . Typing hepatitis B Virus by homology in nucleotide sequence: Comparison of surface antigen subtypes. J Gen Virol. 1988;69:2575-83. http://dx. doi.org/10.1099/0022-1317-69-10-2575 [ Links ]
22. Vernet G, Tran N. The DNA-Chip technology as a new molecular tool for the detection of HBV mutants. J Clin Virol. 2005;34 (Suppl.1):S49-53. http://dx.doi.org/10.1016/S1386-6532(05)80010-1 [ Links ]
23. Mizokami M, Nakano T, Orito E, Tanaka Y, Sakugawa H, Mukaide M, et al . Hepatitis B virus genotype assignment using restriction fragment length polymorphism patterns. FEBS Lett. 1999;450:66-71. http://dx.doi.org/10.1016/S0014-5793(99)00471-8 [ Links ]
24. Naito H, Hayashi S, Abe K. Rapid and specific genotyping system for hepatitis B virus corresponding to six major genotypes by PCR using type-specific primers. J Clin Microbiol. 2001;39:362-4. http://dx.doi.org/10.1128/JCM.39.1.362-364.2001 [ Links ]
25. Usuda S, Okamoto H, Iwanari H, Baba K, Tsuda F, Miyakawa Y, et al . Serological detection of hepatitis B virus genotypes by ELISA with monoclonal antibodies to type-specific epitopes in the preS2-region product. J Virol Methods. 1999;80:97-112. http://dx.doi.org/10.1016/S0166-0934(99)00039-7 [ Links ]
26. Osiowy C, Giles E. Evaluation of the INNO-LiPA HBV genotyping assay for determination of hepatitis B virus genotype. J Clin Microbiol. 2003;41:5473-7. http://dx.doi.org/10.1128/JCM.41.12.5473-5477.2003 [ Links ]
27. Lindh M, Andersson AS, Gusdal A. Genotypes, nt 1858 variants, and geographic origin of hepatitis B virus--Large-scale analysis using a new genotyping method. J Infect Dis. 1997;175:1285-93. http://dx.doi.org/10.1086/516458 [ Links ]
28. Lindh M, González JE, Norkrans G, Horal P. Genotyping of hepatitis B virus by restriction pattern analysis of a pre-S amplicon. J Virol Methods. 1998;72:163-74. http://dx.doi.org/10.1016/S0166-0934(98)00026-3 [ Links ]
29. Zeng GB, Wen SJ, Wang ZH, Yan L, Sun J, Hou JL. A novel hepatitis B virus genotyping system by using restriction fragment length polymorphism patterns of S gene amplicons. World J Gastroenterol. 2004;10:3132-6. http://dx.doi.org/10.3748/wjg.v10.i21.3132 [ Links ]
30. Venegas M, Muñoz G, Hurtado C, Álvarez L, Velasco M, Villanueva RA, et al . Prevalence of hepatitis B virus genotypes in chronic carriers in Santiago, Chile. Arch Virol. 2008;153:2129-32. http://dx.doi.org/10.1007/s00705-008-0231-6 [ Links ]
31. Gulube Z, Chirara M, Kew M, Tanaka Y, Mizokami M, Kramvis A. Molecular characterization of hepatitis B virus isolates from Zimbabwean blood donors. J Med Virol. 2011;83:235-44. http://dx.doi.org/10.1002/jmv.21954 [ Links ]
32. Ouneissa R, Bahri O, Ben Yahia A, Touzi H, Azouz MM, Ben Mami N, et al . Evaluation of PCR-RFLP in the pre-S region as molecular method for hepatitis B virus genotyping. Hepat Mon. 2013;13:e11781. http://dx.doi.org/10.5812/hepatmon.11781 [ Links ]
33. Hall T. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95-8. [ Links ]
34. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28: 2731-9. http://dx.doi.org/10.1093/molbev/msr121 [ Links ]
35. Nabuco LC, Mello FC, Gomes S de A, Perez RM, Soares JA, Coelho HS, et al . Hepatitis B virus genotypes in Southeast Brazil and its relationship with histological features. Mem Inst Oswaldo Cruz. 2012;107:785-9. http://dx.doi.org/10.1590/S0074-02762012000600013 [ Links ]
36. Gopalakrishnan D, Keyter M, Shenoy KT, Leena KB, Thayumanavan L, Thomas V, et al . Hepatitis B virus subgenotype A1 predominates in liver disease patients from Kerala, India. World J Gastroenterol. 2013;19:9294-306. http://dx.doi.org/10.3748/wjg.v19.i48.9294 [ Links ]
37. Tran TT, Trinh TN, Abe K. New complex recombinant genotype of hepatitis B virus identified in Vietnam. J Virol. 2008;82:5657-63. http://dx.doi.org/10.1128/JVI.02556-07 [ Links ]
38. Pourkarim MR, Amini-Bavil-Olyaee S, Lemey P, Maes P, van Ranst M. Are hepatitis B virus "subgenotypes" defined accurately? J Clin Virol. 2010;47:356-60. http://dx.doi.org/10.1016/j.jcv.2010.01.015 [ Links ]
39. Hübschen JM, Mbah PO, Forbi JC, Otegbayo JA, Olinger CM, Charpentier E, et al . Detection of a new subgenotype of hepatitis B virus genotype A in Cameroon but not in neighbouring Nigeria. Clin Microbiol Infect. 2011;17:88-94. http://dx.doi.org/10.1111/j.1469-0691.2010.03205.x [ Links ]
40. Thedja MD, Muljono DH, Nurainy N, Sukowati CH, Verhoef J, Marzuki S. Ethnogeographical structure of hepatitis B virus genotype distribution in Indonesia and discovery of a new subgenotype, B9. Arch Virol. 2011;156:855-68. http://dx.doi.org/10.1007/s00705-011-0926-y [ Links ]
41. Mulyanto, Pancawardani P, Depamede SN, Wahyono A, Jirintai S, Nagashima S, et al . Identification of four novel subgenotypes (C13-C16) and two inter-genotypic recombinants (C12/G and C13/B3) of hepatitis B virus in Papua province, Indonesia. Virus Res. 2012;163:129-40. http://dx.doi.org/10.1016/j.virusres.2011.09.002 [ Links ]
42. Abdou Chekaraou M, Brichler S, Mansour W, Le Gal F, Garba A, Dény P, et al . A novel hepatitis B virus (HBV) subgenotype D (D8) strain, resulting from recombination between genotypes D and E, is circulating in Niger along with HBV/E strains. J Gen Virol. 2010;91:1609-20. http://dx.doi.org/10.1099/vir.0.018127-0 [ Links ]
43. Alvarado-Mora MV, Romano CM, Gomes-Gouvêa MS, Gutiérrez MF, Carrilho FJ, Pinho JR. Dynamics of hepatitis D (delta) virus genotype 3 in the Amazon region of South America. Infect Genet Evol. 2011;11:1462-8. http://dx.doi.org/10.1016/j.meegid.2011.05.020 [ Links ]
44. Alvarado-Mora MV, Romano CM, Gomes-Gouvêa MS, Gutiérrez MF, Carrilho FJ, Pinho JR . Phylogenetic analysis of complete genome sequences of hepatitis B virus from an Afro-Colombian community: Presence of HBV F3/A1 recombinant strain. Virol J. 2012;9:244. http://dx.doi.org/10.1186/1743-422X-9-244 [ Links ]
45. Bautista-Amorocho H, Castellanos-Domínguez YZ, Rodríguez-Villamizar LA, Velandia-Cruz SA, Becerra-Peña JA, Farfán-García AE. Epidemiology, risk factors and genotypes of HBV in HIV-infected patients in the Northeast region of Colombia: High prevalence of occult hepatitis B and F3 subgenotype dominance. PLoS One. 2014;9: e114272. http://dx.doi.org/10.1371/journal.pone.0114272 [ Links ]
46. Cortés-Mancera F, Loureiro CL, Hoyos S, Restrepo JC, Correa G, Jaramillo S, et al . Etiology and viral genotype in patients with end-stage liver diseases admitted to a hepatology unit in Colombia. Hepat Res Treat. 2011;2011:363205. http://dx.doi.org/10.1155/2011/363205 [ Links ]
47. Devesa M, Loureiro CL, Rivas Y, Monsalve F, Cardona N, Duarte MC, et al . Subgenotype diversity of hepatitis B virus American genotype F in Amerindians from Venezuela and the general population of Colombia. J Med Virol. 2008;80:20-6. http://dx.doi.org/10.1002/jmv.21024 [ Links ]
48. Ríos-Ocampo WA, Cortés-Mancera F, Olarte JC, Soto A, Navas MC. Occult hepatitis B virus infection among blood donors in Colombia. Virol J. 2014;11:206. http://dx.doi.org/10.1186/s12985-014-0206-z [ Links ]
49. Ríos D, di Filippo D, Insuasty M, Rendón JC, Ríos WA, Medina C, et al . Hepatitis B infections in individuals with exposure factors in Quibdó and Apartadó, Colombia. Rev Col Gastroenterol. 2015;30:11-8. [ Links ]
50. Alvarado-Mora MV, Romano CM, Gomes-Gouvêa MS, Gutiérrez MF, Botelho L, Carrilho FJ, et al . Molecular characterization of the hepatitis B virus genotypes in Colombia: A Bayesian inference on the genotype F. Infect Genet Evol. 2011;11:103-8. http://dx.doi.org/10.1016/j.meegid.2010.10.003 [ Links ]
51. di Filippo Villa D, Cortés-Mancera F, Payares E, Montes N, de la Hoz F, Arbeláez MP, et al . Hepatitis D virus and hepatitis B virus infection in Amerindian communities of the Amazonas state, Colombia. Virol J. 2015;12:172. http://dx.doi.org/10.1186/s12985-015-0402-5 [ Links ]
52. Departamento Administrativo Nacional de Estadística (DANE) . Censo general. Bogotá, D.C.: DANE; 2005. [ Links ]
53. Quintero A, Martínez D, Alarcón De Noya B, Costagliola A, Urbina L, González N, et al . Molecular epidemiology of hepatitis B virus in Afro-Venezuelan populations. Arch Virol. 2002;147:1829-36. http://dx.doi.org/10.1007/s00705-002-0842-2 [ Links ]
54. Motta-Castro AR, Martins RM, Yoshida CF, Teles SA, Paniago AM, Lima KM, et al . Hepatitis B virus infection in isolated Afro-Brazilian communities. J Med Virol. 2005;77: 188-93. http://dx.doi.org/10.1002/jmv.20435 [ Links ]
55. Martinot-Peignoux M, Lapalus M, Laouénan C, Lada O, Netto-Cardoso AC, Boyer N, et al . Prediction of disease reactivation in asymptomatic hepatitis B e antigen-negative chronic hepatitis B patients using baseline serum measurements of HBsAg and HBV-DNA. J Clin Virol. 2013;58:401-7. http://dx.doi.org/10.1016/j.jcv.2013.08.010 [ Links ]
56. Gupta E, Kumar A, Choudhary A, Kumar M, Sarin SK. Serum hepatitis B surface antigen levels correlate with high serum HBV DNA levels in patients with chronic hepatitis B: A cross-sectional study. Indian J Med Microbiol. 2012;30:150-4. http://dx.doi.org/10.4103/0255-0857.96664 [ Links ]
57. Portilho MM, Baptista ML, da Silva M, de Sousa PS, Lewis-Ximenez LL, Lampe E, et al . Usefulness of in-house PCR methods for hepatitis B virus DNA detection. J Virol Methods. 2015;223:40-4. http://dx.doi.org/10.1016/j.jviromet.2015.07.010 [ Links ]














