Toxocariasis is a soil-transmitted zoonotic disease caused mainly by ingestion of larvated eggs of Toxocara canis that are shed unembryonated with feces of dogs and other canids and reach the infectious stage in the soil. Following ingestion of infective eggs by man the larvae penetrate the small intestine, reach the circulation and spread via the systemic route to different organs where they can encyst for several years 1,2. Infection by ingestion of larvae present in the viscera and undercooked meat from infected paratenic hosts has been demonstrated experimentally in animal models 3.
The migrating larvae invade several organs in man most commonly lungs, liver, muscles, eye and nervous tissue. Human toxocariasis can be asymptomatic or might be present in different clinical syndromes depending on the organ involved, intensity of the infection and the immune status of the host. Allergic manifestations are also common 4. The disease may be classified as visceral larva migrans, ocular larva migrans, neurotoxocariasis and covert or common toxocariasis 5. Toxocariasis can persist for several years as a chronic infection and reactivation of the encysted larvae can occur in immunocompromised individuals leading to further larval migration and exaggeration of the clinical symptoms 6.
Their thick shell enables egg survival in the external environment for many years and confers them resistance to the harsh environmental conditions in soil, chemical treatment of wastewater and sew-age sludge 7,8. This leads to high environmental contamination, making the control of the infection a difficult task and increases the chance of dogs becoming infected at any time 9. Contamination of soil with Toxocara spp. eggs is common, reaching figures as high as 90% in some cases 10.
Toxocara caniseggs can enter sewage through toilets or storm runoff and have been shown to pass through municipal sewage treatment and remain viable. Accordingly, there is a disease transmission risk if sludge containing parasitic eggs is used in topical soil applications 11. Thus, determining the viability of T. canis eggs in soil and wastewater re-used in agriculture is particularly important to public health as a preventative measure.
Several techniques have been used to assess the viability of Toxocara spp. eggs, including animal infectivity, the accuracy of the morphological structure during observation under a microscope, internal structural changes, larval motility induction, use of dyes and PCR techniques 7,12,13. In the two widely used methods for wastewater and sewage sludge treatment, the standard United States Environmental Protection Agency method and that recommended by the World Health Organization, the viability is determined according to the presence or absence of larvae inside the eggs 14,15. However, the two methods do not consider a developing embryo prior to larval stages of development as potentially viable 16.
A limited number of studies were concerned with the early developmental stages of T. canis or were interested only on the early larval development till reaching maturity 7,17-19. There is no inclusive information about the morphological changes of the eggs from the one cell stage to the infective stage. Furthermore, there is a debate concerning the infectious stage of this important zoonotic helminth, whether it is the egg containing the second or the third stage larva 2,18,20.
The objective of the present work was to study the morphology of the intraovular developmental stages of T. canis in culture including the differentiation between viable and non-viable eggs as well as the sequences of larval molting. Furthermore, a comparison of the viability of eggs at the early stages of division and at reaching full maturation was carried out. This will help in determining which larval stage is the infective stage and will help also in estimating the public health hazards of presence of eggs with developing embryos in suitable environments.
Material and methods
Cultivation of Toxocara canis eggs
Adult T. canis worms were collected from the intestines of naturally infected puppies after they were euthanized. The worms were washed in physiological saline and the eggs were extracted from the vagina and the distal third of the uteri. Eggs were suspended in physiological saline solution, and mixed well to remove any effect of individual worm variation and then passed through a stainless steel mesh with 1 mm aperture size. The eggs were washed several times in physiological saline solution by centrifugation at 50gfor five minutes and were placed in five ml of 1% formalin in slightly covered 50 ml Erlenmeyer flasks at a final concentration of approximately 4,000 eggs/ml. The level of the solution was marked on the flask as a reference, and distilled water was added if evaporation was observed to maintain the desired concentration. Cultivation of eggs was performed at 28°C with continuous aeration for 30 min/day for 30 days 21. At this time the eggs were found to be at their maximum infectivity 18,22. A working egg solution with approximately 200 eggs/ml was prepared by suspending the eggs in distilled water.
Observation of the developmental stages of the embryo daily during the experiment
Approximately 200 eggs were placed on three microscope slides; each slide was covered with a cover glass, sealed with wax on the edges to prevent evaporation and examined using light microscopy. During examination, attention was given to the motility of the larvae inside the eggs under intense transmitted illumination of the microscope. The number of each developmental stage inside the eggs and the number of eggs with non-viable embryos from the first 100 eggs examined was recorded. When the first stage larvae had developed inside the eggs, examination was performed by gentle pressure using a cover slip to release the larvae. Examination of ten larvae was done every day until day 30. Daily examination was repeated three times.
Assessment of egg viability
The viability of the eggs was assessed during the early embryonic stage of development from day 5 to 21 of incubation, and compared with the viability detected at day 30.
At the early stages of development, viable eggs are those containing dividing embryos with clearly defined structure. Non-viable eggs are those that do not divide or are arrested in development at any stage. Eggs with abnormal morphology, collapsed shell, poorly defined internal structure and with degenerated contents are also non-viable. For eggs containing larvae, viability was determined by observing larval structure and motility inside the eggs under microscope light stimulation 16,23,24.
Statistical analysis
The viability of T. canis eggs used in this experiment from day 5-21 of cultivation was compared to the viability of eggs after one month of incubation (day 30). We performed a chi-square test for the proportions of viable embryos observed each day (between days five and 21), and the proportion of viable embryos at day 30 of incubation.
Results
Morphology of embryonic stages in Toxocara canis eggs
The fresh released eggs from the vagina and uteri of T. canis worms are ovoid to spherical. The egg shell consists of four layers; the outermost layer is pitted. The eggs are filled entirely with a cytoplasmic mass. After several hours of cultivation, the cytoplasmic mass is found condensed, forming a clear space between the embryo and the inner shell layer. The developmental stages identified inside the eggs are shown in figure 1, a-m. Some larvae were found to emerge naturally from a small hole in the eggshell (figure 1n).
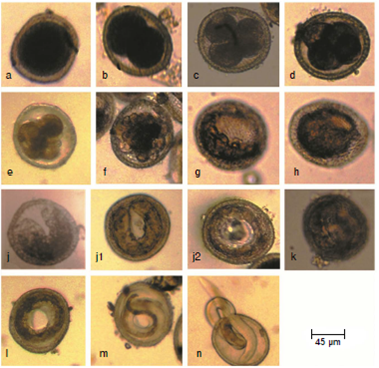
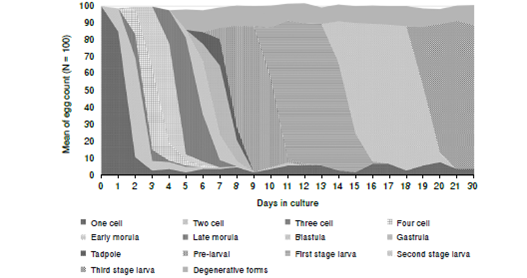
Figura 1 Embryonic stages of Toxocara canis eggs: a: One cell stage. b: Two cell stage. c: Three cell stage. d: Four cell stage. e: Early morula. f: Late morula. g: Blastula. h: Gastrula. i: Tadpole. j: Pre-larva j1: The embryo becomes long enough allowing the two ends of the “U” to meet each other. j2: With further growth, the embryo forms a close ring and starts a new one). k: First-stage larva. l: Second-stage larva. m: Third stage larva. n: Larva naturally emerged from a small hole in the egg shell
The rate of development of the embryos
Cell division commenced after 24 hours (day one) of cultivation. The rate of development is shown in figure 2. Each stage was found to be present over a period of 3-5 days except the first and the second stage larvae that were present for longer time. Usually, at a certain time there is more than one stage but there is a peak of one developmental stage. However, the three-cell stage was observed for 36 hours only.
Morphology of Toxocara canis larvae by light microscopy
First stage larvae released after artificial hatching of eggs using the cover-slip method at day 10 were delicate and could not tolerate being pressed. They had a blunt rounded anterior end. The region of the esophagus and that of the tail contained scattered large refractive globules and dark granules and in between a more dense intestinal region. However, the three regions were difficult to differentiate. The cuticle and the lips were not recognized. By days 12 and 13, first stage larvae were showing reduced density of granules in the esophageal and caudal regions (figure 3a). At day 13, the start of the first molt was observed where 8% of the larvae had a sheath at the buccal or caudal ends (figure 3b). At day 15 a loose sheath was seen at both extremities. The larvae showed reduce density of the granules in the esophageal and caudal regions. By day 16, the sheath is usually lost and most of the second stage larvae had no sheath (figure 3c). However, about 10% of the larvae retained the sheath.

Figura 3 Morphology of Toxocara canis larvae by light microscopy. a: First stage larva with poor demarcation of the esophageal, the intestinal and the tail regions. b: First stage larva with a sheath at the buccal ends. c: Second stage larva showing reduced density of the esophageal and caudal regions. d: Second stage larva with a second sheath characterized by a granular cone like structure at the anterior end. e: Second stage larva emerging from the egg surrounded by two sheaths (arrows). f: Third stage larva at day 21 of cultivation having well developed lips, granular intestinal region and distinct excretory pore. g: Third stage larva at day 30 of cultivation
At day 19, the second molt had commenced and the larvae that had lost the 1st sheath were surrounded by a new one at day 20 indicating a second molt. The second sheath differs from the first one, being much thicker and, also, by presence of a granular cone-like structure at the anterior end (figure 3d). The larvae that had retained the first sheath had now acquired an additional sheath from the second molt thus having two sheaths, proving that two molts had occurred (figure 3e). The most differentiated third stage larvae were seen by day 21. Most of the third stage larvae could withstand the hatching pressure and were intact. They were characterized by having well developed lips, a granular intestinal region and a distinct excretory pore (figure 3f). No change in the larval morphology was detected until the end of the experiment at 30 days of cultivation (figure 3g).
Egg viability
Viable eggs contain dividing embryos with a clearly defined structure. Throughout the experiment, non-viable eggs had been observed. Some eggs did not divide and remained at one or rarely two-cell stage. Others showed condensed (figure 4a), vacuolated (figure 4b) or degenerated cytoplasm (figure 4c). The shell was either thin (figure 4c) or collapsed (figure 4d). Eggs with deformed embryos were also encountered (figure 4e). After larval development non-viable eggs contained ill-defined larvae (figure 4f) or larvae that did not move after exposure to microscope light stimulation. The first stage larvae inside the eggs responded immediately by movement after light stimulation during examination by light microscopy. The second and third stage larvae responded to light stimulation after three to five minutes.
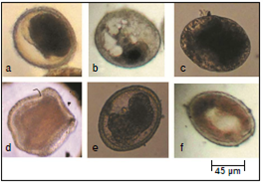
Figura 4 Morphology of non-viable eggs. a: Egg with condensed cytoplasm. b: Egg with vacuolated cytoplasm. c: Egg with degenerated cytoplasm and a thin shell. d: Collapsed egg. e: Egg with deformed embryo. f: Egg contains ill-defined larva
The viability of T. canis eggs at day 30 of incubation was 84.7% ± 2.1. Resulting p-values (>0.5%) showed no significant differences between the proportions of viable eggs from day 5 to day 21as compared to the proportion of viable eggs at day 30 of incubation, as shown in figure 5.
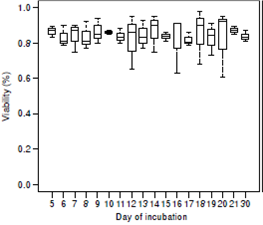
Figura 5 Viability proportions for Toxocara canis eggs during incubation. The y-axis represents the number of embryos viable in percentages. The x-axis represents the day of incubation (from day five to day 21 and day 30). Day 30 is used as a reference. The bottom and top of the box of each box represent the 25th and 75th percentiles, respectively. The band within the box is the 50th percentile (the median). The lines extending out from the top and bottom of the box represent the maximum and minimum values
Discussion
Daily observation of T. canis eggs incubated at 28°C in the present study revealed many developmental stages and lead to expanding the stages of intraovular development reported previously 7,19,25,26. These stages are one cell, two cells, three cells, four cells, early morula, late morula, blastula, gastrula, tadpole, pre-larva, first, second and third stage larvae. Different nomenclatures were previously given concerning some developmental stages of certain ascarids in various publications, in particular those developed just before the appearance of first stage larvae. Both in the current study and the study of the intraovular development of Toxocara pteropodis, the U shaped embryo was given the name of tadpole 27, while Cruz, et al. 16, gave the name pre-larva1 to this stage during Ascaris suum cultivation.
The present study demonstrates for the first time the pre-larval stage in the embryogenesis of T. canis. In this stage, there is elongation of the U shaped embryo allowing the two ends of the “U” to meet each other and, with further growth, the embryo forms a closed ring and starts another new ring. The morphology of the embryo that forms a closed ring and starts a new stage was described before as pre-larva 2 during cultivation of A. suum eggs by Cruz, et al. 16.
The shell of T. canis eggs as demonstrated in the present study is thick and is formed by four layers which correspond to those of Ascaris lumbricoides as described by Foor 28. The shell consists of an inner lipid layer, a middle chitinous layer, a protein layer and an outer layer which is pitted similar to that of most ascarids 28-30. The function of these layers is to minimize water loss so its thickness varied in different ascarids according to the environment in which they survive. Toxocara pteropoides, the parasite of the grey headed flying foxes which live in tropical environment on mangrove branches, has a thicker outer layer than that of T. canis eggs that live in soil and are less exposed to desiccation 30. Furthermore, eggs of Anisakis species, which develop in aquatic environments, lack the pitted outer layer 31. In the present work natural hatching of larvae was occasionally seen where larvae emerged from a thin area in the shell. This observation was also noticed in A. suum32.
The time intervals needed for the appearance of each developmental stage of T. canis varies between different studies, mainly because of variations in incubating temperatures. Temperature is a capital factor influencing the rate of T. canis egg development and the mean daily development rate increased significantly with temperature 7,19. O’Lorcain 25 and Azam, et al. 7, found that the eggs of T. canis cannot reach full embryonation neither at 37°C nor below 11.8°C and the highest percentage of viable larvated eggs is obtained after incubation at 28°C 21.
In the present study the larvae with the first cuticular sheath appeared at day 14 and the second sheath appeared at day 19. Brunaská, et al. 18, found that cuticular sheaths of T. canislarvae have the same electron microscopic structure of the cuticles. Most of the examined larvae at day 20 had one sheath. However, 10% of the larvae was found covered by two sheaths, which provides strong evidence of intraovular development of the third larval stageof T. canis. Some authors described a second molt within the eggs and referred to the third larva of T. canisas the infectious one 18,33. Two larval molts were also observed in T. pteropoides and A. suum inside the eggs proving that the infective stage is the third stage larvae 27,32,34. There is some controversy about the infectious stage. Some authors reported only one molt within T. caniseggsso that the second stage larva is considered as the infectious stage 20,35.
The most differentiated larvae observed in the present study were the third stage larvae which were present at day 21 of incubation. At day 30 of culture no change in the morphology was observed. Infectivity of eggs was not tested in the present study. Brunaská, et al. 18 and Abou-El-Naga 22 found that the maximum infectivity of larvated eggs is reached by day 30 of incubation. Prociv 27 and Green, et al. 32, found that the infectivity of T. pteropoides and A. suum appeared after the second molt and a certain time is needed before maximum infectivity is reached. Green, et al. 32, found that most of the third stage larvae lost their second sheath after a period of cultivation.
In the current study, the viability of the embryos at the early stage of development was determined by their continuous division and the clear defined structure. Some non-viable eggs were observed having a thin shell. Foor 28 found that the lipid droplets and granules of the infertile A. lumbricoides eggs persist in the cytoplasm and do not contribute in the shell formation resulting in a thin layer formation. The lipid layer plays an essential role in embryo protection 36. Few eggs were also found with abnormal morphology and were retarded in their embryogenesis which may be the result of impaired protein biosynthesis 37.
After larval development, larval integrity and motility in response to light stimulation were used as indicators of viability 15,16,24. Larvae in the eggs showed different motility intensities, where first stage larvae were very sensitive to light and showed active movement immediately after exposure to the light of the microscope during examination. The second and third stage larvae required 3-5 min of viewing time before any movement could be noticed. In agreement with the present results, Cruz, et al. 16, while examining A. suum eggs found that the first stage larvae showed active motility immediately while second stage larvae required 5-10 min of light stimulation. Johnson, et al. 24,observed that in most cases A. suum larval movement was detected within a few minutes, but some fields required 5-10 min of observation.
Determination of the viability of parasite eggs, including Toxocara spp. eggs, is necessary to access the hazards of environmental contamination, to monitor the safety of treated sanitary materials and to study the effect of different disinfectants. In the present work no significant difference was detected by comparing the viability of the eggs from day 5-21 with that recorded at day 30. Working on A. suum, Cruz, et al. 16,did not find any significant difference in the viability between the eggs containing embryos and those containing early larvae. Therefore, the early developing embryos of T. canis have the potential of achieving mature stage. Therefore, the presence of any developing embryos in T. canis eggs in the soil, sewage sludge and wastewater must be considered as a potential threat to the public health and hence treatment of these materials is necessary.
The eggs used in the present study were obtained from the uteri of mature female worms. These eggs are similar to eggs obtained from the feces of the definitive host as regards their infectivity and inactivation but there is a doubt as to whether the tanning process may lead to increase the resistance of the eggshell to environmental conditions 36,38. Ascaridia galli eggs isolated from chicken feces were found to have a higher capacity for embryonic development than uterine eggs due to maturation differences 39.
According to the results of the current experiment it could be concluded that two molts take place inside T. canis eggs and therefore the egg with the third stage larva is the infective stage. The precise identification of T. canis developmental stages in the present study and the clear differentiation of viable and non-viable parasite eggs can helpin determining an accurate baseline rate of development that could be used in studies of ovicidal compounds. Furthermore, the findings of the present work may contribute to the study of larval surfaces of the nematodes and their important role in the interaction with the host. Finally, the results high-light the public health importance of the presence of early developing embryos of T. canis in the soil, sewage sludge and wastewater