Clinical case
We report the case of a 48-year old woman who had presented for 15 years with red plaques that started in the legs and spread to arms, thorax, and abdomen. She had auricular nodules, cheek papules, perforation of the nasal septum, bilateral ulnar thickening, thenar and hypothenar atrophy, and ulcers in the legs and ankles. Lesions were anesthetic. Bacilloscopy revealed a bacillary index of 4 and the skin biopsy showed diffuse dermal infiltrate of foamy macrophages, (Virchow´s cells) with acid-fast bacilli (AFB) and globii. Nerves had lamellar perineural thickening with intra-neural inflammation and abundant phagocyted bacilli in Schwann’s cells.
As the diagnosis was lepromatous leprosy, multidrug therapy was prescribed with rifampin, clofazimine, and dapsone 1,2. On her last medical appointment, the patient reported that she had taken multidrug therapy monthly for 32 months. Some brown plaques and papules persisted in several parts of the body, but no new lesions had appeared. The bacillary index was 2 in the skin smear.
A biopsy was taken from a forearm papule to determine the existence of active lepromatous leprosy. It was a 4 x 4 mm cylindric biopsy without hypodermis, which was processed with hematoxylin-eosin (HE) and Fite- Faraco staining (FF staining), as well as immunohistochemistry (IHC) for S100, CD68, and BCG.
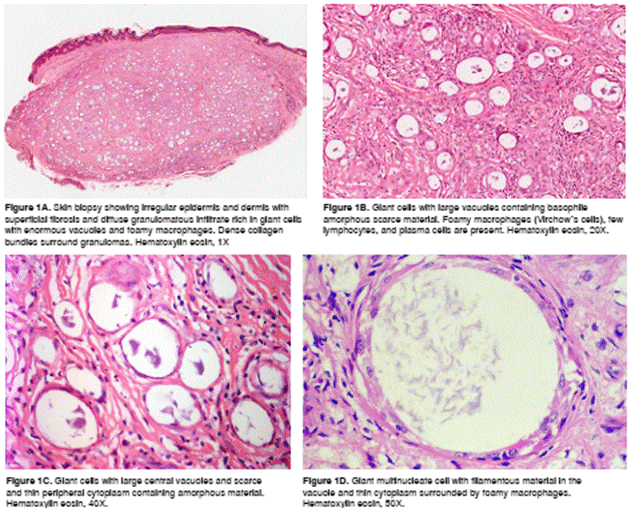
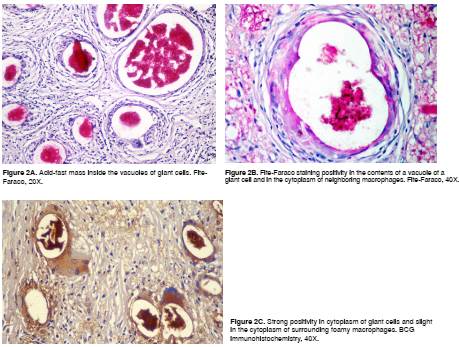
The biopsy showed diffuse dermal infiltration mostly consistent of prominent multinucleated giant cells with large cytoplasmic vacuoles, some of them of more than 100 µm diameter, containing amorphous or granular, slightly basophilic material with occasional granular forms that were diffusely positive with the FF staining and BCG IHC (figure 1 and 2).
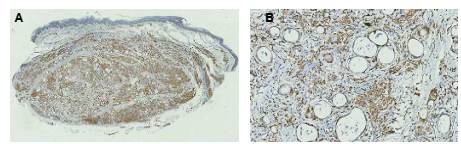
There were large groups of giant cells separated by thick collagen septa. We observed numerous vacuolated macrophages (Virchow´s cells) and few lymphocytes and plasma cells. No nerves were identified by S100 IHC. Immunohistochemistry for CD68 was positive in the scarce cytoplasm surrounding the vacuoles inside the giant cells and in the vacuolated macrophages of the infiltrate (figure 3).
Discussion
We report the case of a woman with long-standing lepromatous leprosy treated with multidrug therapy during 32 months because she had persistent papular and infiltrated lesions in the skin, although the World Health Organization (WHO) recommends a twelve-month therapy 1,2.
We have observed similar histological findings in cases where active disease is suspected. The cytoplasmic vacuoles of more than 100 µm in foreign body giant cells contain granular or filamentous material that is positive with FF staining and BCG immunohistochemistry and is indicative of residual fragments of Hansen bacilli destroyed by the treatment. BCG immunohistochemistry stains most of the mycobacteria and their dissociated components 3. We did not find complete bacilli.
Fibrosis inside the granulomas is a sign of lesion regression. All the inflammatory process explains the clinical lesions, but it also indicates there is not active lepromatous disease and that multidrug therapy does not need to be continued. The physician continues the therapy based on bacilloscopy, which is positive due to the presence of acid-alcohol resistant granules in the smear with a bacillary index of 1 to 2 that is lower than the value registered at admission. Physicians usually try to eliminate the clinical lesions with multidrug therapy, but these bacterial remains are not eliminated with this therapy and macrophages end up eliminating them over the years 4.
Lipid accumulation in the macrophages in lepromatous leprosy is induced by the bacillus, which promotes the formation of lipid vacuoles or adiposomes that constitute an intracellular ecologic niche protecting the bacillus from immunological response, making it impermeable to several compounds and medications, supplying nutrients, and allowing it to survive inside the macrophage for years 5-8.
Lipid components of the bacillus cell wall adhere to TLR2 and TLR6 receptors of the macrophage to be internalized in its cytoplasm 5-9. This process gives origin to molecular messages that propitiate local accumulation of cholesterol and its esters, which facilitate the entrance of new bacilli to the macrophage. Lipid cytoplasmic vacuoles, rich in diverse lipids, are also formed; they join the phagosome and contribute to an ideal environment for bacillary survival 5-9. These lipid vacuoles or adiposomes are cellular organelles induced by the bacillus, and they include eicosanoids such as prostaglandin E2, which induces the macrophage to carry out a Th2 immune response with production of interleukin 10 (IL-10) and abolition or decrease of interleukin 12 (IL-12), gamma interferon (IF ﻻ ) and nitric oxide leading to the inhibition of the Th1 immune response in order to avoid the macrophage attacking the bacillus 5. The macrophage genes activated by the bacillus also induce the production of phospholipases, which help to produce oxidized phospholipids and inhibit the immune response of the host 8.
Mycobacterium leprae induces the expression of the protein related with the adipose/adipophilin differentiation (ADRP) and with the peripilin, which promotes the accumulation of lipids in the macrophage and suppresses the expression of hormone-sensitive lipases so that lipids are not digested 5,6. Oxidized phospholipids also form in the phagosomic vacuole surrounding the cellular wall of the bacillus, which is one of its more protective components 9. An essential component of the cellular wall of M. leprae is phenolic glycolipid-I (PGL-I), which allows the entrance of the bacillus to cells, including the Schwann cells, and induces immune Th2 response, prevents phagosome-lysosome fusion and is antigenic. The antibody IgM titles against PGL-I diminish significantly when the antileprosy treatment is successful and explains its utility as a diagnostic and treatment control test in leprosy 5-7.
As the result of the lepromatous leprosy treatment, the size of the vacuolated macrophages increases as prominent cytoplasmic vacuoles are formed full of lipids, which suggests that they originate in bacillary disintegration due to the treatment 10. Sixty percent of the dry weight of the Hansen bacillus corresponds to lipids 5. Nevertheless, in cultured macrophages infected with M. leprae, it has been shown that clofazimine activates the formation of lipases and diminishes the accumulation of lipids inside the macrophage and that it also induces the production of interferons, all of which contributes to bacillary elimination 9. Macrophages may take an ochre look resulting from clofazimine accumulation in the HE stain.
The bacillary fragments appear in these vacuoles as granular acid-fast forms. There is a confluence of macrophages forming these voluminous cells, which may also contain cholesterol crystals. The rupture of macrophages and their phagocytosis contribute to this increase in cell size and to the formation of giant cells with the aspect of those appearing by the phagocytosis of free cholesterol 11.
As of 2007, WHO recommends treating multibacillary leprosy with three medications: rifampin, clofazimine, and dapsone for one year. This treatment is considered sufficient and relapses are few 1,2,12,13. Patients should have a bacilloscopy and skin biopsy at the end of the treatment and in annual follow-ups to compare the histologic findings and bacillary index values 4. Together with clinical findings, these methods are very helpful to determine if there is persistence or recurrence of the disease.
The viability studies of M. leprae in skin samples of patients with treated multibacillary leprosy carried out with mouse footpad inoculation and fluorescent microscopy have shown the possibility of recurrence of the disease in 3.3% of patients with high bacillary index and viable bacilli in the skin after 12 doses of treatment 12,13. The appearance of erythema nodosum leprosum is also possible, but the characteristics of the clinical lesions and the skin biopsy allow to clarify the diagnosis.
Conclusion
Lepromatous leprosy patients may present with residual clinical lesions when they have received the appropriate treatment or prolonged treatment for more than one year. The histological study of these lesions shows a diffuse pattern of vacuolated macrophages rich in giant cells of the foreign body type resulting from host lipolysis and mycobacteria lipids.
Without the medical history of the patient, the images from the histological study are a diagnostic challenge for the pathologist. The lesions disappear gradually and do not require anti-leprous treatment. FF staining may show acid-fast conglomerates or granular forms in the vacuoles, but not complete bacilli. BCG IHC does not identify them either.
The clinical and histological signs of inactivity and healing of leprosy should be known in order to avoid prolonged unnecessary medication. Clinical findings, comparative studies of smear tests, and biopsies, as well as IgM anti-PGL-I titles, are useful procedures to determine the cure or the persistence of the disease.