Chagas disease (CD) is caused by the parasite Trypanosoma (Schizotrypanum) cruzi1, and mainly transmitted by blood-sucking vectors of the Reduviidae family. This disease is considered unattended and a serious public health problem in Latin America 2 due to the number of persons at risk and the lack of adequate and accessible treatment 3.
Trypanosoma cruzi exhibits a broad intraspecific genetic diversity and is classified into six discrete typing units (DTUs) identified from TcI to TcVI 4,5. TcI presents the broadest geographical distribution, which covers from the southern United States of America to northern Argentina and Chile. This DTU can be found in the sylvatic and domestic transmission cycles 5-7 and is known to infect 150 species belonging to 24 families of domestic and wild animals 8.
The main vectors of T. cruzi in the Andean region, the southern cone, and some areas of Central America are: Rhodnius prolixus, Triatoma dimidiata, Triatoma infestans, and Panstrogylus megistus9. A total of 26 species has been reported in Colombia and 15 of them have been found naturally infected with T. cruzi. R. prolixus, and T. dimidiata are considered primary vectors, whereas Panstrogylus geniculatus, Triatoma maculata, Rhodnius pictipes, and Rhodnius pallescens are considered secondary vectors 8.
In Colombia, the prevalence of Chagas disease has been estimated between 700,000 and 1,200,000 infected people and 3,500,000 people at risk 10. The Andean region is the most densely populated of the country and it harbors the municipalities with the highest Chagas disease transmission risk followed by the Orinoquia region 8,11. The departments with the highest infection rates are Arauca (21.1%), Casanare (10%), Santander (6.3%), Norte de Santander (5.2%), Boyacá (3.7%), Cundinamarca (1.9%), and Meta (1.7%) 11.
However, it is important to mention that as a neglected tropical disease, and due to the delay in the time of appearance of clinical symptoms (that can be diagnosed after 40 years of infection), the prevalence and incidence of Chagas disease are highly underestimated due to case underreport 12.
In the Caribbean coast, at least one case of the disease has been reported in every department 13-15. The known vectors found infected with T. cruzi are R. prolixus, T. maculata, T. dimidiata, R. pallescens, and Eratyrus cuspidatus, which are distributed in seven departments there 8.
The department of Córdoba is located north of Colombia on the Caribbean plains between 09°26’16’’ and 07°22’05’’ N, and 74°47’43’’ and 76°30’01’’ W, with an altitude between 260 and 2,200 m.a.s.l. It has 1,709,644 inhabitants distributed over an approximate total area of 23,980 km2, with a climate that goes from semi-wet to arid, and it includes 30 municipalities 16.
There are three main types of ecosystems in Córdoba: savannas, forests, and aquatic (marine and freshwater) systems. These ecosystems form a tropical wet biome with a high variety of native flora and fauna 17. Such diversity makes it suitable for the presence of Chagas disease vectors and reservoirs and for the establishment of different cycles of Chagas disease transmission. Furthermore, Córdoba socioeconomic characteristics favor the establishment of the disease, especially poverty (46.6%) and extreme poverty (12.9%), and a Gini coefficient of 0.465 18.
The main vector species in Córdoba is R. pallescens, which has been reported naturally infected with T. cruzi and T. rangeli in this region 8. Panstrongylus geniculatus and E. cuspidatus have also been reported. There is no information on P. geniculatus infection for this region so far, while E. cuspidatus has been reported naturally infected with T. cruzi8. Between 2012 and 2016, 20 cases of Chagas disease were reported in Córdoba by the Sistema Nacional de Vigilancia en Salud Pública (Sivigila) with an average of 4.2 cases per year 15 (figure 1).
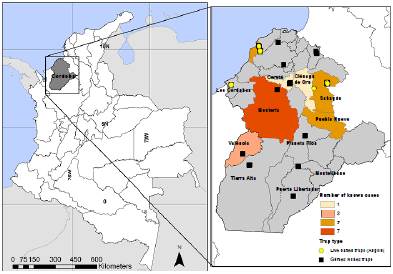
Figure 1 Location of Córdoba department in Colombia and distribution of municipalities and dispersed rural settlements (veredas) where sampling was performed. Chagas disease cases distribution by municipality (2012-2016) is also shown. In each of the 24 houses visited per dispersed rural settlement, two Gómez-Núñez traps were placed. Additionally, Angulo traps were placed in five dispersed rural settlements.
Regarding the entomological surveillance of the disease, community participation has proven to be the most appropriated strategy to develop a sustainable, effective, and efficient Chagas disease prevention program 19. An essential aspect to achieve this participation is that community members that interact with triatomines recognize them as Chagas disease vectors 19. Dumonteil, et al. 20 demonstrated that community-based collection was the most sensitive and cost-effective method as compared with timed manual searches for monitoring domestic and peridomestic infestation by non-domiciliated triatomines 20.
Due to the low number of cases occurring in Córdoba, little is known regarding vectors involved in Chagas disease transmission and circulating parasite strains. In 2011, the program “Diseño de un programa de estudios en infecciones y salud tropical para el departamento de Córdoba” received funds from the Sistema General de Regalías (general system of royalties) to determine the baseline for the study of tropical neglected diseases in the department.
In the context of this project, the aim of this study was to establish the triatomine species with which people come in contact and recognize as Chagas disease vectors, their infection with trypanosomes, and the insects host feeding preferences with the participation of local community members. Our results put in evidence that Chagas disease is present in Córdoba and contributed new records of triatomine insects, their spatial distribution, their infection with different T. cruzi strains, and information on their food sources in order to provide health authorities with relevant information for decision-making regarding tropical neglected diseases in the department of Córdoba.
Materials and methods
Insect collection
As a part of the above-mentioned program, fieldwork was performed in 12 municipalities and 15 dispersed rural settlements in Córdoba, Colombia, where 24 houses were randomly selected to conduct sampling of hematophagous insects. For triatominae screening, two Gómez-Núñez traps 21 were placed inside each of the 24 houses selected in each settlement from 2011 to August, 2016. The traps were checked for the presence of triatomines once a month.
Since triatominae collection in urban settings is challenging, we first visit each municipality to educate people on the importance of Chagas disease and to ask if they had seen triatomine bugs inside their houses. In three municipalities (Los Córdobas, Sahagún, and Moñitos), where human cases had been reported during the time of the study, we provided collection jars to the dwellers after explaining the general aspects of the disease and the risk of vector transmission and people were asked to deliver all collected specimens to the local health authorities.
A total of 96 houses were initially included for community sampling and, finally, triatomines were obtained from 45 houses. In each municipality, one inhabitant was designated to gather the insects obtained from different houses, to record the information, and to deliver the insects to the health authorities once a month.
Collected insects were then delivered to Universidad del Sinú to perform parasite detection in the rectal ampulla by dissection and then to the Centro de Investigaciones en Microbiología y Parasitología Tropical, CIMPAT at Universidad de Los Andes, where species identification and molecular analyses were performed.
Additional sampling with Angulo traps was performed in the three municipalities where collection jars were provided 22. In Moñitos, two Angulo traps were placed in palm trees on the night of May 12, 2015 (09°14’45.9’’ N, 76º04’ 54.6’’ W, 79 m.a.s.l.). In Sahagún dispersed rural settlements of Salitral (June 28 to July 1, 2016) and Villa Lucía (August 8 to 11, 2016), and in Los Córdobas and Guaimaro Abajo (August 1 to 3, 2016), three traps were placed during three consecutive nights in different species of palm trees around houses where infection had been presumably acquired (figure 1).
Collected specimens were identified by external morphological characters using Lent and Wygodzinsky taxonomic key 23 and Weirauch, et al., illustrated key 24.
Parasite detection
To perform parasite detection and blood meal analyses using molecular methods, DNA was extracted from triatomine intestinal content using the High Pure PCR Template Preparation Kit™ (Roche, USA) following manufacturer instructions.
Parasite detection by k-PCR was performed through amplification of a kinetoplastid DNA sequence with PCR using the 121 (5’AAATAATGTACGGGKGAGATGCATGA 3’) and 122 (5’ GGTTCGATTGGGGTTGGTGTAATATA 3’) primers 25 in a final volume of 25 μL containing 1X Go Taq Green Master Mix M7123™ (Promega, USA), 0.5 μM of each primer, and 3 μL of DNA.
The thermal profile consisted of an initial denaturation at 94°C for 3 min followed by 5 cycles at 94ºC for 1 min, annealing for 1 min at 68°C and 1 min at 72°C, then 35 cycles at 94°C for 45 s, annealing for 45 s at 64°C and 45 s at 72°C followed by a final extension step at 72°C for 10 min. The PCR products were resolved by 2% agarose gel electrophoresis and stained with SYBR Safe™ (Invitrogen, Ref. S33102) generating a product with a length of 330 bp for T. cruzi and of 400-450 bp for T. rangeli.
According to the diagnostic algorithm for T. cruzi26, the k-PCR results were confirmed with an SAT-PCR assay as follows: A satellite DNA sequence was amplified with PCR using the Cruzi 1 (5’ ASTCGGCTGATCGTTTTCGA 3’) and Cruzi 2 (5’ AATTCCTCCAAGCAGCGGATA 3’) primers 27 in a final volume of 25 μL containing 1X Go Taq Green Master Mix M7123™ (Promega, USA) and 0.5 μM of each primer and 3 μL of DNA.
The thermal profile consisted of an initial denaturation at 94°C for 5 min followed by 40 cycles at 94°C for 1 min, annealing for 30 s at 64°C and for 1 min at 72°C. The PCR products were resolved by 2% agarose gel electrophoresis and stained with SYBR Safe™ (Invitrogen, Ref. S33102) generating a product with a length of 166 bp for T. cruzi.
Genotyping
To perform T. cruzi molecular genotyping, the mini-exon gene was amplified with PCR using the TCC (5’ CCCCCCTCCCAGGCCACACTG 3’), TC1M (5’ GTGTCCGCCACCTCCTTCGGGCC 3’) and TC2 (5’ CCTGCAGGCACACGTGTGTGTG 3’) primers 28 in a final volume of 25 μL containing 1X Go Taq Green Master Mix M7123™ (Promega, USA), 0.5 μM of each primer, and 3 μL of DNA.
The thermal profile consisted of an initial denaturation at 94°C for 5 min followed by 5 cycles at 94°C for 1 min, annealing for 1 min at 67°C and 1 min at 72°C, then 5 cycles at 94°C for 1 min, annealing for 1 min at 65°C and 1 min at 72°C; 5 cycles at 94°C for 1 min, annealing for 1 min at 63°C and for 1 min at 72°C, and 30 cycles at 94°C for 1 min, annealing for 1 min at 61°C and for 1 min at 72°C followed by a final extension step at 72°C for 10 min. The PCR products were resolved by 2% agarose gel electrophoresis and stained with SYBR Safe™ (Invitrogen, Ref. S33102), generating a product with a length of 350 bp for TcI and 300 bp for TcII.
An SL-IR (spliced-leader intergenic region) sequence was amplified with PCR using the 1 Am (5’ TGTGTGTGTATGTATGTG 3’) 29 and the 1 B (5’ CGGAGCGGTGTGTGCAG 3’) primers 30 in a final volume of 20 μL containing 1X Buffer Taq™ (Invitrogen, USA), 0.25 mM of each dNTP, 1.5 mM MgCl2, 2,5 μM of each primer, 1 unit of Taq DNA polymerase (Invitrogen, USA), and 3 μL of DNA.
The thermal profile consisted of an initial denaturation step at 94°C for 4 min followed by 35 cycles at 94°C for 30 s, 20 s at 55°C, and 30 s at 72°C with a final extension at 72°C for 10 min. The PCR products were resolved by 2% agarose gel electrophoresis and stained with SYBR™ Safe (Invitrogen, Ref. S33102) generating a product with a length of 231 bp for TcIDOM and 450- 550 bp for TcI sylvatic.
Blood source detection
A PCR-based blood source detection was performed using the primers for the cytochrome b gene previously described 31. For detection of avian-derived blood source, the PCR was first conducted with the primers Avian-3 (5’ GACTGTGAYAAAATYCCMTTCCA 3’) and Avian-4 (5´GYCTTCAITYTTTGGYTTACAAGAC 3’) followed by a nested PCR with Avian-3 and Avian-8 (5’ TCTTTGGTTTACAAGACCAATGTTT 3’). For mammalian derived blood source, the PCR was first conducted with primers Mammalian-1 (5’ TGAYATGAAAAAYCATCGTTG 3’) and Mammalian-2 (5’ TGTAGTTRTCWGGGTCKCCTA 3’) and followed by nested PCR with Mammalian-2 and Mammalian-7 (5’ AAAAACCATCGTTGTATTTCAACTA 3’).
The PCRs were performed in a final volume of 25 μL containing 1X Go Taq Green Master Mix M7123™ (Promega, USA), 0.5 μM of each primer, and 3 μL of DNA.
The thermal profile consisted of an initial denaturation at 94°C for 2 min followed by 35 cycles at 94°C for 30 sec, annealing for 30 sec at 55°C and for 1 min at 72°C, and a final extension step at 72°C for 4 min. The PCR products were resolved by 2% agarose gel electrophoresis and stained with SYBR Safe™ (Invitrogen™ Ref. S33102) generating a product with a length of 530 bp for avian blood sources and 810 bp for mammalian blood sources.
All PCR positives products were bidirectionally sequenced at the DNA sequencing laboratory of Universidad de Los Andes using the ABI-3500 Genetic Analyzer™ (Life Technologies).
Results
Insect collection
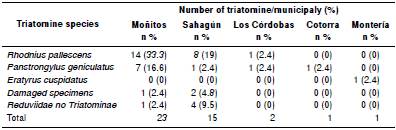
In total, 46 insects were collected, 45 (97.8%) by community members from five municipalities and one (2.1%) was captured using Angulo traps. No insects inside the houses were collected with Gómez-Núñez traps. All the collected specimens belonged to order Hemiptera, 37 to subfamily Triatominae, tribes Rhodniini and Triatomini, five to Reduviidae no Triatominae (predators), and four non Reduviidae (phytophagous) (table 1).
From 37 collected Triatominae, 34 were identified as belonging to three species: R. pallescens, P. geniculatus, and E. cuspidatus. In the remaining three, species identification wasn’t performed due to the damage of specimens during transport. Rhodnius pallescens was the species with the largest number of captured individuals 23 followed by P. geniculatus with 10 and E. cuspidatus with 1. From these 34 identified specimens, 31 were adults and 3 were nymphal instars. Out of the 37 collected triatomines, 36 (97.3%) were collected in dwellings at night and only one (2.7%), a nymphal instar, was collected in the peridomicile.
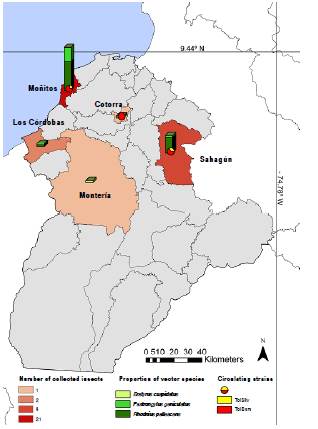
The triatomines were sent from five municipalities: Sahagún, Moñitos, Los Córdobas, Cotorra, and Montería (these two last municipalities were not previously included for community sampling, but two specimens were sent to the health authorities during the present study). The highest number of insects was captured in Moñitos (22 individuals) followed by Sahagún 11, Los Córdobas 2 Cotorra 1, and Montería 1 (figure 2).
Parasite detection
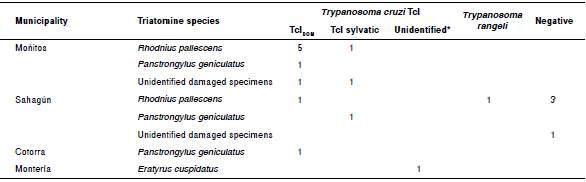
Eighteen triatomines were processed to establish possible infection by trypanosomes using PCR: Four were negative, 13 were positive for T. cruzi, and one for T. rangeli. From the 13 T. cruzi positive individuals, seven were R. pallescens, three P. geniculatus, and one E. cuspidatus. The remaining two specimens could not be identified due to the damage of taxonomic characters during transport. The insect infected with T. rangeli was identified as R. pallescens.
Genotyping
Genotyping showed that all the T. cruzi positive insects belonged to the TcI genotype. Nine were infected with the genotype T. cruzi TcIDOM: six R. pallescens (five from Moñitos and one from Sahagún), two P. geniculatus (one from Moñitos and one from Cotorra), and one specimen that could not be identified from Moñitos. The remaining infected insects had TcI sylvatic genotype: One R. pallescens, another specimen that could not be identified from Moñitos, and one P. geniculatus from Sahagún (figure 2, table 2).
Blood source detection
From 37 triatomine insects, 13 had blood and all these were processed. For these 13 insects, it was possible to establish the blood source in six of them using only conventional PCR; of the five R. pallescens specimens, three were positive for mammalian blood and two for both mammalian and avian blood, while one P. geniculatus insect was positive for mammalian blood. No sequence was obtained for any of the samples.
Discussion
Most of the collected triatomines (80.5%) corresponded to species known as Chagas vectors. The three species detected, R. pallescens, P. geniculatus, and E. cuspidatus, have been previously reported in the department of Córdoba (8). Rhodnius pallescens was the most abundant species (62.2%) followed by P. geniculatus (27%) and E. cuspidatus (2.7%). These findings are consistent with the few reports known for this region that show R. pallescens as the predominant species 8. Most of the R. pallescens specimens were collected in two previously reported municipalities, Moñitos and Sahagún, and only one individual was captured in Los Córdobas municipality.
In total, ten specimens of P. geniculatus were collected. Most of the specimens (seven) were collected in Moñitos. One specimen was collected in each of the following dispersed rural settlements: Sahagún, Cotorra, and Los Córdobas. This species had been previously reported in Moñitos and Sahagún 8, but it is the first time it has been reported in Cotorra and Los Córdobas. New records for two municipalities of the department were a P. geniculatus specimen collected in Cotorra municipality and one collected in Los Córdobas.
The species E. cuspidatus has been previously reported in Córdoba, but only in the municipality of Buenavista 8; in the present study, one specimen was collected in Montería. This is the first time that the presence of triatomines in Montería has been reported.
Three municipalities where triatomines were found (Sahagún, Cotorra, and Montería) have a savannah ecosystem. Two municipalities (Moñitos and Los Córdobas) have a coastal ecosystem 32. It is interesting to note that Sahagún is characterized by a dry environment and the presence of plant associations formed by palm trees, especially Attalea butyracea32. The presence of palm trees may facilitate the establishment of triatomines in dwellings in this municipality. In a different palm tree species, Cocos nucifera, one nymphal instar of R. pallescens was collected using Angulo traps in Moñitos municipality.
In the present study, the majority of the triatomines (89.1%) were collected in two municipalities, Moñitos and Sahagún. Most of the insects in these two municipalities were captured indoors. However, no sign of domiciliation was evident, as was shown by the absence of insects in the Gómez-Núñez traps. Three Chagas cases have been reported in each of these two dispersed rural settlements between 2012 and 2016 15, which represent 30% of the reported Chagas cases for the department.
Seven Chagas cases have been reported in Montería in the last five years. This is the municipality with the highest number of cases (33%) 15. However, the presence of triatomines had not been reported previously. In the present study, one E. cuspidatus infected with T. cruzi was collected and it is the first report of triatomines presence in this municipality.
The overall infection rate of T. cruzi among processed triatomines was 72% (13/18). The infection rate for R. pallescens was 64% (7/11), for P. geniculatus it was 100% (3/3), and for E. cuspidatus, 100% (1/1) (the remaining two T. cruzi positive specimens were not identified due to damage of the specimens). These indexes demonstrate that people in these areas are at high risk of T. cruzi infection, especially having in mind that most of the triatomines, 97.3% (36/37), were found by community members in their dwellings. Two new reports of natural infection for the department of Córdoba were recorded in this study: one P. geniculatus infected with T. cruzi and one R. pallescens infected with T. rangeli.
All collected insects positive for infection with trypanosomes presented T. cruzi DTU TcI, except for one that was positive for infection with T. rangeli. Currently, T. cruzi is recognized as a complex of six lineages or DTUs named TcI to TcVI 4,33. Each DTU can be loosely associated with a particular ecological and/or geographical framework. TcI has been found from Argentina to the United States, while TcII to VI are distributed from the Amazon basin to the south of Argentina 33,34. Nevertheless, it is possible to find regions where all DTUs are present, as it is the case of Colombia, where TcI has been predominantly reported while TcII, TcIII, TcIV, and TcVI have been reported in a low proportion 34.
TcI DTU is ubiquitous among arboreal sylvatic foci throughout the geographic distribution of T. cruzi. It is the major DTU associated with Chagas disease in humans and cardiomyopathies in northern South America 33,35. Several molecular tools now identify substantial genetic diversity within TcI; these new approaches consistently reveal the presence of a genetically divergent and homogeneous TcI group, currently called TcIDOM, and a second TcI group called sylvatic genotype 33,35.
In the present study, from 11 triatomines positive for T. cruzi TcI infection, nine were infected with the genotype TcIDOM. This conclusion is supported by recent findings where the circulation of TcIDOM is emerging in Colombia. In this study, sylvatic TcI was observed in 48% and TcIDOM in 52% of the isolates, concluding that this genotype is still adapting to the domestic cycle of transmission 36.
The remaining two T. cruzi TcI infected insects presented the TcI sylvatic genotype. This genotype is associated with the sylvatic cycle and with the peridomestic cycle of Chagas disease in Colombia. It has been isolated from synanthropic reservoirs like Didelphis marsupialis and Rattus rattus35. In this study, feeding preferences were determined in six triatomines. All of them had fed on mammalian blood and two of them had also fed on avian blood. It was not possible to establish the mammalian or avian species due to the lack of correspondence between the sequences in the GenBank. In some cases, it was not possible to obtain a consensus sequence probably due to the euryphagic behavior exhibited by the triatomines 37.
For R. pallescens, different mammalian blood sources, including opossum, anteater, sloth, rodent, and human, have been reported. Christensen, et al.38 demonstrated that slightly more than half of the R. pallescens collected in houses and nearby palm trees and bird nests feed on humans in Panamá 38. Opossums, which are important reservoirs of T. cruzi in Panamá and Colombia, were the second most frequently selected host 38.
The community participation in entomological surveillance could be a good strategy to determine the presence of triatomines, establish infection indexes, and detect circulating DTUs and genotypes in the area. The community-based collection method allowed to report the presence of triatomines and infected triatomines for the first time in some dispersed rural settlements. The time (15- 20 days) between the collection and the delivery to the entomology technicians was a problem of this strategy. During this period of time, triatomines died and the specimen dried affecting further molecular procedures.
Our results revealed the presence of T. cruzi infected triatomines inside the dwellings and the predominance of TcIDOM, a T. cruzi DTU associated with Chagas disease in humans and cardiomyopathies in Colombia 35. We also concluded that R. pallescens, the most abundant vector species in the area, apparently feeds on mammalian blood. According to the literature, this species has an anthropophagic affinity, especially in rural areas 38. All these results together demonstrate that Chagas disease is a threat for the inhabitants of these five municipalities, especially in Moñitos and Sahagún dispersed rural settlements. It is necessary to implement entomological surveillance programs that incorporate the community-based collection component.
Finally, our results highlight the participation of local community members in entomological surveillance tasks and confirm that community-based collection is an excellent tool for monitoring non-domiciliated triatomines 20. All captured insects were Hemiptera, which shows that people from these five municipalities are aware of this important group of insects.