Rheumatoid arthritis is a chronic, complex, and heterogeneous autoimmune disease. It is characterized by the presence of long-standing inflammation of the diarthrodial joints resulting in a symmetric polyarthritis and synovial membrane hypertrophy, followed by progressive joint damage, bone and cartilage destruction, as well as deformity 1.
Joint damage and physical disability are the two major adverse outcomes of the disease reducing quality of life and causing premature mortality 2. Less joint damage and better physical function have been unequivocally shown to be a consequence of the early implementation of conventional synthetic disease-modifying anti-rheumatic drugs (csDMARD) in addition to glucocorticoids 3. However, some patients in therapy with csDMARD (even with the concomitant use of two drugs) still present clear signs of the disease, which has led to the research for novel drug therapies that would prevent the progression of the disease and have a positive impact on their quality of life.
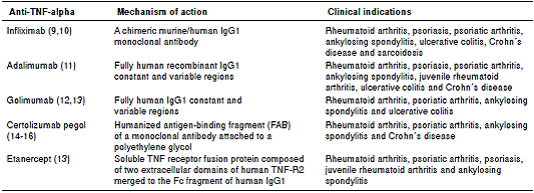
In the last few decades, pathophysiological pathways related to autoimmune diseases have shown that the tumor necrosis factor (TNF) is one of the most relevant targets for treatment and remission induction, thus opening the way to the development of drugs such as adalimumab, etanercept, infliximab, certolizumab pegol, and golimumab, all of which act as TNF inhibitors 4.
In spite of the benefits associated with these new drugs, the risks related to blocking the TNF pathway must be acknowledged. A high predisposition to certain infections, particularly tuberculosis, is one of the most frequent complications of such blocking 5.
We present a case of disseminated tuberculosis in a rheumatoid arthritis patient under anti-TNF-alpha therapy and discuss the topic in the light of updated recommendations for tuberculosis prevention when administering this treatment.
Case report
A 58-year-old woman with a six-year history of rheumatoid arthritis was examined for a 10- day history of headaches with 7/10 intensity and presence of unstable gait. Immunomodulatory
therapy included methotrexate 10 mg/week starting five years before and biological therapy for the last year (anti-TNF-alpha therapy: infliximab, 200 mg every 6 weeks). Infliximab was started due to the failure of remission with csDMARD.
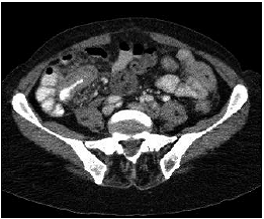
At the moment of hospitalization, the patient did not present any sign of rheumatoid arthritis activity (Clinical Diseases Activity Index: 0). A computed tomography (CT) scan of the brain was done showing a hypodense basal right lesion associated with vasogenic edema in the left frontal lobe upon which extension studies were requested. Brain magnetic resonance imaging showed supraand infratentorial lesions highly suggestive of caseous granulomas (figure 1). A chest CT showed centrilobular micronodules with soft tissue density and branched centrilobular linear densities (figure 2). Abdomino-pelvic CT showed iliac mesenteric and retroperitoneal lymphadenopathy and wall thickening of the distal ileum (figure 3), which is highly suggestive of tuberculosis as a first diagnostic possibility. Lymph node biopsy reported granulomatous lymphadenitis with acid-alcohol resistant bacilli, thus confirming the diagnosis.
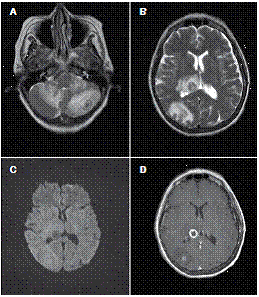
Figure 1 A, B. Axial T2-weighted brain MRI image showing multiple irregular intra-axial lesions with double central hypointense halo surrounded by vasogenic edema. C. Diffusion-weighted image showing no restriction on the diffusion of water. D. T1-weighted image showing an irregular thick ring enhancement
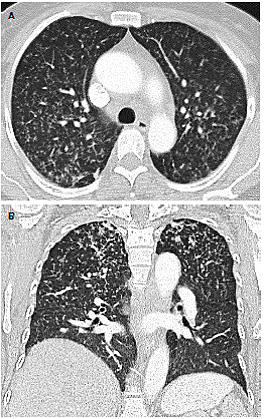
Figure 3 Chest CT showing centrilobular micronodules with apical dominance branched linear densities associated with “tree-in-bud” pattern
Tuberculin skin tests (TST), interferon gamma release assays (IGRA) or chest x-rays were not done to screen for latent tuberculosis infection prior to the administration of infliximab.
The patient was started off with the classic four- drug regime for the empiric treatment of tuberculosis (rifampicin, isoniazid, ethambutol, and pyrazinamide), and was discharged one week after admission. Anti-TNF-alpha therapy administration was suspended and maintenance management with prednisolone 7.5 mg/day in addition to chloroquine 250 mg/day was established.
Discussion
Given that an important fraction of rheumatoid arthritis patients was unable to achieve remission on treatment with csDMARD alone, and the better understanding of the pathophysiological pathways of the disease, new drugs have been developed to achieve clinical remission and improve patients’ quality of life 6. However, these therapies are not 100% safe and innocuous, and can have severe side effects that put patients’ lives at risk 7 as their action is not limited to blocking the abnormal pathways that lead to autoimmune disease development. There is no doubt about the benefits of using anti-TNF agents in rheumatoid arthritis patients. In fact, anti-TNF-alpha therapy for rheumatoid arthritis is now standard care, and it typically follows methotrexate (MTX) in patients who are not doing well. In the majority of cases (70-80%), it is used jointly with MTX since the additional benefit of the combination has been demonstrated 4, i.e., organic chemicals of ~400 Da absorbable via the gut, this is no longer the case. There are now a plethora of important medicines which are proteins and injectable, which have dramatically improved the therapy of many inflammatory diseases and of cancer. Most of these are monoclonal antibodies, some are receptor Ig Fc fusion proteins, others are cytokines or enzymes. The key to this new aspect of therapeutics has been the filling of unmet needs, and the consequent commercial success, which promoted further research and development. The first ‘biologic’ for a common disease, rheumatoid arthritis (RA). Anti-TNF-alpha therapy also critically alters the regulation of inflammatory processes and the pathways of cell apoptosis, activation, recruitment and differentiation 8.
Five anti-TNF-alpha drugs are currently in clinical use. They have structural differences and, thus, their therapeutic indications differ 6,7 (table 1).
The initiation of anti-TNF-alpha therapy is related to the development of multiple adverse effects including cardiovascular disease, malignancies and serious infections that may endanger the patient’s life 8,17,18. Among them, opportunistic infections are a major cause of morbidity and mortality, with a greater incidence of tuberculosis due to the pathophysiological role of TNF. In humans, TNF increases the phagocytic capacity of macrophages that ultimately go into apoptosis leading to the death of mycobacteria and stimulating the production of chemokines in the process. TNF also regulates the expression of adhesion molecules on endothelial cells which are important for recruiting mononuclear cells, an event that plays an important role in the formation of granulomas 19,20. The absence of TNF prevents the formation of granulomas and, thus, infection cannot be contained 20.
According to the evidence available, the risk of developing tuberculosis increases 1.6-25 times after the start of anti-TNF-alpha therapy depending on the drug used 7,21, a fact that is enhanced when tuberculosis incidence is very high 22.
In 1993, the World Health Organization (WHO) declared tuberculosis a health emergency potentially affecting one third of the world’s population. This translates into additional costs in healthcare services and a negative impact on the economy. After AIDS, tuberculosis is currently the second- leading cause of death worldwide among diseases caused by infectious agents 23. According to the latest WHO statistics from 2013, the annual incidence of tuberculosis in Colombia is 32 per 100,000 in the general population 24. About one-third of the world’s population is estimated to have latent tuberculosis infection 23,25.
The incidence of tuberculosis in patients receiving anti-TNF alpha drugs varies depending on the type of therapy, as the specific characteristics of each biological agent differ 26. In the case of soluble TNF-alpha interaction with TNF-alpha receptor 1, this receptor is essential for both granuloma formation and containment of intracellular pathogens unlike membrane-bound TNF-alpha, which exerts its action via TNF receptor 2, and whose role is less significant in infection containment and formation of granulomas 20,27. These processes explain the following three key points:
The increased risk of tuberculosis in patients receiving monoclonal antibodies (infliximab and adalimumab) vs. those patients receiving etanercept 28.
The earlier appearance of tuberculosis in patients receiving therapy with infliximab 8,19,20
The use and benefits of infliximab in granulomatous diseases such as Crohn’s disease and sarcoidosis 12,29
Various international studies have shown the incidence rate of tuberculosis in patients receiving anti-TNF-alpha therapy (table 2), but currently there are no local Colombian statistics available on the risk of active tuberculosis in patients with rheumatic diseases receiving anti-TNF-alpha therapy. However, several pulmonary and extrapulmonary tuberculosis cases in patients under anti-TNF-alpha therapy have been reported 34-41.
There have been multiple case reports worldwide of patients on biological therapy who have developed pulmonary and/or extrapulmonary tuberculosis 42,43. In a multicenter retrospective study in the Netherlands on the importance of tuberculosis screening prior to the administration of biological therapy conducted in 611 patients with Crohn’s disease, 75.8% of them were screened for tuberculosis (TST, IGRA or chest radiography), and 3% were positive. Half of this group received treatment for latent tuberculosis infection (isoniazid), and one untreated patient with a positive test developed pulmonary tuberculosis 44.
A retrospective study in the United States found that 130 patients had developed tuberculosis after the start of therapy with infliximab. However, only 67 of those who had started infliximab therapy in 2001, when the FDA issued the recommendation of tuberculosis screening prior to anti-TNF-alpha therapy, were evaluated. The study showed that 20 of the patients had not been screened for tuberculosis with a TST test while 23 patients had a chest x-ray, of whom 22 had normal results and one was reported as having unknown results. Among this group of patients, 14 developed pulmonary tuberculosis, five extrapulmonary tuberculosis and three disseminated tuberculosis 45.
The conclusion from these two studies is that some patients were not routinely screened for tuberculosis, and others did not receive prophylactic treatment in spite of the fact that they had a positive screening result compatible with latent tuberculosis infection. Another remarkable conclusion is that although initial tests were negative, the development of active tuberculosis infection during the treatment should not have been ruled out.
Clinical presentation of tuberculosis in a patient under anti-TNF-alpha therapy may vary from pulmonary to extrapulmonary or disseminated tuberculosis with an increased incidence of the latter two due to immunosuppression, as occurred with the case presented in this article 46,47.
Likewise, it is clear that the diagnosis of an autoimmune disease, the use of csDMARD and steroid therapy represent by themselves an increased risk of concomitant tuberculosis 5,48-50 psoriatic arthritis (PsA, with a relative risk of 3.68 for rheumatoid arthritis, 3.4 for metothrexate, 11.7 for leflunomide and 2,4 for corticosteroids 47.
Taking into account these statistics and the role of TNF in tuberculosis control, as well as the increased risk of tuberculosis in patients with autoimmune diseases, as well as the use of DMARD therapy, tuberculosis screening before starting anti-TNF-alpha therapy is essential and mandatory 5,8,29. Unfortunately, this was not observed in the case we present. The Centers for Disease Control and Prevention (CDC) recommend screening patients for Mycobacterium tuberculosis risk factors before initiating anti-TNF-alpha therapy 30 in the following cases:
People living in a congregate setting (e.g., jail, homeless shelter or chronic care facility)
TST positive >5 mm
Substance abuse
Health care employment in settings with tuberculosis patients
Chest X-ray findings consistent with previous tuberculosis
We also searched for tuberculosis screening protocols prior to initiating treatment with anti-TNF-alpha drugs 51,52, and we found that routine chest X-ray was a common, widespread recommendation in various guidelines and studies. Regarding the use of TST, most guidelines and protocols favor its widespread use with the exception of the Swiss guidelines 52. German guidelines propose that a TST be done only if discrepancy exists between strong epidemiological evidence of prior tuberculosis exposure and negative IGRA 53. UK guidelines do not recommend TST for immunosuppressed patients 51 and the European guidelines establish that a TST be done on individuals without a history of BCG vaccination 12.
Regarding TST positivity, three study groups considered a result of >5 mm to be positive 31,33,53. The French guidelines considered a >10 mm 33 result to be positive, while in the UK, the positivity corresponds to 5 mm in unvaccinated patients and 15 mm in vaccinated patients 52. In the USA, a result of more than 5 mm is considered positive if the patient is immunosuppressed, more than 10 mm is positive for patients in certain risk groups, and 15 mm if the patient is at low risk 46
Due to the presence of false positives when screening with TST among individuals with prior exposure to BCG vaccine 54, more reliable diagnostic tools have been developed in recent years. One of the most widely used nowadays in some settings are the IGRA, which have better sensitivity than TST in immunocompromised subjects, as well as higher specificity. IGRA also have the advantage of not being influenced by prior vaccination with BCG or contact with nontuberculous mycobacteria 8,52,54,55.
The IGRA quantify interferon-gamma production from T cells and are based on the principle that T cells already sensitized to tuberculosis antigens produce high IFN-γ levels when they are exposed again. As a consequence, IGRA can improve latent tuberculosis infection detection specificity in BCG- vaccinated populations 55. This testing method had been adopted as evidence in tuberculosis screening in only three of seven guidelines consulted (12, 19,52,53). Despite the evidence on the use of IGRA shown in some studies, some research has demonstrated that TST and IGRA responses vary substantially among different groups of immuno-compromised patients, and are poor predictors of the development of tuberculosis in them 56.
Regarding the treatment for latent tuberculosis infection or active tuberculosis, each consensus suggests a specific type of regimen, differing in the drugs used and the duration of treatment. The different treatment protocols are summarized in table 3.
Table 3 Recommendations for the treatment of tuberculosis in patients requiring anti-TNF-alpha therapy
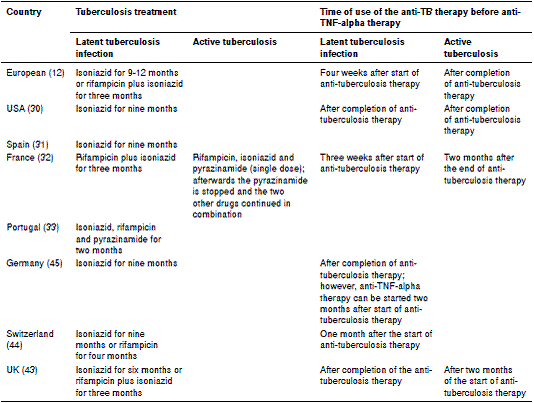
The clinical guidelines for the management of patients with rheumatoid arthritis in Colombia were issued in 2014 57, and they do not include a screening protocol for active tuberculosis nor latent tuberculosis infection despite the high incidence of tuberculosis in the country. Nevertheless, the guidelines recommend ruling out latent or active tuberculosis in patients under treatment with conventional or biologic DMARD as a good clinical practice. It is of great importance to develop recommendations on this topic and to encourage further local studies to evaluate the most appropriate treatment in cases of latent tuberculosis infection and active tuberculosis when anti-TNF-alpha therapy is required. As shown in table 3, there is no international consensus, and proposed therapies are tailored to the characteristics of each country.
It is necessary to adopt a strict and well-established public health policy in Colombia to enable a decrease in tuberculosis incidence among patients receiving biological therapy. This is the only possible way to reduce tuberculosis-associated morbidity and mortality.
The use of anti-TNF-alpha therapy has shown that disease progression in rheumatoid arthritis can be reduced satisfactorily, but it is essential to carry out suitable screening prior to its administration. Patients should also be monitored for any respiratory symptoms and, if found, clinicians should suspect the presence of pulmonary tuberculosis, especially in cases where clinical, laboratory, and image findings allow for a wide range of different diagnoses.
However, even if proper screening is done prior to the start of anti-TNF-alpha therapy, the risk of tuberculosis remains, including cases with prior normal chest X-ray and negative TST 58-60. In this sense, some groups have suggested the use of new screening tests after the start of anti-TNF-alpha therapy 28,45,60. Some institutions are adopting protocols that promote the periodic search for tuberculosis in patients under biological therapy, as proposed by Tae Sun, et al. 25, who did tuberculosis screening three, six and nine months after the start of anti-TNF-alpha therapy, and annually after that. Other authors recommend tuberculosis screening every year 12,61,62.
To achieve a significant reduction in morbidity and mortality, which ultimately would improve the patient’s quality of life, clinicians need to be very meticulous in the search for risk factors increasing the incidence of infectious diseases, and decide which screening test is most suitable for each individual patient 5,8. Lastly, patients’ safety and quality of life should be the main objectives of every rheumatologic treatment.
In conclusion, patients with autoimmune or auto- inflammatory diseases that benefit from anti- TNF-alpha therapy should be screened for infectious agents, especially tuberculosis. Different tests have been developed to detect latent tuberculosis infection (PPD, IGRA and chest radiography), as its proper and timely detection is key to preventing morbidity and mortality in patients with anti-TNF- alpha therapy, given that these drugs mechanism of action decreases the immune system ability to form the tuberculosis granuloma. This interference with the granulomatous immune response increases the risk and predisposes patients to pulmonary, extrapulmonary or disseminated tuberculosis in the context of immunosuppression, with its subsequent complications, such as in the case reported here. Likewise, it is essential to establish internal and national guidelines to clearly define all the parameters and periodicity of tuberculosis screening once the anti-TNF-alpha is introduced.