Tuberculosis (TB) continues to be an important cause of morbidity and mortality in individuals that are positive for the human immunodeficiency virus (HIV). Worldwide, 11.2% of the 10.4 million new TB cases in 2015 occurred in HIV-infected individuals. HIV co-infection is an important risk factor for the acquisition and development of active TB 1. Tuberculosis was reported to cause 1.4 million of deaths in 2015, 0.4 million of them among HIVpositive patients. In 2015, 268,000 active TB cases were reported in the Americas, 12% were coinfections with HIV, and 19% of patients died due to the co-infection. In that same year, 15,000 active TB cases were reported in Colombia, of which 14% were co-infections with HIV, similar to the 16% reported in 1995 in our institution (Velásquez G, García H, Arboleda C, Castro J, Díaz S, Garcés MC, et al. Características de la enfermedad tuberculosa y cumplimiento con las normas de aislamiento en un hospital universitario entre 1995 y 1998. Resumen A3. II Encuentro Nacional de Investigación en Enfermedades Infecciosas. Infectio. 2000;4:17).
Many co-infected patients are diagnosed only when they reach a third-level center; therefore, the diagnosis is late and leads to prolonged hospitalizations. Exploring risk factors for late diagnosis in 191 co-infected patients, Rossato, et al. 2 found that extrapulmonary TB and smear-negative TB contributed significantly to this delay.
HIV coinfection increases the risk of tuberculosis drug-induced liver injury (DILI) 3, complicating management and frequently forcing the discontinuation of first-line therapy and the use of alternative medications. On the other hand, TB/HIV co-infection also increases the risk of drug-resistant tuberculosis 4. These diagnostic and management challenges, adverse effects, and drug interactions all contribute to increase hospitalization rates and duration, as well as the cost of the therapy, with associated worse outcomes.
Recently, the advent of new molecular diagnostic tests for TB and a greater availability of antiretroviral therapy (ART) in Colombia may have changed the characteristics of TB/HIV co-infection in our patients. This study aimed to evaluate the current features of the co-infection, tuberculosis drug effects starting therapy, the impact of TB resistance and mortality in a cohort of TB/HIV co-infected patients hospitalized in a high-complexity hospital in Medellín, Colombia.
Materials and methods
Study design and population
We conducted a retrospective clinical chart review of hospitalized patients with TB/HIV co-infection in the Hospital Universitario San Vicente Fundación, (HUSVF) (a reference healthcare center for these kind of patients) in Medellín, Colombia, between 2007 and 2015, and of registers from Antioquia’s health information systems (Dirección Seccional de Salud de Antioquia, DSSA).
Inclusion criteria were age 15 years or older with a confirmed diagnosis of HIV and active TB infection, and complete access to the medical record of everyone. Patients were excluded in the absence of confirmed HIV or TB infection when charts were not available, or when any other mycobacteria different from Mycobacterium tuberculosis grew in the culture. All patients were under the medical care of the infectious diseases section of the Hospital.
Data collection and definitions
We collected all the demographic and epidemiological variables, as well as clinical, laboratory and treatment information regarding the co-infection. The confirmation of HIV infection required a positive Western blot or HIV viral load. In patients with a compatible clinical picture and no alternative diagnosis to explain it, TB was defined by the positivity of at least one of the following: i) Ziehl-Neelsen (ZN) stained smear; ii) M. tuberculosis culture; iii) ZN positive in compatible histopathology, and iv) M. tuberculosis molecular tests according to the diagnostic criteria of the World Health Organization 5. We used Ziehl-Neelsen stain for the entire duration of the study. Regarding the media for mycobacterial cultures, Ogawa was used between 2007 and 2012, Lowenstein from 2012 to 2015 and MGIT liquid culture as of 2015. Molecular tests have been performed since 2011 in the Hospital. Lymphocytes CD4+ count, HIV viral load, hepatitis B and C, and syphilis serology were obtained during the same hospitalization period in which co-infection was diagnosed.
Anemia was defined as a hemoglobin level of less than 13 g/dl in men and less than 12 g/dl in women; leukopenia as cell counts less than 4,000/μL, and thrombocytopenia as platelet count less than 100,000/μL. Clinical instability at admission was defined as a patient with at least three of the following vital signs out of normal range: Heart rate, 60-100/minute; spontaneous respiratory rate, 12-22/minute; systolic blood pressure, 90-120 mm Hg; temperature, 36.5-38.2°C, and pulse oximetry ≥90% without supplementary oxygen.
The TB clinical presentation was classified as pulmonary, extrapulmonary or mixed according to the compromised organs. TB treatment was categorized in two groups: i) First-line scheme [isoniazid (H), rifampicin (R), pyrazinamide (Z) and ethambutol (E)], and ii) a modified scheme when due to the clinical condition, adverse events, comorbidities, drug interactions or resistance to any TB medication, treatment included medications different from the first-line scheme.
Hepatotoxicity was defined as serum values of alanine aminotransferase (ALT) five times above the upper limit of normal (ULN) values in asymptomatic patients, or more than three times in patients with jaundice or gastrointestinal symptoms 6. Due to the retrospective character of the study, other clinical complications and adverse events associated with TB medications were only considered if they were verified by the specialist in infectious diseases in charge and documented in the clinical chart.
Poor adherence to treatment was defined as partial or total interruptions of treatment for more than a week prior to hospitalization, either by the patient’s own decision or due to difficulties in accessing health services. Due to the nature of the chart review, we were not able to differentiate between them.
Positive cultures for mycobacteria were sent to Antioquia’s laboratory of public health (Laboratorio de Salud Pública) for first-line drugs susceptibility testing. There, the susceptibility to rifampicin was confirmed by molecular methods and to all the first-line drugs by the proportions method. If MDR was found, these resistant strains were sent to the laboratory of the Instituto Nacional de Salud for evaluating the susceptibility to second-line drugs.
Statistical analysis
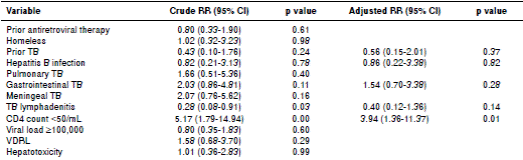
After we screened the database of all HIV patients for co-infection with TB, we did descriptive statistics of collected data. All variables were expressed as percentage or median and interquartile range (IQR). We evaluated risk factors associated with mortality using a binomial regression analysis adjusted by age. Covariates included in the analysis were previous ART, homelessness, previous TB, hepatitis B infection, main organs affected by TB, CD4 count cells <50/mm3, HIV viral load ≥100,000, reactive VDRL, and hepatotoxicity. Results were expressed as adjusted relative risk with their 95% confidence interval. Data were analyzed in the statistic program SPSS™, version 22, and Stata™, version 12.0.
Ethics statement
The ethical committees of the Universidad de Antioquia and the HUSVF approved the study.
Results
We screened 221 hospitalized patients with suspected TB/HIV co-infection within the 2007-2015 period of whom 178 were included and 43 excluded. The main baseline features of these patients are shown in table 1.
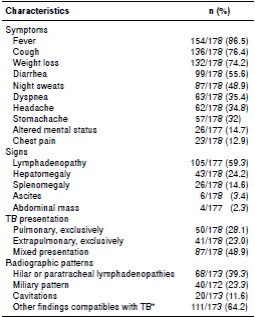
Table 1 Baseline characteristics of 178 hospitalized patients with TB/HIV co-infection in a university hospital in Medellín, Colombia
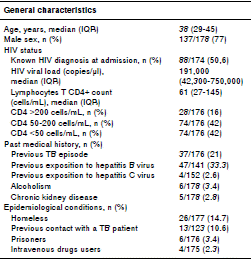
TB and HIV diagnosis were confirmed simultaneously in 49.4% patients (86/174); of the remaining individuals with previous HIV diagnosis, only 38 had been on ART, the majority of them with poor adherence and advanced immunosuppression.
Other co-infections diagnosed during hospitalization were toxoplasmosis in 10% of patients, histoplasmosis in 5.6%, cryptococcosis in 4.5%, and pneumocystosis in 3.4%. More than half (56.6%) of the patients had prior history of syphilis, 33% had been naturally exposed to wild hepatitis B virus (reactive AbHBc IgG), 8.9% were chronic carriers of AgsHB, and 19% had developed protective titers of AbHBs (>10 mIU/mL); 2.6% had evidence of previous exposition to hepatitis C (positive anti-HCV) (table 1).
At admission, 15% of patients had evidence of clinical instability, 86% anemia, 20% leucopenia, 23.5% thrombocytopenia, and 111 of 177 (63%) had liver function tests within normal range.
Clinical characteristics of TB in HIV-positive patients
Table 2 shows the clinical features at admission. Around 28% (50) of patients had pulmonary TB (PTB), 23% (41) extrapulmonary TB (EPTB) and 49% (87) disseminated disease. The organs and systems commonly affected in EPTB were lymphatic nodes (55.4%), peritoneum or gastrointestinal tract (35.9%), central nervous system (18.7%), and pleura (9.3%). Other TB presentations were genito-urinary (6 cases), bone marrow (6 cases), osteoarticular (3 cases), muscular (3 cases), pericardial (2 cases), laryngotracheal (1 case), ophthalmic (1 case), dorso-lumbar myelitis (1 case), mastoiditis (1 case), and cutaneous (1 case).
Microbiological diagnosis of TB
Table 3 shows the laboratory tool used for the diagnosis of TB. In general, Ziehl-Neelsen (ZN) stain was positive in 137 patients (77%), culture in 121 (68%), and M. tuberculosis PCR in 85 of the patients in whom this test was done. However, PCR supported the TB diagnosis in 25 patients with negative ZN. In 29 (16.3%) of the 178 patients the diagnosis was made only with ZN, and three of them died during the follow-up. In the two cases with negative ZN, culture and PCR, TB diagnosis was based on histopathology with positive ZN in tissues, clinical picture compatible with TB, and absence of an alternative diagnosis.
Table 3 Percentage of HIV/TB co-infected patients whose microbiological TB diagnosis was obtained by one or more diagnostic methods
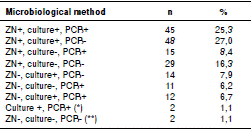
ZN: Ziehl-Neelsen stain; culture: Mycobacterium culture; PCR: Polymerase chain reaction
* No ZN available for two patients
** In two of the patients, diagnosis was made by compatible histopathology with positive ZN in tissues
Drug-susceptibility testing
Among all first-line antibiotics tested, resistance to rifampicin was detected in six of 123 cases with available information (4.9%): Four had multidrugresistant tuberculosis (MDR-TB), one had preextensively drug resistance (pre-XDR), resistance to INH, SM, RMP, and EMB (HRSE), ethionamide and levofloxacin, and no susceptibility test to pyrazinamide available (this patient had prior history of treated gastrointestinal TB and drug addiction disorder). The sixth case had resistance to rifampicin by a genotyping test (Gene Xpert™ MTB/RIF), but no susceptibility testing to other anti-tuberculous drugs was done.
Adverse events associated with anti-TB drugs
TB therapy was started in the hospital in 169 patients, 159 with the first-line scheme (HRZE), three with HZE plus moxifloxacin, one with RZE plus moxifloxacin, one with HRZ plus levofloxacin, two with HZE plus rifabutin, and three with secondline medications. Five did not receive in-hospital TB therapy because acid-fast stains had been negative and they were discharged before culture results were available, two died before the treatment could be initiated, and for two of the patients we had no available information.
An ALT increase was the most frequent adverse event associated with anti-TB therapy. ALT was measured in 177 patients and more than once in 152 (median=4.0, IQR: 2.0-6.0). Before starting TB treatment, the median of ALT was 26 U/L (IQR: 18-48). ALT was followed up for 18 days (IQR: 12-29) and 12 days was the time to get the maximal ALT value (IQR: 8-17). Among the 152 patients with more than one value available, 98 started TB treatment with normal ALT (64%), and 76 (50%) had an increase during the treatment; in eight the ULN increased between 3 and 5 times, in seven, more than 5 times, while none of the other 54 patients (36%) who started with an ALT level higher than the ULN increased more than twice the basal value.
Of the 159 patients who started on HRZE, 134 (84%) continued on the same medications at discharge, but 25 (16%) were switched to a modified scheme.
Hepatotoxicity, according to the established definition, was present in 15 cases (10%); three of them restarted the same medications once normal values were reached, and 12 begun a modified treatment. In addition to liver toxicity, in 13 patients there were other reasons to change TB treatment: Nine cases due to drug interactions, two cases because of in-hospital detection of drug-resistant TB, and one case because of bone marrow toxicity and another discontinuation attributed to arthralgias. Other reported side effects that did not require interruption or changes on anti-TB treatment were bone marrow toxicity in 6%, skin allergy in 3%, and neurotoxicity and nephrotoxicity in three and two cases, respectively. The immune reconstitution inflammatory syndrome (IRIS) was detected in 4% of the patients.
Risk factors associated with mortality
The median hospital stay was 19 days (IQR: 13-28), and the in-hospital mortality was 11.3% (20 of 177). According to registers of the local health information systems, at the end of February of 2015, 61 of the patients included in this study had died (34.3%). By multivariate analysis, having a CD4 count below 50 cells/mL was the only risk factor associated with in-hospital mortality (RR=3.94, 95% CI 1.36-11.37) (table 4).
Discussion
Our results highlight the fact that TB, a common opportunistic infection, led to the diagnosis of advanced HIV disease in about half of co-infected patients included in the study. Most of them were very immunocompromised and presented with disseminated and extrapulmonary disease, and almost 5% were resistant to rifampicin (generally associated with other resistances). During hospital stay, 16% had to interrupt or modify the TB first-line drugs (10% by hepatotoxicity), and CD4 cells<50/ μL at admission was the single factor associated with in-hospital mortality.
Despite of the knowledge amassed about HIV infection, the progress in ART and a better access of patients to it worldwide, as well as the availability of rapid diagnostic and drug-susceptibility tests for TB in the last years, our results show that even in countries with moderate TB incidence like Colombia, TB is still frequently diagnosed among patients with advanced HIV-associated immunosuppression (Velásquez G, Mejía P, Restrepo N, Betancour J. Comportamiento de la tuberculosis en paciente infectados por el virus de la inmunodeficiencia humana. Resúmenes del Segundo Encuentro Nacional de Investigación en Enfermedades Infecciosas. Medellín, Sociedad Colombiana de Infectología, 2000:4(1) Abs. E3:32.) 7-14.
Some possible reasons include late access to health systems (our hospital serves a community with low resources and poor access to health services) and structural delays in access to diagnosis and care. Barriers for antiretroviral therapy initiation are present despite global access to free antiretrovirals, which partially explains the fact that only 38 of the previously diagnosed individuals were on ART. The lack of early access to ART, even in the face of opportunistic infection, leads to repeated admissions, high costs, and poor outcomes. Individual characteristics such as alcohol and drug addiction, poor awareness of risk behaviors, misinformation regarding HIV disease (both among doctors and patients), and the fear of HIV test contribute as well to this situation. The fact that more than half of our patients are young adults previously exposed to other sexually transmitted diseases as syphilis and hepatitis B suggests poor knowledge about preventive measures and no awareness about risk behaviors, as well as limited access to HIV testing, which is a very important issue in our city.
Methods to diagnose TB are imperfect, therefore, many studies have shown that the association of fever, cough and weight loss should prompt diagnostic investigations in search for TB among HIV infected patients 15,16. In our study, confirmation of TB required the simultaneous use of multiple ZN stained smears and mycobacterial cultures, not only in pulmonary samples but also in many extrapulmonary specimens. In that sense, the study draws attention to the good diagnostic yield of the conventional methods such as ZN stain in all kind of samples (70% in respiratory samples and 51% in other samples), which could be explained by the multiple studies conducted in all affected organs 17-19.
On the other hand, the systematic study of all bronchoalveolar lavage (BAL) samples with very well trained staff increases the diagnostic yield of pulmonary TB in HIV patients 17. Our findings are in keeping with previously published reports suggesting that traditional microbiologic tests are still effective to diagnose the TB/HIV co-infection 20-22, which highlights the importance of sample quality and their systematic study.
The rate of rifampicin resistance in our co-infected patients was more than three times the 1,5% observed in the general population 23, similar to the 5% estimated globally for MDRTB 24, but different to the 19% reported in Eastern Europe, 7% in the Western Pacific region 25, and up to 18-26% of newly diagnosed TB cases in some countries 26. According to these findings, testing for TB drugs susceptibility, especially with molecular methods for first- and second-line ones, are mandatory for the timely management of all co-infected patients.
Resistance to rifampicin is usually associated with resistance to isoniazid and other drugs. However, monoresistance to rifampicin has been described in HIV-co-infected patients when they have been treated with intermittent rifampicin therapy and their CD4+ count is less than 100 cells/μL 27. It does not seem to be the problem in our patients, whose greatest risk of resistance is the poor adherence to the TB therapy 27-29; in fact, in the single patient whose resistance to rifampicin was confirmed only by Gene Xpert MTB, it was not possible to exclude resistance to other antibiotics.
Even though 50% of our patients experienced increases in ALT values after beginning the firstline TB treatment, only 9.4% (15 out of 159) had to interrupt it because of liver toxicity; however, contrary to expectations, in our study high basal ALT values did not predict hepatotoxicity due to TB therapy and, therefore, it did not lead to changes in the treatment. This prevalence is similar to the rates reported in India by Saha, et al. 30, who identified co-infection with hepatitis B or hepatitis C as risk factors for liver toxicity. We measured ALT values before starting TB treatment and could follow them closely until patients were discharged. In this study, neither co-infection with hepatitis B or C were associated with hepatotoxicity due to TB drugs. As biomarkers to predict liver toxicity have not been developed yet, the findings highlight the importance of closely monitoring the ALT levels in all TB/HIV patients, and not only in those with chronic hepatic diseases.
As demonstrated in other studies, we found that CD4 counts below 50 cells/μL were associated with in-hospital mortality 31. Again, this underpins the need to address the factors underlying late access to therapy. This subgroup of patients should be closely monitored, and considering their high mortality risk and the non-significant relevance of the immune reconstitution inflammatory syndrome (IRIS) in most of them (only described in 4% of our patients), it is imperative to initiate the ART as soon as possible, usually two weeks after starting the TB treatment 32,33.
The main limitations of our study are related to its retrospective nature, and to the lack of a systematic database of co-infected patients in the city, which made it difficult to obtain results regarding the final outcome of TB treatment, patient survival or availability of antiretroviral drugs in the outpatient program. The date of death was not available either, so survival time could not be estimated. Bearing in mind that this study was conducted in a tertiarycare hospital, it would be necessary to conduct additional studies in centers with different levels of complexity to better characterize the co-infection in our population and, of course, to identify the main challenges for their diagnosis and treatment. Given that approximately half of the patients was previously diagnosed with HIV and, yet, they advanced to severe immunosuppression, studying this group and identifying missed opportunities for linkage to care and antiretroviral therapy initiation is a public health imperative.
In conclusion, our study shows that even after 35 years of HIV pandemic, TB is still a leading cause of advanced HIV diagnosis in our patients. The coinfections affected young adults not aware of risky sexual behaviors who had limited access to HIV testing and presented with both extrapulmonary and pulmonary TB. As their resistance to rifampicin and/or MDRTB is around three times higher than in the general population, and patients frequently have to stop TB drugs because of liver toxicity (about 10%), which cannot be predicted by available biomarkers, it is always necessary to monitor liver function closely during TB therapy and rule out drug resistance.