Pure neural leprosy is a clinical form of Hansen’s disease that affects peripheral nerves without causing skin lesions in the patient 1,2. Therefore, its diagnosis is challenging due to the subjectivity and complexity of the clinical manifestations 3. Although its prevalence is low (5 to 18% of all leprosy diagnosis), it may be underestimated because simple, reliable and non-invasive diagnostic tools to detect Mycobacterium leprae in patients with this kind of leprosy are currently unavailable. Thus, its detection depends on the clinical experience of the physician 3,4.
The neural condition caused by leprosy results from the asymmetric invasion by M. leprae in peripheral nerve fibers, an interaction that causes an inflammatory response at the axon level, resulting in inflammation of peripheral nerve trunks (neuritis) and damaging sensory fibers responsible for exteroceptive sensitivity 5-7. Early pure neural leprosy symptoms occur when infection of the compromised sensory fibers exceeds 30% 8.
The diagnosis of the condition is clinical, and it should be suspected in household contacts of patients with Hansen’s disease who display only peripheral damage as clinical symptom. Neuropathic symptoms and signs should be a sufficient criterion of diagnosis when additional diagnostic studies are not possible, and when common causes of peripheral neuropathy (e.g. diabetes mellitus, alcoholism and carpal tunnel syndrome) have been ruled out 1,9
While the absence of skin lesions complicates pure neural leprosy diagnosis, detecting M. leprae in biopsy smears (skin and peripheral nerve) is also difficult. Given that histopathological findings tend to be nonspecific, implementation of serological tests (such as the detection of IgM anti-bodies against phenolic glycolipid - I (PLG-I) in patients with peripheral neuropathy may support its diagnosis 10.
We describe here four pure neural leprosy cases diagnosed in the Sanatorium of Contratación in Santander, Colombia.
Case 1
A 64-year-old male patient exhibiting 2 years of clinical symptoms characterized by muscle weak-ness in the eyes, hands, and feet with associated sensory loss in these areas.
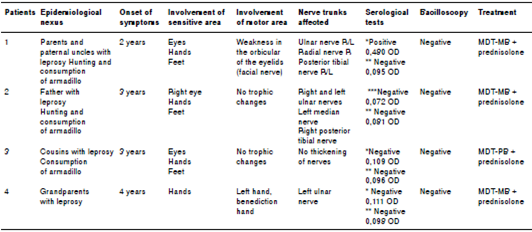
The patient did not have a pathological, pharmacological, surgical, or history of trauma to explain these symptoms. As regards the epidemiological nexus, the patient’s father and paternal uncles were both leprosy patients. In addition, the patient had lived for 20 years with a woman suffering from leprosy. Finally, the patient hunted and ate armadillo.
The physical examination did not reveal skin lesions. A neurological assessment of the face, head, and neck revealed an absence of the bilateral corneal reflex and weakness in the orbicular muscle of the eyelids involving the trigeminal and facial nerve.
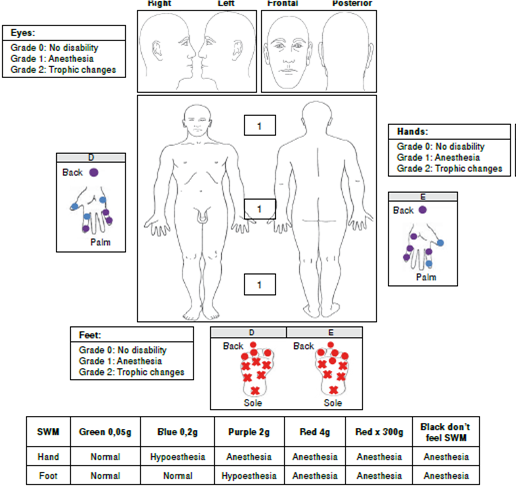
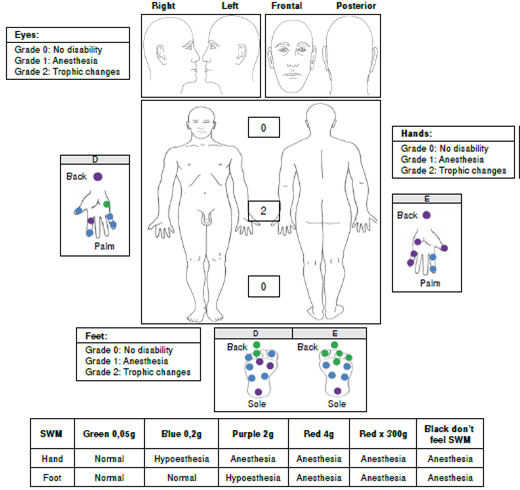
We performed a sensitivity evaluation using Semmes-Weinstein monofilaments. In the upper limbs, we observed sensory loss (anesthesia) in areas innervated by the radial median nerve and in the hands, in areas innervated by the ulnar nerve. We also found evidence of a bilateral thickening of the ulnar and right cutaneous radial nerves. We did not observe trophic changes in the hands (figure 1).
In the lower limbs, sensory loss (anesthesia) was evident in both feet in areas innervated by the tibial posterior and fibular common nerves. During palpation of the nerve trunks, we detected a thickening of both tibial posterior nerves without trophic changes in the feet (figure 1).
As regards laboratory tests, the bacilloscopy of the mucus and interstitial fluid did not detect acid-alcohol resistant bacilli (BAAR). Serology to detect IgG LID1-NOD (cut-off>0.146 optical density, OD) waspositive (0.480 OD). IgM anti-PGL-1 (cut-off>0.162OD) was negative (0.095 OD). We ruled out damage to the central nervous system (CNS), as computerized axial tomography reported a normal status.
Given the patient’s epidemiological nexus, evidence of peripheral neuropathy and the presence of IgG antibodies against M. leprae proteins, we diagnosed the patient with pure neural leprosy.
The patient exhibited grade 1 disability of the eyes, hands, and feet and began multidrug therapy for multibacillary leprosy (MDT-MB). In addition, the patient began physical therapies plus cortico therapy with prednisolone in a descending scheme (60 mg initial doses) and four months later the progression of neural damage stopped.
Table 1 shows the immunological, bacteriological, epidemiological, clinical, and diagnostic characteristics of each of the patients reported in this study.
Case 2
A 33 year-old male patient exhibiting 3 years of clinical symptoms characterized by muscle weak-ness in the hands with associated sensory loss in both his hands and feet.
The patient did not have a pathological, pharmacological, surgical, or trauma history to explain these symptoms. Regarding the epidemiological nexus, his father is a leprosy patient. In addition, the patient also hunted and ate armadillo.
Upon physical examination, we did not observe skin lesions. In the neurological assessment of face, head, and neck, we detected that the corneal reflex in the right eye involving the trigeminal nerve was absent.
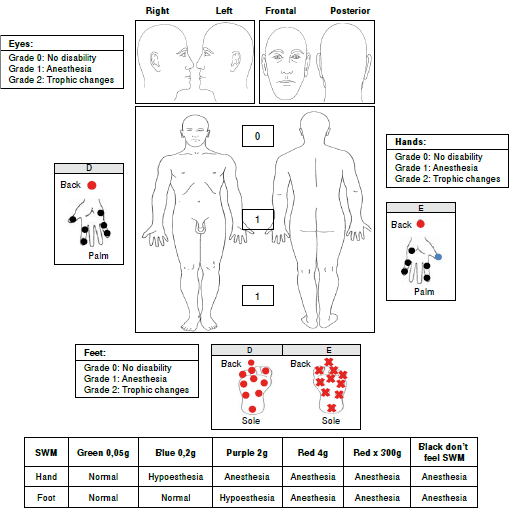
In the upper limbs, we observed sensory loss (anesthesia) in areas innervated by the median, ulnar and radial nerves in both hands (figure 2) and found evidence of a bilateral thickening of the ulnar nerve and percussing pain in the left median nerve. The Froment test was positive in both hands; we did not observe trophic changes in this area.
In the lower limbs, we found evidence of sensory loss (anesthesia) in areas innervated by the tibial posterior and fibular common nerves in both feet. During palpation of the nerve trunks, we detected a thickening of the right posterior tibial nerve without trophic changes in the feet (figure 2).
The bacilloscopy of the mucus and interstitial fluid did not detect BAAR. Serology to detect IgM against LID 1-NOD (cut-off>0.153 OD) was negative (0.072 OD). IgM anti PGL-1 (cut-off>0.162 OD) was negative (0.081 OD).
Considering that this patient had a positive epidemiological nexus with Hansen’s disease and an asymmetrical peripheral neuropathy, he was diagnosed with pure neural leprosy with a grade 1 disability (hands/feet) and started MDT-MB with prednisolone in a descending scheme (50 mg initial doses). Currently, the patient has finished MDT-MB in combination with prednisolone; the clinical outcome was good since the disability grade did not increase (table 1).
Case 3
A 42-year-old female patient exhibiting 4 years of clinical symptoms characterized by weakness in the hands and lower limbs associated with sensory loss in those areas.
She was initially diagnosed with carpal tunnel syndrome, and received surgical treatment (decompression of the median nerve) in both hands. This diagnosis was established without considering the clinical symptoms, which were compatible with multiplex mononeuropathy. The patient did not have a pathological, pharmacological, or trauma history to explain these symptoms.
Regarding the epidemiological nexus, the patient had cousins suffering from leprosy and, besides, she ate armadillo.
During the physical examination, no skin lesions were detected. In the neurological assessment of the face, head, and neck, we observed damage of the trigeminal nerve in both eyes.
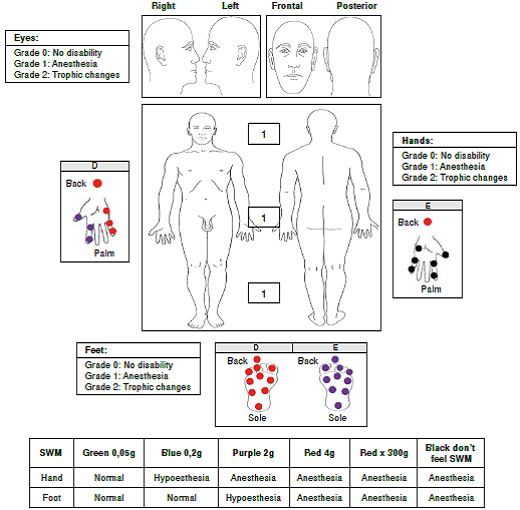
In the upper limbs, we observed sensory loss (anesthesia) in both hands associated with areas innervated by the median, ulnar, and radial nerves. We found no evidence of a thickening of the peripheral nerve or of trophic changes in the hands (figure 3).
In the lower limbs, we found evidence of sensory loss (anesthesia) in areas innervated by the tibial posterior and fibular common nerves in both feet, but no evidence of a thickening of the peripheral nerve or trophic changes in the feet (figure 3).
With regard to laboratory tests, the bacilloscopy did not detect BAAR. Serology to detect IgG anti-LID 1-NOD (cut-off>0.146 OD) was negative (0.109 OD), as well as for IgM anti-PGL-1 (cut-off>0.162 OD;0.096 OD).
Considering this patient had a positive epidemiological nexus with Hansen’s disease, as well as multiplex mononeuropathy without other possible pathologies to explain a neuropathic condition that differed from leprosy, shewas diagnosed with pure neural leprosy and grade 1 disability (eyes/hands/feet), and MDT for paucibacillary leprosy (PB) was started combined with physical therapy. Currently, the patient has improved her strength in hands and lower limbs and she continues with physical therapy (table 1).
Case 4
A 42-year-old female patient exhibiting 4 years of clinical symptoms characterized by decreased sensitivity in her hands and a retraction of the fourth and fifth finger of the left hand.
The patient did not have a pathological, pharmacological, surgical, or trauma history to explain these symptoms. As regards the epidemiological nexus, the patient’s grandparents had leprosy.
The physical examination did not reveal skin lesions and in the neurological assessment of her face, head, and neck, we did not detect damage in the trigeminal or facial nerve. In her upper limbs, we observed sensory loss (anesthesia) in areas innervated by the median, ulnar, and radial nerves of the left hand, as well as a thickening of the left ulnar nerve. The Froment test was positive for her left hand and she had trophic changes (clawed left hand) (figure 4). In the lower limbs, we did not observe sensory ortrophic changes in the feet (figure 4).
The bacilloscopy was negative for BAAR. Serology to detect IgG anti-LID 1-NOD (cut-off>0.146 OD) was negative (0.111 OD), and IgM anti-PGL-1 (cut-off>0.162 OD) was also negative (0.098 OD).
Given that the neurological impairment caused a grade 2 disability in her left hand, the patient was diagnosed with pure neural leprosy and began MDT-MB in combination with prednisolone in a descending scheme (40 mg initial doses), as well as physical therapies. Currently, she has received 18 blisters of MDT-MB and she continues with prednisolone and physical therapy. However, despite the treatment, her disability in the left hand is worst because of impairment of the radial nerve (table 1).
Electromyography test was not available for any of these patients.
Discussion
These cases show thatpure neural leprosy represents a diagnostic challenge for healthcare personnel, even for those with extensive expertise, due to the fact that it typically affects the peripheral nervous system (PNS) as a mononeuropathy or multiplex mononeuropathy without causing skin lesions 11. Furthermore, confirming diagnosis by bacteriological and histopathological methods remains difficult, even via neural biopsy, which is considered as the gold standard for the diagnosis. The difficulty is primarily explained by the low bacillary load that is typical of this form of leprosy, and the fact that the histopathological changes associated with this condition are also present in other neuropathies, particularly vasculitis, and in chronic inflammatory demyelinating polyneuropathy (CIDP) 12. However, histopathological findings such as endoneural inflammation and epithelioid granulomas in the peripheral nerves of patients with epidemiological nexus and clinical manifestations of neural impairment are the major criteria for the diagnosis of pure neural leprosy 13. Furthermore, the electromyography can be an important tool in the detection of the nerve damage, and to determine the best place to take the biopsy, improving the sensitivity of the histopathology 1,6.
Today, the presence of M. leprae may be detected using other diagnostic alternatives, such as polymerase chain reaction (PCR), serological methods to detect antibodies against PGL-I or fine needle aspiration (FNA) cytology 14.
Reja, et al. 4, conducted a study using FNA in 13 patients with pure neural leprosy to demonstrate the presence of M. leprae through multiplex PCR and Ziehl-Neelsen (ZN) staining. Multiplex PCR detected the presence of mycobacteria in 11 patients (84.6%) and ZN staining showed BAAR in 5 patients (38.4%). The authors concluded that a combination of multiplex PCR and ZN staining (from FNA) provided a rapid and reliable diagnosis of the disease 4. Grossi, et al. 15, showed that serology can be useful in detecting and monitoring the disease given that subjects with more than one nerve trunk are four times more likely to be seropositive 15.
The use of serological tests such as PGL-I or NDO-LID could also be useful in the detection of pure neural leprosy as positivity of these serological tests is considered a diagnostic criterion for this condition. Moreover, it is important to mention that these tests are positive only in less than 50% of the patients with pure neural leprosy. Therefore, a negative serological test does not discard its presence 1.
Regarding the personal history of these four cases, it should be mentioned that all of them were both house hold contacts of patients with Hansen’s disease and blood-related. Thus, the patients had a 10 to 14 times more risk of contracting the disease than the general population 16. It is important to note that three of the four patients examined had contact with armadillos, that leprosyis considered a zoonotic disease and that they have tested positive for M. leprae in Colombia 17. Truman, et al. 18 confirmed the zoonotic transmission of leprosy: 25 patients with Hansen’s disease and 28 armadillos infected with leprosy shared the same M. leprae strain 18. Therefore, in patients with peripheral neuropathy of unknown etiology, it is important that physicians inquire about the personal history of their leprosy patients and whether there has been contact with animals that might be related to the transmission of the disease 16,19,20.
The classification of pure neural leprosy isusually paucibacillary (PB) 17, although Rodríguez, et al. 1, have recommended to consider the number of peripheral nerve trunks affected to begin the treatment: patients witht woor more affected peripheral nervetrunks should start MDT-MB for 12 months 1. Furthermore, patients can improve their neurological condition using steroids such as prednisolone in 40 to 60 mg doses. However, Smith, et al. 21, observed that the use of prednisolone in 20 mg doses had similar effects to avoid the impairment of nerves in leprosy patients.
In conclusion, pure neural leprosy is an enigmatic and often silent entity that primarily affects house-hold contacts of Hansen’s disease patients and its diagnosis is clinical. The disease is often disregarded for the differential diagnosis of possible peripheral neuropathy. It is, therefore, imperative that the medical personnel include it in their diagnostic possibilities.
Finally, it is necessary to continue working on reliable, non-invasive diagnostic techniques, possibly through a combination of serological and genomic tools. The combined use of these tests and of medical devices for the early detection of peripheral neuropathy would facilitate the early diagnosis of pure neural leprosy and mitigate the progression of disabilities caused by neural damage.